Abstract
Although many details remain still elusive, it became increasingly evident in recent years that mechanosensing of microenvironmental biophysical cues and subsequent mechanotransduction are strongly involved in the regulation of neuronal cell development and functioning. This review gives an overview about the current understanding of brain and neuronal cell mechanobiology and how it impacts on neurogenesis, neuronal migration, differentiation, and maturation. We will focus particularly on the events in the cell/microenvironment interface and the decisive extracellular matrix (ECM) parameters (i.e. rigidity and nanometric spatial organisation of adhesion sites) that modulate integrin adhesion complex-based mechanosensing and mechanotransductive signalling. It will also be outlined how biomaterial approaches mimicking essential ECM features help to understand these processes and how they can be used to control and guide neuronal cell behaviour by providing appropriate biophysical cues. In addition, principal biophysical methods will be highlighted that have been crucial for the study of neuronal mechanobiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For a long time, research on neuronal cell development and functioning was principally concentrated on the influence of biochemical factors, but in recent years, accumulating evidence made clear that taking a biophysical perspective on these processes is intriguing and promising for various reasons. One outstanding attribute of neuronal cells is the extreme polarisation and compartmentalisation that is taking place during neuronal differentiation and maturation. It requires highly coordinated and dynamic cytoskeletal actions and cell/microenvironment interactions to realise neuronal migration, neuritogenesis and synaptogenesis, and neural network formation and plasticity (Flynn 2013; Kerstein et al. 2015; Leterrier et al. 2017; Park and Goda 2016; Lilja and Ivaska 2018). Developing neurons possess growth cones which are sensory compartments at the tip of neurites or axons, built for the exploration of the microenvironment by their capacity to integrate chemical and mechanical cues (Chan and Odde 2008; Lowery and Van Vactor 2009; Myers et al. 2011; Vitriol and Zheng 2012; Franze et al. 2013; Kerstein et al. 2015) and specialised to operate in the soft brain tissue (Chan and Odde 2008; Betz et al. 2011; Kerstein et al. 2015). Also when neurons reached their terminal differentiation and maturation stage with a complex morphology, characterised by an axon and several dendrites equipped with numerous fine structures such as synapses and spines, they maintain a remarkable plasticity to enable the processing of incoming information. This plasticity is highly dependent on integrin-mediated interaction with the microenvironment (Park and Goda 2016; Lilja and Ivaska 2018).
Biophysical aspects and in particular integrin-mediated mechanotransductive processes, involved in the regulation of neuronal cell development and functioning, will be a focus of this review. Special attention will be drawn to what is happening in the cell/microenvironment interface as these events are, literally and functionally, at the base of the mechanotransductive signalling and its impact on cellular behaviour. Throughout the review, various examples of bioengineering approaches are indicated that were useful to gain insight into the influence of mechanotransduction on neuronal differentiation and/or exploit mechanotransductive mechanisms to guide neuronal cell behaviour in a controlled manner. Finally, this review will highlight some methods that are used to study biophysical aspects of (neuronal) cells and their environment. For further reading, the reader will be pointed to reviews that accentuate specific aspects of the different arguments in more detail.
Microenvironmental cues influencing neuronal cell development
Biophysical, structural, and compositional peculiarities of the extracellular matrix of the central nervous system
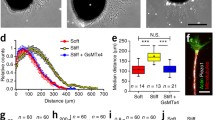
The extracellular matrix (ECM) of the central nervous system (CNS) has some distinctive and unique features regarding mechanics, structure, and composition that differ substantially from the ECM of other organs and tissues (Fig. 1a–d). Regional mechanical heterogeneity within brain and spinal cord compartments has been reported (Elkin et al. 2007; Christ et al. 2010; Koser et al. 2015; Antonovaite et al. 2018) (Fig. 1b), but the CNS is characterised by a general softness compared with other tissues (Franze et al. 2013; Barnes et al. 2017) (Fig. 1c). Interesting observations in this regard are the stiffening of the human brain tissue due to ageing (Sack et al. 2011) and changes in mechanical properties in neurodegenerative diseases (e.g. in multiple sclerosis, amyotrophic lateral sclerosis, and Alzheimer’s disease) and brain cancer (e.g. in glioblastoma) (Tyler 2012; Barnes et al. 2017; Tanner 2018) (Fig. 1c).
Compositional, mechanical, and structural features of the brain extracellular matrix. (a) The cartoon illustrates principal components of the brain extracellular matrix (ECM). Image with permission from Lau et al. (2013), Copyright (2013) Springer Nature. (b) Two examples of the mechanical properties (elastic modulus) in different brain compartments (top: mouse hippocampus, bottom: rat cerebellum, scale bar: 400 μm) are shown that were obtained by atomic force microscopy-based recordings. Images from Antonovaite et al. (2018), and with permission from Christ et al. (2010), Copyright (2010) Elsevier. (c) The graphic highlights the general softness of the brain tissue in comparison with other body tissues and indicates the stiffening in case of brain tumours. Image with permission from Barnes et al. (2017), Copyright (2017) Company of Biologists LTD. (d) The upper image demonstrates a stochastic optical reconstruction microscopy super-resolution recording of perineuronal nets (chondroitin sulphate proteoglycans stained with Wisteria floribunda agglutinin-Dy749P1). Image courtesy of Xiaowei Zhuang (Harvard University, Cambridge, MA, USA) (Sigal et al. 2019). The lower image shows a scanning electron microscopic recording of the configuration of decellularised hippocampal ECM. Image from Tajerian et al. (2018).
The ECM composition of the CNS is furthermore characterised by a high abundance of hyaluronic acid and other glycosaminoglycans, glycoproteins (such as tenascins, reelin, or laminins, the latter particularly in the region of the blood-brain barrier), and proteoglycans (such as lectican family members) (Fig. 1a) which are highly intertwined at the nanoscale (Fig. 1d). The non-fibrillary type IV collagen is present in the brain ECM but the amount of fibrillary proteins (such as collagen I) is instead relatively low (Ruoslahti 1996; Dityatev et al. 2010b; Lau et al. 2013).
Apart from the interstitial neural matrix, the brain ECM possesses also some special partitions with specific tasks and locations. In the subventricular zone of the adult brain, heparan sulphate proteoglycan and laminin-rich structures called fractones can be found close to the brain blood vessels that serve as neural stem cell niches and neurogenic zones (Kerever et al. 2007; Mercier 2016) (Fig. 1a). Another particular brain ECM structure is the perineuronal net; a specialised scaffold surrounding the neuronal cell body and proximal processes of many neurons in the CNS that is essential for the synaptic structure and regulates synaptic plasticity (especially crucial components, such as tenascin-C and tenascin-R, brevican, and neurocan) (Pizzorusso et al. 2002; Geissler et al. 2013; Sorg et al. 2016) (Fig. 1a, d).
ECM constituents and integrin/microenvironment interactions are indeed strongly involved in all steps of neuronal development (Long and Huttner 2019), which will be further outlined throughout the review, in particular in "Mechanotransductive processes and signalling in neuronal cell development and functioning." Furthermore, alterations in the brain ECM composition and organisation have been found in neurodegeneration and brain cancer (Bonneh-Barkay and Wiley 2009; Lau et al. 2013; Miyata and Kitagawa 2017; Barnes et al. 2017; Tanner 2018).
Decellularised brain ECM (an example can be seen in Fig. 1d) that are applied as substrates (either as 2D coating or 3D hydrogel) foster neurite outgrowth (Medberry et al. 2013) and functional neural network formation (Lam et al. 2019) better than equivalent decellularised ECM substrates from other tissues. A recent study furthermore shows that decellularised brain ECM increases the neuronal reprogramming efficiency of mouse embryonic fibroblasts into induced neurons, compared with 2D laminin-coated substrates. The promotive effect was already observable when the brain-derived ECM was also presented as a 2D coating, but strongly pronounced when it was applied as a 3D hydrogel environment (Jin et al. 2018). This emphasised the importance of appropriate microenvironmental biophysical and topographical cues, even in the presence of the same biochemical components.
The ECM building blocks and their interactions/crosslinking define the biophysical configuration of the intricate meshwork and determine its specific mechanical and structural properties, i.e. rigidity and nanotopography (Gasiorowski et al. 2013; Young et al. 2016) (Fig. 1a–d). In the next paragraphs, it will be detailed how these two principal biophysical cues deriving from the in vivo microenvironment, or from engineered biomaterials that are mimicking these pivotal features, impact on the neuronal cell behaviour and functioning.
Rigidity
Generally speaking, the interaction of (neural) stem cells (shown for embryonal and induced pluripotent stem cells, as well as foetal and adult neural stem or progenitor cells) with substrates that possess brain-like rigidity (usually ≤ 1 kPa elastic modulus, Fig. 1c) favours neuronal viability (Georges et al. 2006) and directs their fate towards neuronal lineage commitment (Saha et al. 2008; Leipzig and Shoichet 2009; Teixeira et al. 2009; Banerjee et al. 2009; Seidlits et al. 2010; Keung et al. 2011, 2012, 2013; Franze et al. 2013; Mammadov et al. 2013; Musah et al. 2014; Sun et al. 2014).
Numerous studies furthermore indicated a promotive effect of softer substrates on neurite outgrowth for a variety of neuronal cell types and different gel materials used as substrates (Balgude et al. 2001; Willits and Skornia 2004; Kostic et al. 2007; Jiang et al. 2008; Teixeira et al. 2009; Sundararaghavan et al. 2009; Cheng et al. 2011; Man et al. 2011; Koch et al. 2012; Hopkins et al. 2013; Franze et al. 2013; Kerstein et al. 2015; Mosley et al. 2017). However, it should be mentioned that also some conflicting results regarding neuron sensitivity towards substrate rigidity have been reported; in some studies (e.g. with PC12 cells (Leach et al. 2007), cortical neurons (Norman and Aranda-Espinoza 2010), or hippocampal neurons (Koch et al. 2012)), neurite outgrowth was insensitive to the tested mechanical substrate properties, or in one case for cortical neurons, an even stronger outgrowth on stiffer substrates was noted (Stabenfeldt and LaPlaca 2011). Furthermore, it has been shown that the mechanosensitivity varies between different neuronal cell types (Koch et al. 2012). Certainly, the plethora of utilised combinations between substrate materials and ligands/adhesive agents (e.g. (hydro)gels made from agarose, collagen I, polyacrylamide, polydimethylsiloxane, silk fibroin, polyethylene glycol, or methylcellulose, functionalised with often varying concentrations of collagen I, laminin, fibronectin, matrigel, or poly-lysine; taking into account only some of the cited references) complicates a comparison of the results. The contradictory effects could be due to not considered aspects such as structural differences in porosity, crosslinking, and mesh size, which could have led to changes in topographical parameters. These topographical parameters affect also strongly neurite outgrowth, as will be highlighted in the next paragraph.
Topography
A widely demonstrated impact of accordingly designed anisotropic micro- or nanotopographical features on neuronal cell behaviour (shown for various neuronal cell types and (neural) stem cells during neuronal differentiation) is the alignment of cell polarity and neurite/axon outgrowth (Hoffman-Kim et al. 2010; Kim et al. 2013; Simitzi et al. 2017), e.g. along ridges (Rajnicek et al. 1997; Johansson et al. 2006; Ferrari et al. 2010; Lee et al. 2010; Ferrari et al. 2011; Béduer et al. 2012; Yang et al. 2013, 2014; Baek et al. 2018), electrospun fibres (Xie et al. 2009; Lim et al. 2010; Wang et al. 2010; Gertz et al. 2010; Smith Callahan et al. 2013), pillar arrays (Park et al. 2016b), or elliptical cones (Simitzi et al. 2015). This contact guidance was attributed to focal adhesion confinement and alignment (Ferrari et al. 2010, 2011; Tonazzini et al. 2013; Yang et al. 2014; Baek et al. 2018), favouring in this manner, the effective neurite outgrowth in a specific direction. This phenomena is of high biomedical interest and already exploited to improve nerve guidance conduits utilised to promote the regeneration of peripheral nerve cells (Hoffman-Kim et al. 2010; Sarker et al. 2018).
However, the potential impact of appropriate nanotopographical features goes beyond these more geometrically guiding effects, since they can also influence the neuronal program, i.e. gene/protein expression and differentiation, as specific instructive cues (Xie et al. 2009; Lee et al. 2010; Lim et al. 2010; Yang et al. 2013; Smith Callahan et al. 2013; Yang et al. 2014); similar to the effects described for soft substrates. In fact, even isotropic and disordered nanotopographies (made by quite different methods and materials, e.g. silica nanobeads, carbon nanotubes, silicon nanowires, assembled zirconia nanoclusters, and nanorough glass surfaces generated by reactive-ion etching or platinum-coated polystyrene nanopattern) have been reported to promote neuritogenesis, neuronal differentiation, and neural network maturation (Migliorini et al. 2011; Kang et al. 2012; Fabbro et al. 2012; Bugnicourt et al. 2014; Schulte et al. 2016c, b; Chen et al. 2018; Baek et al. 2018; Schulte et al. 2018). Nanotopographies with a suitable dimensionality have the ability to modulate integrin adhesion complexes (IAC) in a way that impacts on neuronal cell decision making, programming, and fate (Yang et al. 2013, 2014; Schulte et al. 2016c, b; Maffioli et al. 2017; Chen et al. 2018; Baek et al. 2018).
In the last decades, it has been unravelled that mechanotransductive processes are at the basis of these biophysical cue effects on (neuronal) cell development. The next paragraphs will therefore focus on how the cellular mechanotransductive machinery actually senses and interprets microenvironmental biophysical features in "Mechanosensing and mechanotransduction" (Fig. 2a–d), highlighting in particular also what is known about the neuronal context in "Mechanotransductive processes and signalling in neuronal cell development and functioning" (Fig. 3).
Integrin-mediated mechanosensing and mechanotransductive sequence with influencing parameters of the extracellular matrix. (a) The cartoon illustrates the initial ECM-integrin-talin-actin linkage in the nascent adhesions and how the force loading within this molecular clutch determines whether this structure disassembles (in case of too low force loading) or reinforces (in case of sufficient force loading) and (b) matures into integrin adhesion complexes (IAC) by recruitment of further proteins. (c) In this graphic, the stratified nanoarchitecture of mature IAC with its different layers is shown. (d) The extent of force loading and IAC maturation is determined by biophysical cues of the extracellular matrix, in particular, the rigidity and the spatial organisation of the integrin adhesion sites (in terms of spacing, distribution, (dis)order, (an)isotropy and nanotopography). Further details on IAC maturation are outlined in "Mechanosensing and mechanotransduction". The figure contains adapted elements of images with permission from Case et al. (2015) and Case and Waterman (2015), Copyright (2015) Springer Nature; Barnes et al. (2017), Copyright (2017) Company of Biologists LTD; and an adapted element with permission from Borghi et al. (2018), Copyright (2018) American Chemical Society.
Mechanotransductive processes in neuronal development and functioning. (a) The graphic illustrates the different phases of neuronal cell development (in this case during cortex formation) starting from self-renewal and neurogenesis, passing to neuronal migration, neuritogenesis, and ending with terminal differentiation and maturation with synaptogenesis and network integration (VZ: Ventricular zone, SVZ: Subventricular zone, IZ: Intermediate zone, CP: Cortical plate). Examples of extracellular matrix and cellular proteins related to mechanotransductive processes that are known to influence different phases of these events are indicated in bold and underlined. Further details can be found in "Mechanotransductive processes and signalling in neuronal cell development and functioning". The figure contains elements of an image from Schulte et al. (2016b). (b) The panel shows in vivo mechanosensitivity of a growing axon in the Xenopus brain. The colour code indicates (a) the stiffness (elastic modulus) of the brain tissue measured by atomic force microscopy-based recordings or (b) the stiffness changes over time in the same region. The fluorescently labelled axon was tracked and outlined in blue and documents the directed axon movement towards the softer region (Scale bars = 100 μm). The image has been reproduced from Thompson et al. (n.d.). (c) The panel demonstrates the modulations along the mechanotransductive sequence induced by the interaction of neuron-like PC12 cells with ECM-mimicking nanotopographical zirconia substrates produced by the nanofabrication technique supersonic cluster beam deposition, compared with flat zirconia surfaces. In the transmission electron images, it can be seen that the cells interact only with the apical part of the nanotopographical asperities which restricts the dimension of the nanometric adhesion sites (indicated by the white arrows) to smaller sizes with respect to the situation on flat zirconia. Also, the integrin adhesion complexes (vinculin staining in green recorded by TIRF microscopy) remain of small dimensions (focal contact/point contact size, see white arrows with dashed lines) whereas mature focal adhesions form only on the flat substrate (see white arrows). Consequently, on the nanostructured zirconia, no stress fibre formation (epifluorescence of phalloidin staining in red) can be noted, while there are abundant stress fibres on the flat zirconia (examples marked by white asterisks). The cells on the nanotopographical substrate are softer than on the flat surface (quantified by atomic microscopy-based analysis, the colour code indicates the Young’s modulus (YM)) and neurite outgrowth was visible (black arrow). The image was adapted with permission from Schulte et al. (2017), Copyright (2017) American Chemical Society.
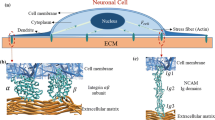
Mechanosensing in the cell/microenvironment interface and neuronal mechanotransductive processes and signalling
The adhesive structures in the cell/microenvironment interface that enable the cell to perceive the biophysical configuration of its microenvironment and to translate the information into appropriate cellular responses are highly intricate. We will concentrate on the fundamental mechanotransducers of the cells, i.e. integrin adhesion complexes (IAC), although various cell surface receptors are known to contribute to mechanosensing and mechanotransduction (such as GPI-anchored proteins (Kalappurakkal et al. 2019) (e.g. uPAR (Ferraris et al. 2014; Schulte et al. 2016a)), CD44 (Seidlits et al. 2010; Kim and Kumar 2014), syndecans (Bass et al. 2007; Morgan et al. 2013), or receptor tyrosine kinases (Yang et al. 2016)), which often cooperate in some way with integrins. First, we will detail processes of mechanosensing in the cell/microenvironment interface in general (Fig. 2a–c) and the decisive extracellular matrix parameters that influence them (Fig. 2d). It should be noted that, for the most part, this understanding on mechanosensing and mechanotransduction reported in paragraph below was not specifically obtained from studies on neurons, but it is likely that the general mechanisms are largely comparable in neurons.
Mechanosensing and mechanotransduction
The integrin family consists of heterodimeric transmembrane receptors with one α- (18 types exist) and one β-subunit (8 types) that can assemble in 24 different combinations. They possess large extracellular domains and short cytoplasmic tails (with the exception of α6β4 integrin that has a longer β-subunit tail). Despite some redundancy, the different integrin heterodimers have distinct binding specificities to ligands (such as the RGD motif) present in the numerous proteins of the ECM (or in some cases, receptors in the membrane of other cells) rendering possible the versatility of integrin signalling and its broad involvement in many cell biological events. However, there are common features of integrin-mediated cell adhesion realising mechanosensing and interpretation of the microenvironment by mechanotransductive processes (Changede and Sheetz 2017; Gauthier and Roca-Cusachs 2018; Sun et al. 2019; Kechagia et al. 2019) (Fig. 2a–d).
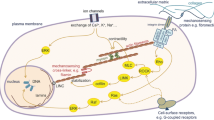
Integrin activation, i.e. the transition from low to high ligand affinity state by changing the integrin conformation from bent and closed to extended and open with separated cytoplasmic tails (Shattil et al. 2010; Zhu et al. 2013), can either be induced and/or stabilised by integrin ligand binding itself (outside-in signalling), by intracellular events (inside-out signalling, often through signals arriving from G-protein coupled receptors) (Sun et al. 2019; Kechagia et al. 2019), or by modulation of the membrane tension (Wang and Ha 2013; Ferraris et al. 2014; Paszek et al. 2014; Schulte et al. 2016a; Gauthier and Roca-Cusachs 2018). In any case, the adaptor proteins talin and kindlin are recruited to the cytoplasmic tail of the integrin β-subunit, which is essential for integrin activation (Jiang et al. 2003; Theodosiou et al. 2016). The glycocalyx, a pericellular sugar coat that surrounds the cell membrane and is attached to proteoglycans, glycolipids, and glycoproteins, is another important player in the cell/microenvironment interface that influences integrin properties. Its compression in the vicinity of integrin/substrate binding sites leads to mechanical loading of the integrins through force application towards the cell membrane. The compressed glycocalyx acts furthermore as a steric kinetic trap that impacts on lateral integrin diffusion and promotes integrin clustering (Paszek et al. 2014). These initial processes are independent of actomyosin contraction (Choi et al. 2008; Wang and Ha 2013; Changede et al. 2015) (Fig. 2a).
However, the talin rod can bind to filamentous actin (f-actin), which connects the ECM to the actin cytoskeleton in the nascent adhesions (Jiang et al. 2003), engaging in this manner also the molecular clutch by linking the integrins to the retrograde actin flow and its forces generated by actin polymerisation and actomyosin contraction (Chan and Odde 2008; Zhang et al. 2008; Schulte et al. 2016a) (Fig. 2a). An interesting historic side note in the context of this review is the fact that the molecular clutch hypothesis was first developed (Mitchison and Kirschner 1988) and decisively elaborated (Chan and Odde 2008) studying neuronal growth cones. However, whether this initial structure disassembles immediately or instead is reinforced and matures by recruitment of further proteins and integrin clustering depends on the extent of force loading within the molecular clutch (Wang and Ha 2013; Oria et al. 2017) (Fig. 2a–b). At sufficient force loading, different stabilising events can take place. Forces in the low piconewton (pN) range are sufficient to maintain integrins in their extended conformation (which can happen very quickly in less than a second) (Strohmeyer et al. 2017; Li and Springer 2017). At forces in the order of tens of pN, the ECM/integrin binding can further strengthen by catch bond formation, shown, e.g. for α5β1 and αVβ3 integrin, increasing thus the lifetime of the bond (Kong et al. 2009; Chen et al. 2017). Talin can be activated by stretching of the talin rod (starting from forces of ~ 5 pN to tens of pN) which leads to unfolding of cryptic binding sites for vinculin, first near the membrane and integrins and later closer to the f-actin. Vinculin is recruited to these uncovered sites, leading to its movement towards f-actin at higher force loading (> 5–25 pN). During these events, vinculin is activated itself and its tail forms a catch bond with f-actin (maximally stable at ~ 8 pN), thereby additionally stabilising the nascent adhesions (del Rio et al. 2009; Grashoff et al. 2010; Ciobanasu et al. 2014; Yao et al. 2014; Case et al. 2015; Elosegui-Artola et al. 2016; Huang et al. 2017) (Fig. 2a). This force-dependent reinforcement and the consequential increase in lifetime allow the recruitment of further essential IAC components (Carisey et al. 2013; Case et al. 2015). The forming IAC organise into modular nanometric units (with dimensions of ~ 80–120 nm containing 20–50 integrins (Changede et al. 2015)) with a stratified nanoarchitecture composed of 3 layers, i.e. an integrin signalling layer (containing, e.g. paxillin, integrin-linked kinase (ILK), focal adhesion kinase (FAK), p130Cas, and src), a force transduction layer (mainly talin and vinculin), and an actin regulatory layer (e.g. f-actin, α-actinin, zyxin, or VASP) (Case et al. 2015) (Fig. 2b–c). In further maturation steps, the modules can group into structures with increasing dimensions, i.e. first in focal complexes then focal adhesions, but their dimension and especially composition are quite versatile depending on the cell biological context. The recruitment of many adaptor and signalling proteins, such as paxillin, ILK, FAK, src, p130cas, PAK, or ERK, transforms the IAC into signalling hubs capable of controlling and influencing cell signalling, decision making, and fate in many ways. Integrin downstream signalling controls actin cytoskeletal dynamics through modulation of RhoGTPase activity (in particular RhoA, Rac1, and Cdc42). Changes in the cytoskeletal organisation, in turn, impact on the localisation of mechanosensitive transcription factors, such as YAP/TAZ (yes-associated protein/transcriptional coactivator with PDZ-binding motif) and MRTF-A (myocardin-related transcription factor-A). RhoGTPase activity influences also proliferation and differentiation by activation of the ERK/MAPK pathway (Sun et al. 2019; Kechagia et al. 2019; Humphries et al. 2019; Green and Brown 2019). The remodelling of the cytoskeleton can furthermore lead to alterations in the nuclear architecture by its connection via the LINC (Linker of Nucleoskeleton and Cytoskeleton) complex, which affects the spatial chromosome organisation and gene expression by mechanoregulatory transcription factors (Uhler and Shivashankar 2017).
Whether sufficient force loading within molecular clutch permits the different steps of reinforcement and IAC maturation depends decisively on critical mechanical and structural microenvironmental parameters, i.e. the substrate rigidity and the spatial organisation of integrin adhesion sites (in particular in terms of ligand spacing, density, and distribution, as well as topography) (Fig. 2d), or their combination (Gauthier and Roca-Cusachs 2018; Kechagia et al. 2019). In general, the lower the rigidity, the (s)lower is the force loading per integrin. If the rigidity is too low, the initial adhesion is likely to disassemble before the mentioned force thresholds in the molecular clutch can be achieved because the integrin/ligand bond lifetime is too short. Higher rigidities instead enable a stronger force transmission along the ECM/integrin/talin/f-actin axis surpassing the force thresholds and favouring thus IAC reinforcement and maturation processes (Elosegui-Artola et al. 2016) (Fig. 2a). Furthermore, it has been observed that IAC maturation is not taking place if the ligand spacing distance exceeds a certain threshold (> 60–70 nm) on rigid substrates (Arnold et al. 2004). Until very recently, this was attributed to a potential direct measurement of the ligand spacing by an adaptor protein (with talin as a potential candidate) that works as a molecular ruler, but new data indicate instead that the force loading within the molecular clutch is actually the principal decisive factor. Considering both parameters (i.e. rigidity and spatial organisation and distribution of adhesion sites) in combination, there are indeed counter-intuitive effects (Oria et al. 2017). High rigidity substrates with ligand spacing distances that are too large can increase the force load per integrin to an extremely high, i.e. eventually too high, level, which causes an adhesion collapse (Liu et al. 2014; Oria et al. 2017); most probably due to limitations in the maximal integrin recruitment impeding sufficient force redistribution. On lower substrate rigidities, an increase in ligand spacing distance can lead to an augmentation of force load per integrin above the critical force thresholds, enabling IAC maturation (Oria et al. 2017). However, a minimal adhesion unit of ~ 3–6 integrin binding ligands in certain vicinity (i.e. in the high tens of nm) promotes integrin clustering, including also the recruitment of unligated integrins. This accentuates the importance of the distribution of the adhesion sites in terms of order and explains why introducing disorder in the adhesion site distribution (and local differences in ligand spacing) can change again the whole outcome in regards to whether IAC maturation takes place or not (Jiang et al. 2003; Huang et al. 2009; Schvartzman et al. 2011; Oria et al. 2017., Changede et al. 2019). Recently, it has furthermore been shown that nanotopographical features impact on cell migration in dependency of actomyosin and RhoGTPase activity (Park et al. 2016a) (Fig. 2a–d).
Actually these processes in the cell/microenvironment interface are even more complex with various further levels of regulation, such as mechanisms to modulate the cell surface availability of integrins by endocytosis (clathrin-dependent or clathrin-independent (CLIC/GEEC) routes), trafficking/recycling, and degradation, as well as talin competitors/inhibitors or talin cleavage. The interested reader can find further details on certain aspects in recent reviews of Gauthier and Roca-Cusachs (2018), Green and Brown (2019), Humphries et al. (2019), Sun et al. (2019) and Kechagia et al. (2019).
These events in the cell/microenvironment interface contribute essentially to the regulation of many cell biological behaviours, and aberrations therein can cause numerous pathophysiological cell states and diseases, also in the CNS (Winograd-Katz et al. 2014). The next paragraph will outline the current understanding about the involvement of the mechanotransductive pathway in neuronal cell behaviour (some examples are highlighted in Fig. 3). Considering the aforementioned local heterogeneity and complexity of biophysical cues in the brain ECM (Fig. 1), these insights are also highly relevant in regards to the optimisation of biomaterial approaches that are based on exploitation of mechanotransductive mechanisms (Fig. 4).
Biomaterial and biophysical approaches to study and/or control mechanotransductive processes that regulate neuronal cell development and functioning. (a) The scheme illustrates important stages during neuronal cell development. (b) The graphic outlines schematically principal (extra)cellular structures and processes of interest within the integrin adhesion complex-mediated mechanotransductive sequence in neuronal cells (on the right the axon/neurite growth cone is highlighted) and (c) some biophysical methods (AFM: atomic force microscopy, TFM: traction force microscopy) that are used to study them (see also "BIOPHYSICAL METHODS TO STUDY NEURONAL MECHANOBIOLOGY"). (d) Examples of different bioengineering approaches are listed that are used for the production of biomaterials which mimic biophysical extracellular matrix (ECM) features, such as hydrogels derived from decellularised brain ECM (Jin et al. 2018), hydrogels made of polymers (in this case, polyacrylamide gels with different stiffness ranges, Young’s modulus: left; < 14.5 kPa, middle: 14.5–29 kPa, right: > 29 kPa) (Hadden et al. 2017), electrospinning, lithographic methods, and pattern transfer, as well as reactive-ion etching (RIE) (Chen et al. 2014), carbon nanotubes (Cellot et al. 2011), and assembly of zirconia nanocluster by supersonic cluster beam deposition (Schulte et al. 2017). Various approaches are referenced throughout the review in which these types of substrates are applied and exploited to study and guide neuronal cell mechanotransduction and development. (a–d) Together, these mechanobiological approaches can contribute to a better understanding of how mechanotransductive processes impact on neuronal cell development and functioning. The figure contains adapted elements of images with permission from Jin et al. (2018), Copyright (2018) Springer Nature; Chen et al. (2014), Copyright (2014) Elsevier; as well as elements from Hadden et al. (2017), Cellot et al. (2011), Schulte et al. (2016b, c), and Maffioli et al. (2017).
Mechanotransductive processes and signalling in neuronal cell development and functioning
Albeit many details remain elusive, it is evident that the ECM configuration and integrin-mediated mechanosensing/transduction participate in the control of neuronal cell development and functioning at all stages (Franze et al. 2013; Stukel and Willits 2015; Park and Goda 2016; Barnes et al. 2017; Lilja and Ivaska 2018; Long and Huttner 2019; Xu et al. 2019) (Figs. 3 and 4).
Integrin/microenvironment interactions and RhoGTPase signalling play an important role during neurogenesis and the subsequent neuronal long-range migration when the neuronal progenitors move towards the final destination and terminally differentiate into neurons (Tate et al. 2004; Fietz et al. 2012; Long and Huttner 2019; Xu et al. 2019). The regulation of neural progenitor proliferation in the stem cell niche during neurogenesis depends on α6β1 integrin-mediated laminin (predominantly laminin-111) binding which activates MAPK signalling (Campos et al. 2004; Flanagan et al. 2006; Haubst et al. 2006; Lathia et al. 2007; Ma et al. 2008; Shen et al. 2008; Long and Huttner 2019). Interestingly, it has been shown that the mechanosensitive protein YAP sustains the proliferation of neural progenitors and negatively regulates their neuronal differentiation (shown for the postnatal mouse retina (Zhang et al. 2012) and chicken neural tube (Cao et al. 2008)). Recently, it was furthermore demonstrated that α3β1 integrin/laminin 511 interaction-dependent YAP activation fosters survival of immature dopaminergic midbrain neurons in their niche (Zhang et al. 2017). Also another prominent mechanosensitive transcription factor, i.e. MRTF-A (together with SRF (serum response factor)), is known to be involved in the regulation of neuronal migration and differentiative processes, in particular neuritogenesis (Mokalled et al. 2010), by controlling the expression of actin cytoskeleton-related target genes (Knöll and Nordheim 2009) (Fig. 3a and Fig. 4b).
During mammalian cerebral cortex development, neuronal progenitors leave the (sub)ventricular zone after neurogenesis and undergo a multipolar-bipolar transition before they start to migrate along the radial glia cell (RGC) fibres. The cytoskeletal and morphological changes of this transition are orchestrated by a complex spatiotemporal regulation of RhoGTPase activity (Konno et al. 2005; Xu et al. 2019). Later on, the brain ECM glycoprotein reelin is decisively involved in the control of neuronal cell adhesiveness. It regulates another shift in neuronal migration mode, by inhibiting the bipolar α3β1 integrin-dependent migration of the immature neurons along the RGC (Anton et al. 1999; Hong et al. 2000; Dulabon et al. 2000; Schmid et al. 2004) and activating instead the RGC-independent α5β1 integrin/fibronectin binding-dependent terminal translocation during lamination of the developing neocortex (Sekine et al. 2012). Dynamic and precisely coordinated RhoGTPase signalling (Xu et al. 2019), actomyosin activity, and traction forces (Solecki et al. 2009; Jiang et al. 2015) are required to enable neuronal migration. Loss of Rac1 in the forebrain leads to apoptosis of neural progenitors and newborn neurons, as well as defects in migratory competence, axonal guidance, and terminal neuronal differentiation (Chen et al. 2007, 2009). Lowering RhoA activity instead fosters neuronal lineage commitment (Keung et al. 2011), as well as neurite initiation and outgrowth, in many neuronal cell models (Yamaguchi et al. 2001; Dergham et al. 2002; Da Silva et al. 2003; Fournier et al. 2003; Schulte et al. 2010; Gu et al. 2013). In line with this, RhoGTPase signalling has been found to be involved in various biomaterial-induced (either rigidity or topography-based approaches) effects on neuronal differentiation (Georges et al. 2006; Saha et al. 2008; Teixeira et al. 2009; Seidlits et al. 2010; Keung et al. 2011; Yang et al. 2014; Schulte et al. 2016c; Maffioli et al. 2017; Chen et al. 2018) (Fig. 3a).
Recently, in vivo data obtained in the developing Xenopus brain demonstrated that retinal ganglion cell axons are sensitive to rapidly changing mechanical properties within the brain tissue that lead to stiffness gradients, causing a directional growth of the axons towards softer areas (Koser et al. 2016; Thompson et al., n.d.) (Fig. 3b). The directional collective migration of Xenopus neural crest cells can instead be induced by stiffening of the underlying head mesoderm via integrin-dependent mechanosensing (Barriga et al. 2018).
Interesting insights on the processes in the neuron/microenvironment interface and mechanotransductive signalling were obtained from studies that use biomaterials with topographical surfaces (examples in Fig. 4d). Nanotopographical features that restrict the maturation of IAC to dimensions beneath focal adhesion size and consequentially also decrease stress fibre formation (Bugnicourt et al. 2014; Schulte et al. 2016c; Baek et al. 2018, 2019) and cell rigidity (Schulte et al. 2016c) foster neuronal differentiation (Fig. 3c). These types of neuron/nanotopography interactions modulated the expression and phosphorylation levels of proteins that are known to be important components of the IAC and mechanotransductive machinery/signalling sequence (e.g. FAK phosphorylation and ILK signalling) (Schulte et al. 2016c, b; Maffioli et al. 2017; Baek et al. 2018). A study performed with a microtopographical substrates indicated that the ubiquitin E3a ligase, a protein which targets several integrin signalling/mechanotransduction-related proteins (such as, e.g. src family members) for degradation and whose deficiency leads to the neurodevelopmental disorder Angelman syndrome, is involved in neurite contact guidance and neuronal topography sensing (Tonazzini et al. 2016). Furthermore, it has been reported that nanotopography- and soft substrate-promoted neuronal differentiation were accompanied by an increase in Ser127 phosphorylation of YAP and its cytoplasmic retention in an actin cytoskeleton-dependent manner (Musah et al. 2014),(Sun et al. 2014),(Baek et al. 2018).
Consistent with their function as explorative compartments of developing neurons, neurite growth cones are particularly influenced and controlled by mechanotransductive processes. The extent of molecular clutch engagement to the retrograde actin flow-generated forces regulates neurite/axon guidance and pathfinding. Within growth cones, dynamic and small (focal complex size) integrin-mediated interaction sites are formed with microenvironmental cues, called point contacts. Their spatiotemporal dynamics are tightly governed by a balanced signalling interplay involving diverse IAC signalling-related components, such as RhoGTPases, src, PAK, and FAK (Robles et al. 2005; Woo and Gomez 2006; Medeiros et al. 2006; Myers et al. 2011; Vitriol and Zheng 2012; Santiago-Medina et al. 2013; Kerstein et al. 2015; Nichol et al. 2016) (Fig. 4b, on the right). Growth cones are soft structures (with low elastic modulus around hundreds of Pa and a tension in the range of hundreds of pN (Betz et al. 2011)) and produce relatively weak forces (compared with other cell types, e.g. fibroblasts or epithelial cells). The traction forces are in the range of tens of pN per μm2, executed in particular in the peripheral region growth cone (such as filopodia and lamellipodial edges) (Hällström et al. 2010; Betz et al. 2011; Koch et al. 2012; Franze et al. 2013; O’Toole et al. 2015), and largely myosin II dependent (Bridgman et al. 2001). Peripheral neurons seem to generate stronger traction forces than CNS neurons (Koch et al. 2012). The protrusive forces, measured for retinal ganglion cell growth cones, are in the order of 100 pN (Fuhs et al. 2013).
Also later on during neuronal maturation, mechanoregulatory processes are potentially highly relevant in controlling the spatiotemporal dynamics of synaptogenesis and activity-dependent synapse plasticity. Postsynaptic terminals of synapses are rich in mechanotransductively active components, such as cell adhesion molecules and regulators of actin dynamics and organisation (e.g. cofilin, drebrin α-actinin, and cortactin) (McGeachie et al. 2011; Sheng and Kim 2011; Kilinc 2018). Cofilin, e.g. is strongly recruited to dendritic spines during long-term potentiation and involved in the remodelling of the synapse structure (Bosch et al. 2014). The configuration and composition of the ECM and dynamic integrin/ECM (dis)engagement modulate the activity-dependent functional plasticity of synaptic connectivity and neural circuitry (Chavis and Westbrook 2001; Dityatev et al. 2010a; McGeachie et al. 2011; Orlando et al. 2012; Kerrisk et al. 2013; Bikbaev et al. 2015; Park and Goda 2016; Kilinc 2018).
More details about brain ECM and RhoGTPase signalling during neuronal development are outlined in reviews by Long and Huttner (2019) and Xu et al. (2019), respectively. Brain tissue mechanics are covered in detail by Franze et al. (2013) and Barnes et al. (2017). The involvement of integrins in synapse formation and plasticity is highlighted in reviews from Park and Goda (2016) and Lilja and Ivaska (2018).
Biophysical methods to study neuronal mechanobiology
The aim of research in cellular mechanobiology is to understand how biophysical properties of the cells and the surrounding microenvironment is intertwined and influence each other mutually to regulate cell morphology and fate (Fig. 4). In light of this premise, it is fundamental to obtain a precise quantification of the structural and mechanical properties of the microenvironment, cells, or tissues. The exploration of mechanotransductive processes relies furthermore on the ability to apply accurately controlled physical stimuli to living cells and to measure the forces of their mechanobiological actions (Iskratsch et al. 2014), in particular also for the understanding of neuronal cell behaviour (Athamneh and Suter 2015).
The study of biophysical aspects of cells and their microenvironment and/or the nature of their interaction requires specific instrumentation (Fig. 4c). This paragraph will give a short overview about some principal experimental techniques that often were essential to gain the insights into mechanotransductive processes outlined throughout this review.
Optical/magnetic tweezers
Optical and magnetic tweezers were amongst the first tools that enabled cell biologists to quantitatively measure the weak forces produced by cells or to apply corresponding forces to the cells (Iskratsch et al. 2014).
The optical tweezer technique consists in using a suitably functionalised μm-sized dielectric bead as a probe and exploiting the restoring force generated by the interaction of a laser with the dielectric sphere to control the position of the bead (Fig. 4c). Measuring the probe displacement in experimental conditions provides a measure of the applied force. The bead diameter can range from few hundreds of nm to tens of μm depending on the contact region and the applied pressure required in the experiments. The limitations in bead dimensions are due to the fact that the trapping force decreases with the bead diameter. The tweezers can be calibrated accurately to know how much force is required to remove the trapped bead from its focal centre. The optical traps can generate forces ranging from tens to hundreds of pN and the effective force constant of the tweezer is typically in the range of pN/m, providing extreme sensitivity to pN forces (Moffitt et al. 2008; Neuman and Nagy 2008; Capitanio and Pavone 2013; Siedlik et al. 2016).
The magnetic tweezer approach relies on the same principle of action as the optical tweezer, but in this case, magnets are used to position or apply forces on para-ferromagnetic beads. The advantage of the magnetic control is that many beads can be affected simultaneously (Neuman and Nagy 2008; Siedlik et al. 2016; De Vlaminck and Dekker 2012; Le et al. 2016), and it is also possible to induce twisting to the beads, allowing to test different degrees of freedom of the system under investigation (Wang et al. 1993; Strick et al. 2000). The drawback is that the spatial variation of the field results in a non-uniform force applied to the beads; careful design of the magnetic tweezer apparatus permits nowadays for nearly constant gradient fields over more than hundreds of microns (Neuman and Nagy 2008; Siedlik et al. 2016; De Vlaminck and Dekker 2012; Le et al. 2016).
An early use of magnetic tweezers in cell biological research led to one of the seminal works for the mechanobiology field by Wang et al. (1993), showing the mechanosensitivity of integrins and mechanotransduction through the actin cytoskeleton. In 1995, Dai and Sheetz (1995) characterised the mechanical properties of the neuronal growth cone by means of optical tweezers, demonstrating the role of the actin cytoskeleton in affecting the elastic properties of the membrane. Similar experiments were performed later on to determine the forces exerted by growing filopodia and lamellipodia (Cojoc et al. 2007) or axons (Moore et al. 2009; Kilinc et al. 2014) during neuronal differentiation, or to compare forces generated by the growth cones of neurons from the central nervous system (hippocampal neurons) and the peripheral nervous system (dorsal root ganglia) (Amin et al. 2013).
Moving the stage in xy-direction or the focus of the laser spot in z-direction in a finely tuned and precisely controlled manner enables also the application of static or oscillatory forces (in the range of a few pN), in order to study, e.g. the rheological behaviour of cells. With this method, it was recently demonstrated that mechanical stimuli with pN forces activate calcium channels (Falleroni et al. 2018) and that viscoelastic properties of soma and neurite differ and that in neurites, these properties change in dependency of the substrate rigidity (Grevesse et al. 2015).
Additional details on optical and/or magnetic tweezer-based approaches, focussing on applications relevant for biophysics and mechanotransduction, can be found in Capitanio and Pavone (2013), Siedlik et al. (2016), and Le et al. (2016).
Atomic force microscopy
AFM belongs to the branch of scanning probe microscopy (SPM). In recent years, it has gained increasing importance in the mechanobiology field as an instrument with versatile application modes that allow high-resolution morphological imaging (i.e. surface topography) and characterisation of mechanical properties (e.g. stiffness or viscoelasticity (Puricelli et al. 2015)) of biological samples, also simultaneously (Haase and Pelling 2015; Gavara 2017; Alcaraz et al. 2018; Krieg et al. 2019).
The probes are attached to an elastic lever (cantilever) that flexes under the interaction forces between the probe and the surface of the sample. The corresponding cantilever deflections are measured by a quadrant photodiode that detects the displacement of a laser reflecting from the exterior part of the cantilever. The probe shape and dimension can be modified according to the needs of the measurement and the information to be obtained. Pyramidal or narrow conical tips are usually exploited for high-resolution imaging application (Krieg et al. 2019). More spherical (Indrieri et al. 2011; Puricelli et al. 2015) or even cylindrical (Rico et al. 2007) probes instead are more suitable for mechanical characterisations of cells, ECM, or tissues due to the fine control of the probe/sample contact geometry (Fig. 4c). This system can be run in different modes depending on which structural and/or mechanical parameters of the measured samples are to be characterised (Haase and Pelling 2015; Gavara 2017; Alcaraz et al. 2018; Krieg et al. 2019).
During the scan, by keeping the cantilever deflection constant through a feedback circuit, the surface morphology of the sample can be reconstructed. This standard imaging application is widely used to image the nanotopography of cells (also neuronal cells and their compartments, such as growth cones (Parpura et al. 1993; Grzywa et al. 2006; Xiong et al. 2009), natural extracellular matrices (Abrams et al. 2000; Last et al. 2010; Gasiorowski et al. 2013), or nanostructured biomaterials (Cellot et al. 2011; Schulte et al. 2016c, b). It is also possible to apply forces by pushing the probe into the sample. Measuring the flexure of the cantilever through the extent of laser dislocation, the upward force acting on the tip can be calculated and mechanical properties of the sample can be determined. Varying the cantilever geometry allows covering more than six orders of magnitude in force sensitivity (from tens of pN to tens of μN). The nanomechanical analysis of soft biological samples (such as cells and many tissues or extracellular matrices) with AFM is still to date not straightforward. Attention must be drawn to the choice of the right experimental conditions for the application of contact mechanics models (such as Hertz (1882) or Sneddon (1965)) and also in the data analyses (Puricelli et al. 2015; Schillers et al. 2017). In the neuroscience field, by means of these types of AFM-based characterisations, it was possible to determine the mechanical properties (Young’s modulus) of substrates that are able to promote neuronal differentiation processes (Saha et al. 2008; Leipzig and Shoichet 2009; Teixeira et al. 2009; Banerjee et al. 2009; Seidlits et al. 2010; Keung et al. 2012, 2013; Musah et al. 2014; Sun et al. 2014), as well as the mechanics of brain tissue or spinal cord (Elkin et al. 2007; Christ et al. 2010; Koser et al. 2016, 2015; Antonovaite et al. 2018) (Fig. 1b and Fig. 3b) and different types of neuronal cells (Lu et al. 2006; Grzywa et al. 2006; Spedden et al. 2012; Schulte et al. 2016c), including changes due to mechanotransductive processes (Koser et al. 2016; Schulte et al. 2016c; Thompson et al., n.d.) (as detailed also in precedent paragraphs) (Fig. 3b, c).
Instead of applying forces to the cell with the AFM probe to deform the cell in order to analyse the cellular mechanical properties, AFM can also be used to measure cellular adhesion forces (e.g. integrin/ligand binding (Strohmeyer et al. 2017)) by the force spectroscopy technique. There are two principal approaches to apply this technique. In one case (single-cell force spectroscopy), a cell is attached to a tipless cantilever (using basically the cell as probe) and brought smoothly into contact with a substrate of interest (Taubenberger et al. 2007; Helenius et al. 2008) or even another cell (Puech et al. 2006). In a second approach, an AFM probe is functionalised in a suitable manner (e.g. with proteins of the ECM) and brought gently into contact with the cell membrane. In both cases, after a sufficient time of contact to enable the desired interaction, the probe is then retracted in order to break the generated bonds. From the obtained force versus distance curves, typically exhibiting a complex pattern of sudden jumps and plateaux, it is possible to measure the number of ruptured bonds and the distribution of their strength. Several configurations have been adopted to study cell adhesion and its mechanisms and biomolecular determinants. Depending on the probe and type of functionalisation, even adhesion forces at the single-molecule level can be measured (single-molecule force spectroscopy) (Müller et al. 2009; Dufrêne et al. 2011).
Further information on AFM methodologies and applications in mechanobiology research is available in more specific reviews from Haase and Pelling (2015), Gavara (2017), Alcaraz et al. (2018), or Krieg et al. (2019).
Traction force microscopy
The first cellular reaction to biophysical stimuli in the microenvironment takes place in the interface between cell membrane (and its embedded receptors) and the ECM.
Traction force microscopy (TFM) aims at measuring the traction forces exerted by the cell towards the substrate it interacts with. This can be achieved by different approaches, either fluorescent microspheres embedded into deformable hydrogel substrates or pillar arrays are used (Fig. 4c). When the cells apply forces on these substrates, as a consequence, the beads or the pillars are displaced. Tracking the displacement microscopically and knowing the stiffness of the hydrogel, or the pillars, permits an estimate of the applied traction forces and the reconstruction of a traction force field. The spatial resolution of TFM is limited by the optical setup and by the relative density of the beads, respectively pillars. Several models have been proposed to accurately convert the strain field into a stress field (Schwarz and Soiné 2015). In the neuronal context, TFM has been used, e.g. to investigate the forces involved in the growth cones of neurons (Chan and Odde 2008; Betz et al. 2011) or during neuronal migration (Jiang et al. 2015). A combined TFM/AFM approach was recently applied to study the correlation between cellular rigidity, viscoelasticity, and the contractile prestress, highlighting the role of the actomyosin machinery (Schierbaum et al. 2019).
Another tool to measure the mechanical forces exerted by the cell is molecular tension probes. The functioning of these probes is based on an extendable linker (which can be built up by adequate polymers or biomacromolecules (oligonucleotide or protein)) flanked by a spectroscopic ruler, consisting of a fluorophore and a quencher (Fig. 4c). If a sufficient force is applied to the probe, bonds within the linker region sequentially break and the linker extents, leading to a displacement and separation of the fluorophore and quencher. The extent of linker extension is usually measured by fluorescence resonance energy transfer. Knowing the bond strengths inside the linker region, the applied force can be quantified (Jurchenko and Salaita 2015; Liu et al. 2017). These tension probes can be utilised as immobilised sensors on a substrate (Liu et al. 2014) or also intracellularly if they are integrated into proteins; an example is the vinculin tension sensor (Grashoff et al. 2010; Jurchenko and Salaita 2015).
Conclusion and outlook
In this review, we attempted to outline the crucial involvement of mechanobiological aspects in physiological brain and neuronal cell development and functioning. In recent years, there are furthermore increasing indications that aberrations in the brain ECM organisation and mechanotransductive processes of neuronal cells strongly contribute to various neuronal pathologies, such as neurodegenerative diseases (e.g. in multiple sclerosis, amyotrophic lateral sclerosis, and Alzheimer’s disease) and neurodevelopmental disorders (such as autism spectrum disorders and schizophrenia) (Tyler 2012; Lau et al. 2013; Franze et al. 2013; Park and Goda 2016; Lilja and Ivaska 2018; Barnes et al. 2017), or primary brain cancers (e.g., in glioblastoma) (Barnes et al. 2017; Tanner 2018). The research in this field is often still in its infancy, gaining a deeper insight into neuronal cell mechanotransduction by means of biophysical approaches is therefore essential (Fig. 4) and will help to understand better how abnormal mechanotransductive processes contribute to the aetiology of brain disorders (Tyler 2012; Franze et al. 2013; Barnes et al. 2017; Lilja and Ivaska 2018; Tanner 2018). The biomedical significance of research in this direction is underlined by a very recent study which suggests a glycocalyx/IAC mechanosignalling feedback loop regulated by tension in glioblastoma multiforme that might be causal for the high recurrence of this tumour after current chemotherapy treatments (Barnes et al. 2018).
References
Abrams GA, Goodman SL, Nealey PF, Franco M, Murphy CJ (2000) Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res 299:39–46
Alcaraz J, Otero J, Jorba I, Navajas D (2018) Bidirectional mechanobiology between cells and their local extracellular matrix probed by atomic force microscopy. Semin Cell Dev Biol, Application of Atomic Force Microscopy in cell biology 73:71–81. https://doi.org/10.1016/j.semcdb.2017.07.020
Amin L, Ercolini E, Ban J, Torre V (2013) Comparison of the force exerted by hippocampal and DRG growth cones. PLoS One 8:e73025. https://doi.org/10.1371/journal.pone.0073025
Anton ES, Kreidberg JA, Rakic P (1999) Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron 22:277–289
Antonovaite N, Beekmans SV, Hol EM, Wadman WJ, Iannuzzi D (2018) Regional variations in stiffness in live mouse brain tissue determined by depth-controlled indentation mapping. Sci Rep 8:12517. https://doi.org/10.1038/s41598-018-31035-y
Arnold M, Cavalcanti-Adam EA, Glass R, Blümmel J, Eck W, Kantlehner M, Kessler H, Spatz JP (2004) Activation of integrin function by nanopatterned adhesive interfaces. Chemphyschem 5:383–388. https://doi.org/10.1002/cphc.200301014
Athamneh AIM, Suter DM (2015) Quantifying mechanical force in axonal growth and guidance. Front Cell Neurosci 9. https://doi.org/10.3389/fncel.2015.00359
Baek J, Cho S-Y, Kang H, Ahn H, Jung W-B, Cho Y, Lee E, Cho S-W, Jung H-T, Im SG (2018) Distinct mechanosensing of human neural stem cells on extremely limited anisotropic cellular contact. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.8b10171
Baek J, Jung W-B, Cho Y, Lee E, Yun G-T, Cho S-Y, Jung H-T, Im SG (2019) Facile fabrication of high-definition hierarchical wrinkle structures for investigating the geometry-sensitive fate commitment of human neural stem cells. ACS Appl Mater Interfaces 11:17247–17255. https://doi.org/10.1021/acsami.9b03479
Balgude AP, Yu X, Szymanski A, Bellamkonda RV (2001) Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials 22:1077–1084
Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, Schaffer DV, Kane RS (2009) The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 30:4695–4699. https://doi.org/10.1016/j.biomaterials.2009.05.050
Barnes JM, Przybyla L, Weaver VM (2017) Tissue mechanics regulate brain development, homeostasis and disease. J Cell Sci 130:71–82. https://doi.org/10.1242/jcs.191742
Barnes JM, Kaushik S, Bainer RO, Sa JK, Woods EC, Kai F, Przybyla L, Lee M, Lee HW, Tung JC, Maller O, Barrett AS, Lu KV, Lakins JN, Hansen KC, Obernier K, Alvarez-Buylla A, Bergers G, Phillips JJ, Nam D-H, Bertozzi CR, Weaver VM (2018) A tension-mediated glycocalyx–integrin feedback loop promotes mesenchymal-like glioblastoma. Nat Cell Biol 20:1203. https://doi.org/10.1038/s41556-018-0183-3
Barriga EH, Franze K, Charras G, Mayor R (2018) Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 554:523–527. https://doi.org/10.1038/nature25742
Bass MD, Roach KA, Morgan MR, Mostafavi-Pour Z, Schoen T, Muramatsu T, Mayer U, Ballestrem C, Spatz JP, Humphries MJ (2007) Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J Cell Biol 177:527–538. https://doi.org/10.1083/jcb.200610076
Béduer A, Vieu C, Arnauduc F, Sol J-C, Loubinoux I, Vaysse L (2012) Engineering of adult human neural stem cells differentiation through surface micropatterning. Biomaterials 33:504–514. https://doi.org/10.1016/j.biomaterials.2011.09.073
Betz T, Koch D, Lu Y-B, Franze K, Käs JA (2011) Growth cones as soft and weak force generators. Proc Natl Acad Sci 108:13420–13425. https://doi.org/10.1073/pnas.1106145108
Bikbaev A, Frischknecht R, Heine M (2015) Brain extracellular matrix retains connectivity in neuronal networks. Sci Rep 5:14527. https://doi.org/10.1038/srep14527
Bonneh-Barkay D, Wiley CA (2009) Brain extracellular matrix in neurodegeneration. Brain Pathol 19:573–585. https://doi.org/10.1111/j.1750-3639.2008.00195.x
Borghi F, Scaparra B, Paternoster C, Milani P, Podestà A (2018) Electrostatic double-layer interaction at the surface of rough cluster-assembled films: the case of nanostructured zirconia. Langmuir 34:10230–10242. https://doi.org/10.1021/acs.langmuir.8b01387
Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M, Hayashi Y (2014) Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron 82:444–459. https://doi.org/10.1016/j.neuron.2014.03.021
Bridgman PC, Dave S, Asnes CF, Tullio AN, Adelstein RS (2001) Myosin IIB is required for growth cone motility. J Neurosci 21:6159–6169. https://doi.org/10.1523/JNEUROSCI.21-16-06159.2001
Bugnicourt G, Brocard J, Nicolas A, Villard C (2014) Nanoscale surface topography reshapes neuronal growth in culture. Langmuir 30:4441–4449. https://doi.org/10.1021/la5001683
Campos LS, Leone DP, Relvas JB, Brakebusch C, Fässler R, Suter U, ffrench-Constant C (2004) Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development 131:3433–3444. https://doi.org/10.1242/dev.01199
Cao X, Pfaff SL, Gage FH (2008) YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev 22:3320–3334. https://doi.org/10.1101/gad.1726608
Capitanio M, Pavone FS (2013) Interrogating biology with force: single molecule high-resolution measurements with optical tweezers. Biophys J 105:1293–1303. https://doi.org/10.1016/j.bpj.2013.08.007
Carisey A, Tsang R, Greiner AM, Nijenhuis N, Heath N, Nazgiewicz A, Kemkemer R, Derby B, Spatz J, Ballestrem C (2013) Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr Biol 23:271–281. https://doi.org/10.1016/j.cub.2013.01.009
Case LB, Waterman CM (2015) Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat Cell Biol 17:955–963. https://doi.org/10.1038/ncb3191
Case LB, Baird MA, Shtengel G, Campbell SL, Hess HF, Davidson MW, Waterman CM (2015) Molecular mechanism of vinculin activation and nanoscale spatial organization in focal adhesions. Nat Cell Biol 17:880–892. https://doi.org/10.1038/ncb3180
Cellot G, Toma FM, Varley ZK, Laishram J, Villari A, Quintana M, Cipollone S, Prato M, Ballerini L (2011) Carbon nanotube scaffolds tune synaptic strength in cultured neural circuits: novel frontiers in nanomaterial–tissue interactions. J Neurosci 31:12945–12953. https://doi.org/10.1523/JNEUROSCI.1332-11.2011
Chan CE, Odde DJ (2008) Traction dynamics of filopodia on compliant substrates. Science 322:1687–1691. https://doi.org/10.1126/science.1163595
Changede R, Sheetz M (2017) Integrin and cadherin clusters: a robust way to organize adhesions for cell mechanics. BioEssays 39. https://doi.org/10.1002/bies.201600123
Changede R, Xu X, Margadant F, Sheetz MP (2015) Nascent integrin adhesions form on all matrix rigidities after integrin activation. Dev Cell 35:614–621. https://doi.org/10.1016/j.devcel.2015.11.001
Changede R, Cai H, Wind SJ, Sheetz MP, (2019) Integrin nanoclusters can bridge thin matrix fibres to form cell-matrix adhesions. Nat Mater 1–10. https://doi.org/10.1038/s41563-019-0460-y
Chavis P, Westbrook G (2001) Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature 411:317–321. https://doi.org/10.1038/35077101
Chen L, Liao G, Waclaw RR, Burns KA, Linquist D, Campbell K, Zheng Y, Kuan C-Y (2007) Rac1 controls the formation of midline commissures and the competency of tangential migration in ventral telencephalic neurons. J Neurosci 27:3884–3893. https://doi.org/10.1523/JNEUROSCI.3509-06.2007
Chen L, Melendez J, Campbell K, Kuan C-Y, Zheng Y (2009) Rac1 deficiency in the forebrain results in neural progenitor reduction and microcephaly. Dev Biol 325:162–170. https://doi.org/10.1016/j.ydbio.2008.10.023
Chen W, Shao Y, Li X, Zhao G, Fu J (2014) Nanotopographical surfaces for stem cell fate control: engineering mechanobiology from the bottom. Nano Today 9:759–784. https://doi.org/10.1016/j.nantod.2014.12.002
Chen Y, Lee H, Tong H, Schwartz M, Zhu C (2017) Force regulated conformational change of integrin αVβ3. Matrix Biol 60–61:70–85. https://doi.org/10.1016/j.matbio.2016.07.002
Chen W, Han S, Qian W, Weng S, Yang H, Sun Y, Villa-Diaz LG, Krebsbach PH, Fu J (2018) Nanotopography regulates motor neuron differentiation of human pluripotent stem cells. Nanoscale 10:3556–3565. https://doi.org/10.1039/c7nr05430k
Cheng C-M, LeDuc PR, Lin Y-W (2011) Localized bimodal response of neurite extensions and structural proteins in dorsal-root ganglion neurons with controlled polydimethylsiloxane substrate stiffness. J Biomech 44:856–862. https://doi.org/10.1016/j.jbiomech.2010.12.006
Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR (2008) Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol 10:1039–1050. https://doi.org/10.1038/ncb1763
Christ AF, Franze K, Gautier H, Moshayedi P, Fawcett J, Franklin RJM, Karadottir RT, Guck J (2010) Mechanical difference between white and gray matter in the rat cerebellum measured by scanning force microscopy. J Biomech 43:2986–2992. https://doi.org/10.1016/j.jbiomech.2010.07.002
Ciobanasu C, Faivre B, Le Clainche C (2014) Actomyosin-dependent formation of the mechanosensitive talin-vinculin complex reinforces actin anchoring. Nat Commun 5:3095. https://doi.org/10.1038/ncomms4095
Cojoc D, Difato F, Ferrari E, Shahapure RB, Laishram J, Righi M, Fabrizio EMD, Torre V (2007) Properties of the force exerted by filopodia and lamellipodia and the involvement of cytoskeletal components. PLoS One 2:e1072. https://doi.org/10.1371/journal.pone.0001072
Da Silva JS, Medina M, Zuliani C, Di Nardo A, Witke W, Dotti CG (2003) RhoA/ROCK regulation of neuritogenesis via profilin IIa-mediated control of actin stability. J Cell Biol 162:1267–1279. https://doi.org/10.1083/jcb.200304021
Dai J, Sheetz MP (1995) Mechanical properties of neuronal growth cone membranes studied by tether formation with laser optical tweezers. Biophys J 68:988–996. https://doi.org/10.1016/S0006-3495(95)80274-2
De Vlaminck I, Dekker C (2012) Recent advances in magnetic tweezers. Annu Rev Biophys 41:453–472. https://doi.org/10.1146/annurev-biophys-122311-100544
del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP (2009) Stretching single talin rod molecules activates vinculin binding. Science 323:638–641. https://doi.org/10.1126/science.1162912
Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L (2002) Rho signaling pathway targeted to promote spinal cord repair. J Neurosci 22:6570–6577
Dityatev A, Schachner M, Sonderegger P (2010a) The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci 11:735–746. https://doi.org/10.1038/nrn2898
Dityatev A, Seidenbecher CI, Schachner M (2010b) Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci 33:503–512. https://doi.org/10.1016/j.tins.2010.08.003
Dufrêne YF, Evans E, Engel A, Helenius J, Gaub HE, Müller DJ (2011) Five challenges to bringing single-molecule force spectroscopy into living cells. Nat Methods 8:123–127. https://doi.org/10.1038/nmeth0211-123
Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, Kreidberg JA, Anton ES (2000) Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron 27:33–44
Elkin BS, Azeloglu EU, Costa KD, Morrison B (2007) Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J Neurotrauma 24:812–822. https://doi.org/10.1089/neu.2006.0169
Elosegui-Artola A, Oria R, Chen Y, Kosmalska A, Pérez-González C, Castro N, Zhu C, Trepat X, Roca-Cusachs P (2016) Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat Cell Biol 18:540–548. https://doi.org/10.1038/ncb3336
Fabbro A, Villari A, Laishram J, Scaini D, Toma FM, Turco A, Prato M, Ballerini L (2012) Spinal cord explants use carbon nanotube interfaces to enhance neurite outgrowth and to fortify synaptic inputs. ACS Nano 6:2041–2055. https://doi.org/10.1021/nn203519r
Falleroni F, Torre V, Cojoc D (2018) Cell mechanotransduction with piconewton forces applied by optical tweezers. Front Cell Neurosci 12:130. https://doi.org/10.3389/fncel.2018.00130
Ferrari A, Cecchini M, Serresi M, Faraci P, Pisignano D, Beltram F (2010) Neuronal polarity selection by topography-induced focal adhesion control. Biomaterials 31:4682–4694. https://doi.org/10.1016/j.biomaterials.2010.02.032
Ferrari A, Cecchini M, Dhawan A, Micera S, Tonazzini I, Stabile R, Pisignano D, Beltram F (2011) Nanotopographic control of neuronal polarity. Nano Lett 11:505–511. https://doi.org/10.1021/nl103349s
Ferraris GMS, Schulte C, Buttiglione V, De Lorenzi V, Piontini A, Galluzzi M, Podestà A, Madsen CD, Sidenius N (2014) The interaction between uPAR and vitronectin triggers ligand-independent adhesion signalling by integrins. EMBO J 33:2458–2472. https://doi.org/10.15252/embj.201387611
Fietz SA, Lachmann R, Brandl H, Kircher M, Samusik N, Schröder R, Lakshmanaperumal N, Henry I, Vogt J, Riehn A, Distler W, Nitsch R, Enard W, Pääbo S, Huttner WB (2012) Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal. Proc Natl Acad Sci 109:11836–11841. https://doi.org/10.1073/pnas.1209647109
Flanagan LA, Rebaza LM, Derzic S, Schwartz PH, Monuki ES (2006) Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res 83:845–856. https://doi.org/10.1002/jnr.20778
Flynn KC (2013) The cytoskeleton and neurite initiation. Bioarchitecture 3:86–109
Fournier AE, Takizawa BT, Strittmatter SM (2003) Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci 23:1416–1423. https://doi.org/10.1523/JNEUROSCI.23-04-01416.2003
Franze K, Janmey PA, Guck J (2013) Mechanics in neuronal development and repair. Annu Rev Biomed Eng 15:227–251. https://doi.org/10.1146/annurev-bioeng-071811-150045
Fuhs T, Reuter L, Vonderhaid I, Claudepierre T, Käs JA (2013) Inherently slow and weak forward forces of neuronal growth cones measured by a drift-stabilized atomic force microscope. Cytoskeleton 70:44–53. https://doi.org/10.1002/cm.21080
Gasiorowski JZ, Murphy CJ, Nealey PF (2013) Biophysical cues and cell behavior: the big impact of little things. Annu Rev Biomed Eng 15:155–176. https://doi.org/10.1146/annurev-bioeng-071811-150021
Gauthier NC, Roca-Cusachs P (2018) Mechanosensing at integrin-mediated cell–matrix adhesions: from molecular to integrated mechanisms. Curr Opin Cell Biol 50:20–26. https://doi.org/10.1016/j.ceb.2017.12.014
Gavara N (2017) A beginner’s guide to atomic force microscopy probing for cell mechanics. Microsc Res Tech 80:75–84. https://doi.org/10.1002/jemt.22776
Geissler M, Gottschling C, Aguado A, Rauch U, Wetzel CH, Hatt H, Faissner A (2013) Primary hippocampal neurons, which lack four crucial extracellular matrix molecules, display abnormalities of synaptic structure and function and severe deficits in perineuronal net formation. J Neurosci 33:7742–7755. https://doi.org/10.1523/JNEUROSCI.3275-12.2013
Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA (2006) Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J 90:3012–3018. https://doi.org/10.1529/biophysj.105.073114
Gertz CC, Leach MK, Birrell LK, Martin DC, Feldman EL, Corey JM (2010) Accelerated neuritogenesis and maturation of primary spinal motor neurons in response to nanofibers. Dev Neurobiol 70:589–603. https://doi.org/10.1002/dneu.20792
Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA (2010) Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466:263–266. https://doi.org/10.1038/nature09198
Green HJ, Brown NH (2019) Integrin intracellular machinery in action. Exp Cell Res 378:226–231. https://doi.org/10.1016/j.yexcr.2019.03.011
Grevesse T, Dabiri BE, Parker KK, Gabriele S (2015) Opposite rheological properties of neuronal microcompartments predict axonal vulnerability in brain injury. Sci Rep 5(9475). https://doi.org/10.1038/srep09475
Grzywa EL, Lee AC, Lee GU, Suter DM (2006) High-resolution analysis of neuronal growth cone morphology by comparative atomic force and optical microscopy. J Neurobiol 66:1529–1543. https://doi.org/10.1002/neu.20318
Gu H, Yu SP, Gutekunst C-A, Gross RE, Wei L (2013) Inhibition of the Rho signaling pathway improves neurite outgrowth and neuronal differentiation of mouse neural stem cells. Int J Physiol Pathophysiol Pharmacol 5:11–20
Haase K, Pelling AE (2015) Investigating cell mechanics with atomic force microscopy. J R Soc Interface 12. https://doi.org/10.1098/rsif.2014.0970
Hadden WJ, Young JL, Holle AW, McFetridge ML, Kim DY, Wijesinghe P, Taylor-Weiner H, Wen JH, Lee AR, Bieback K, Vo B-N, Sampson DD, Kennedy BF, Spatz JP, Engler AJ, Choi YS (2017) Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc Natl Acad Sci 114:5647–5652. https://doi.org/10.1073/pnas.1618239114
Hällström W, Lexholm M, Suyatin DB, Hammarin G, Hessman D, Samuelson L, Montelius L, Kanje M, Prinz CN (2010) Fifteen-piconewton force detection from neural growth cones using nanowire arrays. Nano Lett 10:782–787. https://doi.org/10.1021/nl902675h
Haubst N, Georges-Labouesse E, Arcangelis AD, Mayer U, Götz M (2006) Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development 133:3245–3254. https://doi.org/10.1242/dev.02486
Helenius J, Heisenberg C-P, Gaub HE, Muller DJ (2008) Single-cell force spectroscopy. J Cell Sci 121:1785–1791. https://doi.org/10.1242/jcs.030999
Hertz H (1882) Ueber die Berührung fester elastischer Körper. J Für Reine Angew Math (92):156–171
Hoffman-Kim D, Mitchel JA, Bellamkonda RV (2010) Topography, cell response, and nerve regeneration. Annu Rev Biomed Eng 12:203–231. https://doi.org/10.1146/annurev-bioeng-070909-105351
Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martin ND, Walsh CA (2000) Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet 26:93–96. https://doi.org/10.1038/79246
Hopkins AM, Laporte LD, Tortelli F, Spedden E, Staii C, Atherton TJ, Hubbell JA, Kaplan DL (2013) Silk hydrogels as soft substrates for neural tissue engineering. Adv Funct Mater 23:5140–5149. https://doi.org/10.1002/adfm.201300435
Huang J, Grater SV, Corbellini F, Rinck S, Bock E, Kemkemer R, Kessler H, Ding J, Spatz JP (2009) Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett 9:1111–1116. https://doi.org/10.1021/nl803548b
Huang DL, Bax NA, Buckley CD, Weis WI, Dunn AR (2017) Vinculin forms a directionally asymmetric catch bond with F-actin. Science 357:703–706. https://doi.org/10.1126/science.aan2556
Humphries JD, Chastney MR, Askari JA, Humphries MJ (2019) Signal transduction via integrin adhesion complexes. Curr Opin Cell Biol 56:14–21. https://doi.org/10.1016/j.ceb.2018.08.004
Indrieri M, Podestà A, Bongiorno G, Marchesi D, Milani P (2011) Adhesive-free colloidal probes for nanoscale force measurements: production and characterization. Rev Sci Instrum 82:023708. https://doi.org/10.1063/1.3553499
Iskratsch T, Wolfenson H, Sheetz MP (2014) Appreciating force and shape—the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol 15:825–833. https://doi.org/10.1038/nrm3903
Jiang G, Giannone G, Critchley DR, Fukumoto E, Sheetz MP (2003) Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 424:334. https://doi.org/10.1038/nature01805
Jiang FX, Yurke B, Firestein BL, Langrana NA (2008) Neurite outgrowth on a DNA crosslinked hydrogel with tunable stiffnesses. Ann Biomed Eng 36:1565–1579. https://doi.org/10.1007/s10439-008-9530-z
Jiang J, Zhang Z, Yuan X, Poo M (2015) Spatiotemporal dynamics of traction forces show three contraction centers in migratory neurons. J Cell Biol 209:759–774. https://doi.org/10.1083/jcb.201410068
Jin Y, Lee JS, Kim J, Min S, Wi S, Yu JH, Chang G-E, Cho A-N, Choi Y, Ahn D-H, Cho S-R, Cheong E, Kim Y-G, Kim H-P, Kim Y, Kim DS, Kim HW, Quan Z, Kang H-C, Cho S-W (2018) Three-dimensional brain-like microenvironments facilitate the direct reprogramming of fibroblasts into therapeutic neurons. Nat Biomed Eng 2:522–539. https://doi.org/10.1038/s41551-018-0260-8
Johansson F, Carlberg P, Danielsen N, Montelius L, Kanje M (2006) Axonal outgrowth on nano-imprinted patterns. Biomaterials 27:1251–1258. https://doi.org/10.1016/j.biomaterials.2005.07.047
Jurchenko C, Salaita KS (2015) Lighting up the force: investigating mechanisms of mechanotransduction using fluorescent tension probes. Mol Cell Biol 35:2570–2582. https://doi.org/10.1128/MCB.00195-15
Kalappurakkal JM, Anilkumar AA, Patra C, van Zanten TS, Sheetz MP, Mayor S (2019) Integrin mechano-chemical signaling generates plasma membrane nanodomains that promote cell spreading. Cell 177:1738–1756.e23. https://doi.org/10.1016/j.cell.2019.04.037
Kang K, Choi S-E, Jang HS, Cho WK, Nam Y, Choi IS, Lee JS (2012) In vitro developmental acceleration of hippocampal neurons on nanostructures of self-assembled silica beads in filopodium-size ranges. Angew Chem Int Ed 51:2855–2858. https://doi.org/10.1002/anie.201106271
Kechagia JZ, Ivaska J, Roca-Cusachs P (2019) Integrins as biomechanical sensors of the microenvironment. Nat Rev Mol Cell Biol 1. https://doi.org/10.1038/s41580-019-0134-2
Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F (2007) Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells Dayt. Ohio 25:2146–2157. https://doi.org/10.1634/stemcells.2007-0082
Kerrisk ME, Greer CA, Koleske AJ (2013) Integrin α3 is required for late postnatal stability of dendrite arbors, dendritic spines and synapses, and mouse behavior. J Neurosci 33:6742–6752. https://doi.org/10.1523/JNEUROSCI.0528-13.2013
Kerstein PC, Nichol RH, Gomez TM (2015) Mechanochemical regulation of growth cone motility. Front Cell Neurosci 9:244. https://doi.org/10.3389/fncel.2015.00244
Keung AJ, de Juan-Pardo EM, Schaffer DV, Kumar S (2011) Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells 29:1886–1897. https://doi.org/10.1002/stem.746
Keung AJ, Asuri P, Kumar S, Schaffer DV (2012) Soft microenvironments promote the early neurogenic differentiation but not self-renewal of human pluripotent stem cells. Integr Biol (Camb) 4:1049–1058. https://doi.org/10.1039/c2ib20083j
Keung AJ, Dong M, Schaffer DV, Kumar S (2013) Pan-neuronal maturation but not neuronal subtype differentiation of adult neural stem cells is mechanosensitive. Sci Rep 3:1817. https://doi.org/10.1038/srep01817
Kilinc D (2018) The emerging role of mechanics in synapse formation and plasticity. Front Cell Neurosci 12. https://doi.org/10.3389/fncel.2018.00483
Kilinc D, Blasiak A, O’Mahony JJ, Lee GU (2014) Low piconewton towing of CNS axons against diffusing and surface-bound repellents requires the inhibition of motor protein-associated pathways. Sci Rep 4:7128. https://doi.org/10.1038/srep07128
Kim Y, Kumar S (2014) CD44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motility. Mol Cancer Res 12:1416–1429. https://doi.org/10.1158/1541-7786.MCR-13-0629
Kim M-H, Park M, Kang K, Choi IS (2013) Neurons on nanometric topographies: insights into neuronal behaviors in vitro. Biomater Sci. 2:148–155. https://doi.org/10.1039/C3BM60255A
Knöll B, Nordheim A (2009) Functional versatility of transcription factors in the nervous system: the SRF paradigm. Trends Neurosci 32:432–442. https://doi.org/10.1016/j.tins.2009.05.004
Koch D, Rosoff WJ, Jiang J, Geller HM, Urbach JS (2012) Strength in the periphery: growth cone biomechanics and substrate rigidity response in peripheral and central nervous system neurons. Biophys J 102:452–460. https://doi.org/10.1016/j.bpj.2011.12.025
Kong F, García AJ, Mould AP, Humphries MJ, Zhu C (2009) Demonstration of catch bonds between an integrin and its ligand. J Cell Biol 185:1275–1284. https://doi.org/10.1083/jcb.200810002
Konno D, Yoshimura S, Hori K, Maruoka H, Sobue K (2005) Involvement of the phosphatidylinositol 3-kinase/rac1 and cdc42 pathways in radial migration of cortical neurons. J Biol Chem 280:5082–5088. https://doi.org/10.1074/jbc.M408251200
Koser DE, Moeendarbary E, Hanne J, Kuerten S, Franze K (2015) CNS cell distribution and axon orientation determine local spinal cord mechanical properties. Biophys J 108:2137–2147. https://doi.org/10.1016/j.bpj.2015.03.039
Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK, Svoboda H, Viana M, Costa LD, Guck J, Holt CE, Franze K (2016) Mechanosensing is critical for axon growth in the developing brain. Nat Neurosci 19:1592–1598. https://doi.org/10.1038/nn.4394
Kostic A, Sap J, Sheetz MP (2007) RPTPα is required for rigidity-dependent inhibition of extension and differentiation of hippocampal neurons. J Cell Sci 120:3895–3904. https://doi.org/10.1242/jcs.009852
Krieg M, Fläschner G, Alsteens D, Gaub BM, Roos WH, Wuite GJL, Gaub HE, Gerber C, Dufrêne YF, Müller DJ (2019) Atomic force microscopy-based mechanobiology. Nat Rev Phys 1:41. https://doi.org/10.1038/s42254-018-0001-7
Lam D, Enright HA, Cadena J, Peters SKG, Sales AP, Osburn JJ, Soscia DA, Kulp KS, Wheeler EK, Fischer NO (2019) Tissue-specific extracellular matrix accelerates the formation of neural networks and communities in a neuron-glia co-culture on a multi-electrode array. Sci Rep 9:4159. https://doi.org/10.1038/s41598-019-40128-1
Last JA, Russell P, Nealey PF, Murphy CJ (2010) The applications of atomic force microscopy to vision science. Invest Ophthalmol Vis Sci 51:6083–6094. https://doi.org/10.1167/iovs.10-5470
Lathia JD, Patton B, Eckley DM, Magnus T, Mughal MR, Sasaki T, Caldwell MA, Rao MS, Mattson MP, ffrench-Constant C (2007) Patterns of laminins and integrins in the embryonic ventricular zone of the CNS. J Comp Neurol 505:630–643. https://doi.org/10.1002/cne.21520
Lau LW, Cua R, Keough MB, Haylock-Jacobs S, Yong VW (2013) Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci 14:722–729. https://doi.org/10.1038/nrn3550
Le S, Liu R, Lim CT, Yan J (2016) Uncovering mechanosensing mechanisms at the single protein level using magnetic tweezers. Methods 94:13–18. https://doi.org/10.1016/j.ymeth.2015.08.020
Leach JB, Brown XQ, Jacot JG, Dimilla PA, Wong JY (2007) Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. J Neural Eng 4:26–34. https://doi.org/10.1088/1741-2560/4/2/003
Lee MR, Kwon KW, Jung H, Kim HN, Suh KY, Kim K, Kim K-S (2010) Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials 31:4360–4366. https://doi.org/10.1016/j.biomaterials.2010.02.012
Leipzig ND, Shoichet MS (2009) The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 30:6867–6878. https://doi.org/10.1016/j.biomaterials.2009.09.002
Leterrier C, Dubey P, Roy S (2017) The nano-architecture of the axonal cytoskeleton. Nat Rev Neurosci 18:713–726. https://doi.org/10.1038/nrn.2017.129
Li J, Springer TA (2017) Integrin extension enables ultrasensitive regulation by cytoskeletal force. Proc Natl Acad Sci U S A 114:4685–4690. https://doi.org/10.1073/pnas.1704171114
Lilja J, Ivaska J (2018) Integrin activity in neuronal connectivity. J Cell Sci 131. https://doi.org/10.1242/jcs.212803
Lim SH, Liu XY, Song H, Yarema KJ, Mao H-Q (2010) The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials 31:9031–9039. https://doi.org/10.1016/j.biomaterials.2010.08.021
Liu Y, Medda R, Liu Z, Galior K, Yehl K, Spatz JP, Cavalcanti-Adam EA, Salaita K (2014) Nanoparticle tension probes patterned at the nanoscale: impact of integrin clustering on force transmission. Nano Lett 14:5539–5546. https://doi.org/10.1021/nl501912g
Liu Y, Galior K, Ma VP-Y, Salaita K (2017) Molecular tension probes for imaging forces at the cell surface. Acc Chem Res 50:2915–2924. https://doi.org/10.1021/acs.accounts.7b00305
Long KR, Huttner WB (2019) How the extracellular matrix shapes neural development. Open Biol 9:180216. https://doi.org/10.1098/rsob.180216
Lowery LA, Van Vactor D (2009) The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol 10:332–343. https://doi.org/10.1038/nrm2679
Lu Y-B, Franze K, Seifert G, Steinhäuser C, Kirchhoff F, Wolburg H, Guck J, Janmey P, Wei E-Q, Käs J, Reichenbach A (2006) Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci U S A 103:17759–17764. https://doi.org/10.1073/pnas.0606150103
Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP (2008) Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol 8:90. https://doi.org/10.1186/1471-213X-8-90
Maffioli E, Schulte C, Nonnis S, Grassi Scalvini F, Piazzoni C, Lenardi C, Negri A, Milani P, Tedeschi G (2017) Proteomic dissection of nanotopography-sensitive mechanotransductive signalling hubs that foster neuronal differentiation in PC12 cells. Front Cell Neurosci 11. https://doi.org/10.3389/fncel.2017.00417
Mammadov B, Sever M, Guler MO, Tekinay AB (2013) Neural differentiation on synthetic scaffold materials. Biomater Sci 1:1119–1137. https://doi.org/10.1039/C3BM60150A
Man AJ, Davis HE, Itoh A, Leach JK, Bannerman P (2011) Neurite outgrowth in fibrin gels is regulated by substrate stiffness. Tissue Eng Part A 17:2931–2942. https://doi.org/10.1089/ten.tea.2011.0030
McGeachie AB, Cingolani LA, Goda Y (2011) A stabilising influence: integrins in regulation of synaptic plasticity. Neurosci Res 70:24–29. https://doi.org/10.1016/j.neures.2011.02.006
Medberry CJ, Crapo PM, Siu BF, Carruthers CA, Wolf MT, Nagarkar SP, Agrawal V, Jones KE, Kelly J, Johnson SA, Velankar SS, Watkins SC, Modo M, Badylak SF (2013) Hydrogels derived from central nervous system extracellular matrix. Biomaterials 34:1033–1040. https://doi.org/10.1016/j.biomaterials.2012.10.062
Medeiros NA, Burnette DT, Forscher P (2006) Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol 8:215–226. https://doi.org/10.1038/ncb1367
Mercier F (2016) Fractones: extracellular matrix niche controlling stem cell fate and growth factor activity in the brain in health and disease. Cell Mol Life Sci 73:4661–4674. https://doi.org/10.1007/s00018-016-2314-y
Migliorini E, Grenci G, Ban J, Pozzato A, Tormen M, Lazzarino M, Torre V, Ruaro ME (2011) Acceleration of neuronal precursors differentiation induced by substrate nanotopography. Biotechnol Bioeng 108:2736–2746. https://doi.org/10.1002/bit.23232
Mitchison T, Kirschner M (1988) Cytoskeletal dynamics and nerve growth. Neuron 1:761–772
Miyata S, Kitagawa H (2017) Formation and remodeling of the brain extracellular matrix in neural plasticity: roles of chondroitin sulfate and hyaluronan. Biochim Biophys Acta Gen Subj 1861:2420–2434. https://doi.org/10.1016/j.bbagen.2017.06.010
Moffitt JR, Chemla YR, Smith SB, Bustamante C (2008) Recent advances in optical tweezers. Annu Rev Biochem 77:205–228. https://doi.org/10.1146/annurev.biochem.77.043007.090225
Mokalled MH, Johnson A, Kim Y, Oh J, Olson EN (2010) Myocardin-related transcription factors regulate the Cdk5/Pctaire1 kinase cascade to control neurite outgrowth, neuronal migration and brain development. Development 137:2365–2374. https://doi.org/10.1242/dev.047605
Moore SW, Biais N, Sheetz MP (2009) Traction on immobilized netrin-1 is sufficient to reorient axons. Science 325:166. https://doi.org/10.1126/science.1173851
Morgan MR, Hamidi H, Bass MD, Warwood S, Ballestrem C, Humphries MJ (2013) Syndecan-4 phosphorylation is a control point for integrin recycling. Dev Cell 24:472–485. https://doi.org/10.1016/j.devcel.2013.01.027
Mosley MC, Lim HJ, Chen J, Yang Y-H, Li S, Liu Y, Smith Callahan LA (2017) Neurite extension and neuronal differentiation of human induced pluripotent stem cell derived neural stem cells on polyethylene glycol hydrogels containing a continuous Young’s Modulus gradient. J Biomed Mater Res A 105:824–833. https://doi.org/10.1002/jbm.a.35955
Müller DJ, Helenius J, Alsteens D, Dufrêne YF (2009) Force probing surfaces of living cells to molecular resolution. Nat Chem Biol 5:383–390. https://doi.org/10.1038/nchembio.181
Musah S, Wrighton PJ, Zaltsman Y, Zhong X, Zorn S, Parlato MB, Hsiao C, Palecek SP, Chang Q, Murphy WL, Kiessling LL (2014) Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc Natl Acad Sci U S A 111:13805–13810. https://doi.org/10.1073/pnas.1415330111
Myers JP, Santiago-Medina M, Gomez TM (2011) Regulation of axonal outgrowth and pathfinding by integrin-ECM interactions. Dev Neurobiol 71:901–923. https://doi.org/10.1002/dneu.20931
Neuman KC, Nagy A (2008) Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat Methods 5:491–505. https://doi.org/10.1038/nmeth.1218
Nichol RH, Hagen KM, Lumbard DC, Dent EW, Gómez TM (2016) Guidance of axons by local coupling of retrograde flow to point contact adhesions. J Neurosci 36:2267–2282. https://doi.org/10.1523/JNEUROSCI.2645-15.2016
Norman LL, Aranda-Espinoza H (2010) Cortical neuron outgrowth is insensitive to substrate stiffness. Cell Mol Bioeng 3:398–414. https://doi.org/10.1007/s12195-010-0137-8
O’Toole M, Lamoureux P, Miller KE (2015) Measurement of subcellular force generation in neurons. Biophys J 108:1027–1037. https://doi.org/10.1016/j.bpj.2015.01.021
Oria R, Wiegand T, Escribano J, Elosegui-Artola A, Uriarte JJ, Moreno-Pulido C, Platzman I, Delcanale P, Albertazzi L, Navajas D, Trepat X, García-Aznar JM, Cavalcanti-Adam EA, Roca-Cusachs P (2017) Force loading explains spatial sensing of ligands by cells. Nature 552:219. https://doi.org/10.1038/nature24662
Orlando C, Ster J, Gerber U, Fawcett JW, Raineteau O (2012) Perisynaptic chondroitin sulfate proteoglycans restrict structural plasticity in an integrin-dependent manner. J Neurosci 32:18009–18017, 18017a. https://doi.org/10.1523/JNEUROSCI.2406-12.2012
Park YK, Goda Y (2016) Integrins in synapse regulation. Nat Rev Neurosci 17:745–756. https://doi.org/10.1038/nrn.2016.138
Park J, Kim D-H, Kim H-N, Wang CJ, Kwak MK, Hur E, Suh K-Y, An SS, Levchenko A (2016a) Directed migration of cancer cells guided by the graded texture of the underlying matrix. Nat Mater 15:792–801. https://doi.org/10.1038/nmat4586
Park M, Oh E, Seo J, Kim M-H, Cho H, Choi JY, Lee H, Choi IS (2016b) Control over neurite directionality and neurite elongation on anisotropic micropillar arrays. Small 12:1148–1152. https://doi.org/10.1002/smll.201501896
Parpura V, Haydon PG, Henderson E (1993) Three-dimensional imaging of living neurons and glia with the atomic force microscope. J Cell Sci 104(Pt 2):427–432
Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, Hudak JE, Lakins JN, Wijekoon AC, Cassereau L, Rubashkin MG, Magbanua MJ, Thorn KS, Davidson MW, Rugo HS, Park JW, Hammer DA, Giannone G, Bertozzi CR, Weaver VM (2014) The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511:319–325. https://doi.org/10.1038/nature13535
Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L (2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298:1248–1251. https://doi.org/10.1126/science.1072699
Puech P-H, Poole K, Knebel D, Muller DJ (2006) A new technical approach to quantify cell-cell adhesion forces by AFM. Ultramicroscopy 106:637–644. https://doi.org/10.1016/j.ultramic.2005.08.003
Puricelli L, Galluzzi M, Schulte C, Podestà A, Milani P (2015) Nanomechanical and topographical imaging of living cells by atomic force microscopy with colloidal probes. Rev Sci Instrum 86:033705. https://doi.org/10.1063/1.4915896
Rajnicek A, Britland S, McCaig C (1997) Contact guidance of CNS neurites on grooved quartz: influence of groove dimensions, neuronal age and cell type. J Cell Sci 110:2905–2913
Rico F, Roca-Cusachs P, Sunyer R, Farré R, Navajas D (2007) Cell dynamic adhesion and elastic properties probed with cylindrical atomic force microscopy cantilever tips. J Mol Recognit 20:459–466. https://doi.org/10.1002/jmr.829
Robles E, Woo S, Gomez TM (2005) Src-dependent tyrosine phosphorylation at the tips of growth cone filopodia promotes extension. J Neurosci 25:7669–7681. https://doi.org/10.1523/JNEUROSCI.2680-05.2005
Ruoslahti E (1996) Brain extracellular matrix. Glycobiology 6:489–492. https://doi.org/10.1093/glycob/6.5.489
Sack I, Streitberger K-J, Krefting D, Paul F, Braun J (2011) The influence of physiological aging and atrophy on brain viscoelastic properties in humans. PLoS One 6:e23451. https://doi.org/10.1371/journal.pone.0023451
Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE (2008) Substrate modulus directs neural stem cell behavior. Biophys J 95:4426–4438. https://doi.org/10.1529/biophysj.108.132217
Santiago-Medina M, Gregus KA, Gomez TM (2013) PAK-PIX interactions regulate adhesion dynamics and membrane protrusion to control neurite outgrowth. J Cell Sci 126:1122–1133. https://doi.org/10.1242/jcs.112607
Sarker M, Naghieh S, McInnes AD, Schreyer DJ, Chen X (2018) Strategic design and fabrication of nerve guidance conduits for peripheral nerve regeneration. Biotechnol J 13:1700635. https://doi.org/10.1002/biot.201700635
Schierbaum N, Rheinlaender J, Schäffer TE (2019) Combined atomic force microscopy (AFM) and traction force microscopy (TFM) reveals a correlation between viscoelastic material properties and contractile prestress of living cells. Soft Matter 15:1721–1729. https://doi.org/10.1039/C8SM01585F
Schillers H, Rianna C, Schäpe J, Luque T, Doschke H, Wälte M, Uriarte JJ, Campillo N, Michanetzis GPA, Bobrowska J, Dumitru A, Herruzo ET, Bovio S, Parot P, Galluzzi M, Podestà A, Puricelli L, Scheuring S, Missirlis Y, Garcia R, Odorico M, Teulon J-M, Lafont F, Lekka M, Rico F, Rigato A, Pellequer J-L, Oberleithner H, Navajas D, Radmacher M (2017) Standardized nanomechanical atomic force microscopy procedure (SNAP) for measuring soft and biological samples. Sci Rep 7:5117. https://doi.org/10.1038/s41598-017-05383-0
Schmid RS, Shelton S, Stanco A, Yokota Y, Kreidberg JA, Anton ES (2004) alpha3beta1 integrin modulates neuronal migration and placement during early stages of cerebral cortical development. Development 131:6023–6031. https://doi.org/10.1242/dev.01532
Schulte C, Racchetti G, D’Alessandro R, Meldolesi J (2010) A new form of neurite outgrowth sustained by the exocytosis of enlargeosomes expressed under the control of REST. Traffic Cph Den 11:1304–1314. https://doi.org/10.1111/j.1600-0854.2010.01095.x
Schulte C, Ferraris GMS, Oldani A, Galluzzi M, Podestà A, Puricelli L, de Lorenzi V, Lenardi C, Milani P, Sidenius N (2016a) Lamellipodial tension, not integrin/ligand binding, is the crucial factor to realise integrin activation and cell migration. Eur J Cell Biol 95:1–14. https://doi.org/10.1016/j.ejcb.2015.10.002
Schulte C, Ripamonti M, Maffioli E, Cappelluti MA, Nonnis S, Puricelli L, Lamanna J, Piazzoni C, Podestà A, Lenardi C, Tedeschi G, Malgaroli A, Milani P (2016b) Scale invariant disordered nanotopography promotes hippocampal neuron development and maturation with involvement of mechanotransductive pathways. Front Cell Neurosci 10:267. https://doi.org/10.3389/fncel.2016.00267
Schulte C, Rodighiero S, Cappelluti MA, Puricelli L, Maffioli E, Borghi F, Negri A, Sogne E, Galluzzi M, Piazzoni C, Tamplenizza M, Podestà A, Tedeschi G, Lenardi C, Milani P (2016c) Conversion of nanoscale topographical information of cluster-assembled zirconia surfaces into mechanotransductive events promotes neuronal differentiation. J Nanobiotechnology 14:18. https://doi.org/10.1186/s12951-016-0171-3
Schulte C, Podestà A, Lenardi C, Tedeschi G, Milani P (2017) Quantitative control of protein and cell interaction with nanostructured surfaces by cluster assembling. Acc Chem Res 50:231–239. https://doi.org/10.1021/acs.accounts.6b00433
Schulte C, Lamanna J, Moro AS, Piazzoni C, Borghi F, Chighizola M, Ortoleva S, Racchetti G, Lenardi C, Podestà A, Malgaroli A, Milani P (2018) Neuronal cells confinement by micropatterned cluster-assembled dots with mechanotransductive nanotopography. ACS Biomater Sci Eng. https://doi.org/10.1021/acsbiomaterials.8b00916
Schvartzman M, Palma M, Sable J, Abramson J, Hu X, Sheetz MP, Wind SJ (2011) Nanolithographic control of the spatial organization of cellular adhesion receptors at the single-molecule level. Nano Lett 11:1306–1312. https://doi.org/10.1021/nl104378f
Schwarz US, Soiné JRD (2015) Traction force microscopy on soft elastic substrates: a guide to recent computational advances. Biochim Biophys Acta 1853:3095–3104. https://doi.org/10.1016/j.bbamcr.2015.05.028
Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, Schmidt CE (2010) The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 31:3930–3940. https://doi.org/10.1016/j.biomaterials.2010.01.125
Sekine K, Kawauchi T, Kubo K-I, Honda T, Herz J, Hattori M, Kinashi T, Nakajima K (2012) Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of integrin α5β1. Neuron 76:353–369. https://doi.org/10.1016/j.neuron.2012.07.020
Shattil SJ, Kim C, Ginsberg MH (2010) The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol 11:288–300. https://doi.org/10.1038/nrm2871
Shen Q, Wang Y, Kokovay E, Lin G, Chuang S-M, Goderie SK, Roysam B, Temple S (2008) Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell 3:289–300. https://doi.org/10.1016/j.stem.2008.07.026
Sheng M, Kim E (2011) The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol 3. https://doi.org/10.1101/cshperspect.a005678
Siedlik MJ, Varner VD, Nelson CM (2016) Pushing, pulling, and squeezing our way to understanding mechanotransduction. Methods 94:4–12. https://doi.org/10.1016/j.ymeth.2015.08.019
Sigal YM, Bae H, Bogart LJ, Hensch TK, Zhuang X (2019) Structural maturation of cortical peineuronal nets and their perforating synapses revealed by superresolution imaging. Proc Natl Acad Sci 116:7071–7076. https://doi.org/10.1073/pnas.1817222116
Simitzi C, Efstathopoulos P, Kourgiantaki A, Ranella A, Charalampopoulos I, Fotakis C, Athanassakis I, Stratakis E, Gravanis A (2015) Laser fabricated discontinuous anisotropic microconical substrates as a new model scaffold to control the directionality of neuronal network outgrowth. Biomaterials 67:115–128. https://doi.org/10.1016/j.biomaterials.2015.07.008
Simitzi C, Ranella A, Stratakis E (2017) Controlling the morphology and outgrowth of nerve and neuroglial cells: the effect of surface topography. Acta Biomater 51:21–52. https://doi.org/10.1016/j.actbio.2017.01.023
Smith Callahan LA, Xie S, Barker IA, Zheng J, Reneker DH, Dove AP, Becker ML (2013) Directed differentiation and neurite extension of mouse embryonic stem cell on aligned poly(lactide) nanofibers functionalized with YIGSR peptide. Biomaterials 34:9089–9095. https://doi.org/10.1016/j.biomaterials.2013.08.028
Sneddon IN (1965) The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int J Eng Sci 3:47–57. https://doi.org/10.1016/0020-7225(65)90019-4
Solecki DJ, Trivedi N, Govek E-E, Kerekes RA, Gleason SS, Hatten ME (2009) Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and soma during CNS glial-guided neuronal migration. Neuron 63:63–80. https://doi.org/10.1016/j.neuron.2009.05.028
Sorg BA, Berretta S, Blacktop JM, Fawcett JW, Kitagawa H, Kwok JCF, Miquel M (2016) Casting a wide net: role of perineuronal nets in neural plasticity. J Neurosci 36:11459–11468. https://doi.org/10.1523/JNEUROSCI.2351-16.2016
Spedden E, White JD, Naumova EN, Kaplan DL, Staii C (2012) Elasticity maps of living neurons measured by combined fluorescence and atomic force microscopy. Biophys J 103:868–877. https://doi.org/10.1016/j.bpj.2012.08.005
Stabenfeldt SE, LaPlaca MC (2011) Variations in rigidity and ligand density influence neuronal response in methylcellulose-laminin hydrogels. Acta Biomater 7:4102–4108. https://doi.org/10.1016/j.actbio.2011.07.026
Strick T, Allemand J-F, Croquette V, Bensimon D (2000) Twisting and stretching single DNA molecules. Prog Biophys Mol Biol 74:115–140. https://doi.org/10.1016/S0079-6107(00)00018-3
Strohmeyer N, Bharadwaj M, Costell M, Fässler R, Müller DJ (2017) Fibronectin-bound α5β1 integrins sense load and signal to reinforce adhesion in less than a second. Nat Mater 16:1262–1270. https://doi.org/10.1038/nmat5023
Stukel JM, Willits RK (2015) Mechanotransduction of neural cells through cell–substrate interactions. Tissue Eng Part B Rev 22:173–182. https://doi.org/10.1089/ten.teb.2015.0380
Sun Y, Yong KMA, Villa-Diaz LG, Zhang X, Chen W, Philson R, Weng S, Xu H, Krebsbach PH, Fu J (2014) Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat Mater 13:599–604. https://doi.org/10.1038/nmat3945
Sun Z, Costell M, Fässler R (2019) Integrin activation by talin, kindlin and mechanical forces. Nat Cell Biol 21:25. https://doi.org/10.1038/s41556-018-0234-9
Sundararaghavan HG, Monteiro GA, Firestein BL, Shreiber DI (2009) Neurite growth in 3D collagen gels with gradients of mechanical properties. Biotechnol Bioeng 102:632–643. https://doi.org/10.1002/bit.22074
Tajerian M, Hung V, Nguyen H, Lee G, Joubert L-M, Malkovskiy AV, Zou B, Xie S, Huang T-T, Clark JD (2018) The hippocampal extracellular matrix regulates pain and memory after injury. Mol Psychiatry 23:2302. https://doi.org/10.1038/s41380-018-0209-z
Tanner K (2018) Perspective: the role of mechanobiology in the etiology of brain metastasis. APL Bioeng 2:031801. https://doi.org/10.1063/1.5024394
Tate MC, García AJ, Keselowsky BG, Schumm MA, Archer DR, LaPlaca MC (2004) Specific beta1 integrins mediate adhesion, migration, and differentiation of neural progenitors derived from the embryonic striatum. Mol Cell Neurosci 27:22–31. https://doi.org/10.1016/j.mcn.2004.05.001
Taubenberger A, Cisneros DA, Friedrichs J, Puech P-H, Muller DJ, Franz CM (2007) Revealing early steps of α2β1 integrin-mediated adhesion to collagen type I by using single-cell force spectroscopy. Mol Biol Cell 18:1634–1644. https://doi.org/10.1091/mbc.E06-09-0777
Teixeira AI, Ilkhanizadeh S, Wigenius JA, Duckworth JK, Inganäs O, Hermanson O (2009) The promotion of neuronal maturation on soft substrates. Biomaterials 30:4567–4572. https://doi.org/10.1016/j.biomaterials.2009.05.013
Theodosiou M, Widmaier M, Böttcher RT, Rognoni E, Veelders M, Bharadwaj M, Lambacher A, Austen K, Müller DJ, Zent R, Fässler R (2016) Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. eLife 5:e10130. https://doi.org/10.7554/eLife.10130
Thompson AJ, Pillai EK, Dimov IB, Foster SK, Holt CE, Franze K (n.d.) Rapid changes in tissue mechanics regulate cell behaviour in the developing embryonic brain. eLife 8. https://doi.org/10.7554/eLife.39356
Tonazzini I, Meucci S, Faraci P, Beltram F, Cecchini M (2013) Neuronal differentiation on anisotropic substrates and the influence of nanotopographical noise on neurite contact guidance. Biomaterials 34:6027–6036. https://doi.org/10.1016/j.biomaterials.2013.04.039
Tonazzini I, Meucci S, Van Woerden GM, Elgersma Y, Cecchini M (2016) Impaired neurite contact guidance in ubiquitin ligase E3a (Ube3a)-deficient hippocampal neurons on nanostructured substrates. Adv Healthc Mater 5:850–862. https://doi.org/10.1002/adhm.201500815
Tyler WJ (2012) The mechanobiology of brain function. Nat Rev Neurosci 13:867–878. https://doi.org/10.1038/nrn3383
Uhler C, Shivashankar GV (2017) Chromosome intermingling: mechanical hotspots for genome regulation. Trends Cell Biol. https://doi.org/10.1016/j.tcb.2017.06.005
Vitriol EA, Zheng JQ (2012) Growth cone travel in space and time: the cellular ensemble of cytoskeleton, adhesion, and membrane. Neuron 73:1068–1081. https://doi.org/10.1016/j.neuron.2012.03.005
Wang X, Ha T (2013) Defining single molecular forces required to activate integrin and notch signaling. Science 340:991–994. https://doi.org/10.1126/science.1231041
Wang N, Butler JP, Ingber DE (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260:1124–1127. https://doi.org/10.1126/science.7684161
Wang HB, Mullins ME, Cregg JM, McCarthy CW, Gilbert RJ (2010) Varying the diameter of aligned electrospun fibers alters neurite outgrowth and Schwann cell migration. Acta Biomater 6:2970–2978. https://doi.org/10.1016/j.actbio.2010.02.020
Willits RK, Skornia SL (2004) Effect of collagen gel stiffness on neurite extension. J Biomater Sci Polym Ed 15:1521–1531
Winograd-Katz SE, Fässler R, Geiger B, Legate KR (2014) The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol 15:273–288. https://doi.org/10.1038/nrm3769
Woo S, Gomez TM (2006) Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J Neurosci 26:1418–1428. https://doi.org/10.1523/JNEUROSCI.4209-05.2006
Xie J, Willerth SM, Li X, Macewan MR, Rader A, Sakiyama-Elbert SE, Xia Y (2009) The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials 30:354–362. https://doi.org/10.1016/j.biomaterials.2008.09.046
Xiong Y, Lee AC, Suter DM, Lee GU (2009) Topography and nanomechanics of live neuronal growth cones analyzed by atomic force microscopy. Biophys J 96:5060–5072. https://doi.org/10.1016/j.bpj.2009.03.032
Xu Z, Chen Y, Chen Y (2019) Spatiotemporal regulation of Rho GTPases in neuronal migration. Cells 8:568. https://doi.org/10.3390/cells8060568
Yamaguchi Y, Katoh H, Yasui H, Mori K, Negishi M (2001) RhoA inhibits the nerve growth factor-induced Rac1 activation through Rho-associated kinase-dependent pathway. J Biol Chem 276:18977–18983. https://doi.org/10.1074/jbc.M100254200
Yang K, Jung K, Ko E, Kim J, Park KI, Kim J, Cho S-W (2013) Nanotopographical manipulation of focal adhesion formation for enhanced differentiation of human neural stem cells. ACS Appl Mater Interfaces 5:10529–10540. https://doi.org/10.1021/am402156f
Yang K, Jung H, Lee H-R, Lee JS, Kim SR, Song KY, Cheong E, Bang J, Im SG, Cho S-W (2014) Multiscale, hierarchically patterned topography for directing human neural stem cells into functional neurons. ACS Nano 8:7809–7822. https://doi.org/10.1021/nn501182f
Yang B, Lieu ZZ, Wolfenson H, Hameed FM, Bershadsky AD, Sheetz MP (2016) Mechanosensing controlled directly by tyrosine kinases. Nano Lett 16:5951–5961. https://doi.org/10.1021/acs.nanolett.6b02995
Yao M, Goult BT, Chen H, Cong P, Sheetz MP, Yan J (2014) Mechanical activation of vinculin binding to talin locks talin in an unfolded conformation. Sci Rep 4:4610. https://doi.org/10.1038/srep04610
Young JL, Holle AW, Spatz JP (2016) Nanoscale and mechanical properties of the physiological cell–ECM microenvironment. Exp Cell Res 343:3–6. https://doi.org/10.1016/j.yexcr.2015.10.037
Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP (2008) Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol 10:1062–1068. https://doi.org/10.1038/ncb1765
Zhang H, Deo M, Thompson RC, Uhler MD, Turner DL (2012) Negative regulation of Yap during neuronal differentiation. Dev Biol 361:103–115. https://doi.org/10.1016/j.ydbio.2011.10.017
Zhang D, Yang S, Toledo EM, Gyllborg D, Saltó C, Villaescusa JC, Arenas E (2017) Niche-derived laminin-511 promotes midbrain dopaminergic neuron survival and differentiation through YAP. Sci Signal 10:eaal4165. https://doi.org/10.1126/scisignal.aal4165
Zhu J, Zhu J, Springer TA (2013) Complete integrin headpiece opening in eight steps. J Cell Biol 201:1053–1068. https://doi.org/10.1083/jcb.201212037
Funding
This project has received funding from the European Union Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 812772 (Phys2BioMed) and under the FET Open grant agreement no. 801126 (EDIT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chighizola, M., Dini, T., Lenardi, C. et al. Mechanotransduction in neuronal cell development and functioning. Biophys Rev 11, 701–720 (2019). https://doi.org/10.1007/s12551-019-00587-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-019-00587-2