Abstract
Fusarium species are common fungal pathogens of maize. Fusarium graminearum and Fusarium verticillioides, among others, can cause maize ear rot, and they are also mycotoxin producers. The aims of this work were to determine the frequency and diversity of Fusarium species in Uruguayan maize kernels, evaluate the toxigenic potential of the isolates, determine toxin contamination levels on freshly harvested grain, and assess the sensitivity of main Fusarium species against fungicides. Fusarium verticillioides was the most frequent species isolated, followed by Fusarium graminearum sensu stricto. Of F. verticillioides isolates studied for fumonisin production, 72% produced fumonisin B1 and 32% fumonisin B2. Considering in vitro toxin production by F. graminearum sensu stricto isolates, deoxynivalenol was the main toxin produced, followed by zearalenone and nivalenol. Fumonisins were the most frequently found toxins on freshly harvested maize samples (98% in 2018 and 86% in 2019), and also, fumonisin B1 was the toxin with highest concentration in both years studied (4860 µg/kg in 2018 and 1453 µg/kg in 2019). Deoxynivalenol and zearalenone were also found as contaminants. Metconazole and epoxiconazole were the most effective fungicides tested on F. verticillioides isolates. Fusarium graminearum sensu stricto isolates also were more sensitive to metconazole compared to other fungicides; nevertheless, epoxiconazole was less efficient in controlling this species. This is the first study that reports Fusarium species and mycotoxin contamination levels associated with maize grain in Uruguay. Its detection is the main step to develop management strategies in order to minimize fungal infection in maize crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maize, Zea mays L., is considered to be one of the most susceptible crops to mycotoxin contamination worldwide. Fusarium species are common fungal pathogens of maize, and these fungi are responsible for various diseases including seedling blight, stalk, and ear rot. Within Fusarium species, Fusarium graminearum and some species of the Fusarium fujikuroi complex (FFSC) such as Fusarium verticillioides, Fusarium proliferatum, and Fusarium subglutinans are considered among the predominant species causing maize ear rot (Logrieco et al. 2002).
Fusarium verticillioides is capable of producing fumonisins, dangerous toxins for animals and humans. They have been classified into four main groups: fumonisins A, B, C, and P. Fumonisin B (FB) is divided into 4 analogs (FB1, FB2, FB3, and FB4) and is the most abundant naturally occurring fumonisin (Picot et al. 2010; Reddy et al. 2010; Sundheim and Tsehaye 2015). Also, FB1 is the most toxic and usually appears in the highest concentrations in grains contaminated with F. verticillioides (Alexander et al. 2009; Baldwin et al. 2014). This mycotoxin is classified as possibly carcinogenic to humans and livestock and also causes equine leukoencephalomalacia, rat hepatocarcinoma, and porcine pulmonary edema (Gelderblom et al. 1988; Marasas et al. 1988).
Species in the Fusarium graminearum species complex (FGSC) are important pathogens of small-grain cereals and maize in many areas of the world. These fungi often cause crop diseases at different stages of development, and they are also mycotoxin producers. Fusarium graminearum species complex can produce different mycotoxins such as zearalenone (ZEN), nivalenol (NIV), and deoxynivalenol (DON). Deoxynivalenol is also divided into two acetylated derivatives, 3-acetyldeoxynivalenol (3-AcDON) and 15-acetyldeoxynivalenol (15-AcDON), where 3-AcDON has shown to be more phytotoxic and isolates that produce it may have higher pathogenic potential than those producing 15-AcDON (Pestka 2010; McCormick et al. 2011). In South America, 15-AcDON-producing isolates are usually predominant, while 3-AcDON and NIV isolates are less frequent (Del Ponte et al. 2022). Zearalenone is a toxin which has an estrogenic effect on humans and animals, causing hyperestrogenism, especially in swine (Sundlof and Strickland 1986).
Fungicides have always been a useful strategy for limiting fungal diseases on important crops. However, long-term and extensive use of chemical compounds in different crops can cause the emergence of fungicide resistance in pathogens. For this reason, monitoring the efficacy of fungicides against Fusarium species plays a key role in managing chemical control strategies. Carbendazim, a benzimidazole fungicide, has been widely applied for Fusarium control, and in recent years, carbendazim-resistant field populations have been increasing in isolates from small-grain cereals (Duan et al. 2014). On the other hand, sterol biosynthesis inhibitors, which include triazoles, are reported to be the most effective chemicals against Fusarium (Pirgozliev et al. 2002; Amarasinghe et al. 2013).
In Uruguay, the area planted with corn has been increasing over the last 3 years, reaching 143,000 hectares in the 2020/2021. At the same time, the productivity of the crop has increased (an accumulated 45% in the last 10 years) due to the professionalization of corn production with the incorporation of technological improvements and the use of late sowing varieties. Maize in Uruguay is mainly used as livestock feed for beef and dairy cattle (Methol 2021). In addition to this, surveys to determine prevalence of different Fusarium species and mycotoxins in maize have not been extensively carried out in Uruguay. This is extremely important, since a comprehensive knowledge of the Fusarium species occurring on maize kernels, as well as the mycotoxins present, has important practical implications for the development of appropriate strategies to limit the presence of fungi in maize and ensure animal health.
Considering the information mentioned above, the aims of this study were (i) to determine the frequency and diversity of Fusarium species in Uruguayan maize kernels, (ii) to evaluate the toxigenic potential of the isolates, (iii) to determine mycotoxin contamination levels on freshly harvested maize grain, and (iv) to evaluate the sensitivity of different fungicides to the main Fusarium species associated to maize.
Materials and methods
Sampling and fungal isolation
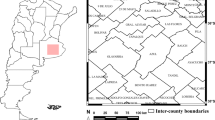
A total of 152 maize kernel samples were collected from fields located in the major maize growing area south-west of Uruguay, at the departments of Paysandú (n = 4), Río Negro (n = 21), Soriano (n = 47), Colonia (n = 71), Flores (n = 1), San José (n = 2), Florida (n = 1), Durazno (n = 3), and Rocha (n = 2) (Fig. 1). Samples from the two crop seasons, 2018 (n = 94) and 2019 (n = 58), were analyzed. Kernels were surface sterilized for 1 min in 0.4% sodium hypochlorite solution, rinsed twice in sterile distilled water, and dried on sterilized filter paper. One hundred kernels from each sample were placed in Petri dishes containing potato dextrose agar (PDA) and incubated at 25 °C under a 12 h white /12 h black fluorescent light photoperiod for 7 days. Fungal colonies presumably belonging to Fusarium spp. were purified and transferred to new Petri dishes with PDA, using a single-spore technique, for subsequent identification to species level.
Identification of Fusarium spp
Identification to species level was performed based on morphological characteristics following mycological methods according to Leslie and Summerell (2006). Subsequently, in order to confirm morphological identification, molecular methods were performed. For this, genomic DNA (gDNA) was extracted from single-spore culture isolates with a cetyltrimethylammonium bromide (2% CTAB) method (Leslie and Summerell 2006). All PCR reactions were carried out in a GeneAmp PCR system 9700 thermocycler (Perkin-Elmer, USA). Fusarium graminearum sensu stricto (Fusarium graminearum s. s.) was identified by RFLP of the TEF-1α gene method using enzyme BsaHI (Garmendia et al. 2018a). Fusarium graminearum s. s. strains were identified by two fragments of 367 bp and 291 bp each. The identification of F. verticillioides strains was carried out by PCR detection assays using sets of species specific primers VER-1 and VER-2 (5′-CTTCCTGCGATGTTTCTCC-3′ and 5′-AATTGGCCATTGGTATTATATATCTA-3′, respectively) (Mulè et al. 2004). Also, elongation factor-1 α (tef-1α) gene sequence was used to identify other Fusarium isolates obtained (Geiser et al. 2004). DNA sequences were analyzed using the SeqMan software (Lasergene, Madison, WI) and compared with the sequences of closely related species in GenBank database by using the Basic Local Alignment Search Tool (BLAST) and in the CBS-KNAW Fungal Biodiversity Centre’s Fusarium MLST website (https://fusarium.mycobank.org/databases). The generated sequences were deposited in the GenBank database under accession numbers OQ948480–OQ948506.
Toxigenic capacity of fungal isolates
A random subsample of all collected isolates of F. verticillioides (n = 86) and FGSC (n = 79) was selected in order to analyze their in vitro toxigenic profile. Toxins were analyzed by high-performance liquid chromatography (HPLC) consisting of a Shimadzu LC-10ADvp pump. A RF-10Axl fluorescence detector and a photodiode array detector were used for fumonisins and trichothecenes, respectively (Shimadzu). For all toxin evaluations, a C18 column (150 mm × 4.6 mm i.d., 5 µm particle size; Nucleodur®, Macherey–Nagel, Düren, Germany) connected to a pre-column security guard (8 mm × 4 mm i.d., 5 µm particle size; Nucleodur®, Macherey–Nagel, Düren, Germany) was used. Fumonisins B1 and B2, DON, NIV, and ZEN standards were supplied by Trilogy Analytical Laboratory Inc., Washington, MO. For FB1 and B2 evaluation, 20 g of rice kernels with 10 ml of sterile water was autoclaved for 30 min twice on alternate days. For DON, NIV, and ZEA production, 25 g of rice with 10 ml of water was used. Bags of autoclaved rice were inoculated with 3 mycelium plugs taken from pure cultures of each selected isolate. Bags were incubated in the dark at 25 °C for 28 days, and developed cultures were then oven dried at 60 °C, finely ground with a laboratory blender, and stored at − 20 °C until use.
Fumonisins extraction was performed according to the AOAC: 995.15 method. Toxins were extracted from 5g of grinded rice with methanol:water (3:1 v/v), filtered extract was applied to an anion-exchange (SAX) column (Strata®SAX, 55μm, 70 Å, Phenomenex), and fumonisins were eluted with acetic acid: methanol (1:99 v/v). Eluate was evaporated to dryness, and the residue was dissolved in methanol. O-Phthaldialdehyde (Sigma-Aldrich, Milan, Italy) reagent solution was added to form fluorescent fumonisin derivatives. A fluorescence detector with excitation and emission wavelengths of 335nm and 440 nm, respectively, was used. The mobile phase was methanol: NaH2PO4 0.1M solution (77:23 v/v) pH 3.5, at a flow rate of 1ml/min; the injection volume was 20 μl. Fumonisin concentration was determined by comparison with external standards.
Zearalenone production was performed based on the methodology described by AOAC 985.18. Five grams of ground rice was used for toxin extraction using methanol: water (80:20 v/v); the filtered extract was cleaned using a MycoSep®226 column (Romer Labs Inc., MO, USA). The eluate was first evaporated and then dissolved in methanol: water (70:30 v/v), the same solution was used as mobile phase, the flow rate was 1ml/min, and the injection volume was 50 μl. ZEN concentration was determined by comparison with external standards.
Deoxynivalenol and NIV were detected using methods described by Reynoso et al. (2011). Toxins were extracted from 7.5 g of ground rice with acetonitrile: methanol (14:1 v/v) and then filtered through an aluminum C-activated (20:1, w/w) laboratory made column. Eluate was evaporated to dryness and resuspended with methanol: water (5:95 v/v). An UV detector (220 nm) was used. The mobile phase was methanol: water (12:88 v/v), at a flow rate of 1.5 ml/min. The injection volume was 50 μl. DON and NIV concentration was determined by comparison with external standards.
Trichothecene genotype determination
For FGSC isolates, trichothecene genotypes were also determined using three different PCR assays. First, two multiplex assays targeted a portion of trichothecene biosynthesis genes Tri3 and Tri12 to determine NIV, 15-AcDON, and 3-AcDON genotypes (Ward et al. 2002), while the third assay targeted portions of the Tri13 gene to determine DON genotypes (Chandler et al. 2003) (Table 1). The Tri3 multiplex included a primer common to all genotypes (3CON) and three genotype-specific primers: 3NA, 3D15A, and 3D3A. This reaction produces amplicons of approximately 840, 610, and 243 bp for isolates that match NIV, 15-AcDON, and 3-AcDON genotypes, respectively. The Tri12 multiplex similarly included a primer common to all genotypes, 12CON, and three genotype-specific primers: 12NF, 12-15F, and 12-3F. This multiplex produced amplicons of approximately 840, 670, and 410 bp for isolates that match NIV, 15ADON, and 3ADON genotypes, respectively. The Tri13 gene amplification was performed using primers Tri13F and Tri13DONR; the PCR amplifies a fragment of 282 bp for DON-producing isolates. The multiplex PCR was conducted in 25 µl reaction mixtures containing 50 ng of DNAg, 1X PCR buffer (Nzytech), 2 mM MgCl2, 0.2 mM dNTPs, 0.5U DNA Taq polymerase (Nzytech), and 0.2 mM each primer. PCR for Tri3 and Tri12 consisted of an initial denaturation of 2 min at 94 °C, followed by 25 cycles of 30 s at 94 °C, 30 s at 52 °C, and 1 min at 72 °C.
PCR assays for Tri13 genes were conducted using 50 ng of fungal DNA in a total volume of 25 µl containing 1.5X PCR buffer (Nzytech), 2 mM MgCl2, 0.2 mM of each dNTPs, 0.75 U of DNA Taq polymerase (Nzytech), and 0.4 mM each primer. PCR amplification of Tri13 consisted of an initial denaturation of 2 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 30 s at 65 °C, and 30 s at 72 °C.
All amplification products were resolved on 1.5% (wt/vol) agarose gels and scored by size in comparison to a 100-bp DNA size ladder (Invitrogen Life Technologies, Carlsbad, CA).
Natural mycotoxin occurrence in maize grain
Sixty-one representative samples of maize grain from the 2018 harvest and 58 samples from 2019 were selected for mycotoxin determination. One kilogram of each sample was ground in a Romer mill, range of 60–200 mesh (Romer Labs Inc., MO, USA) and stored in the freezer at − 80 °C until analysis. Before the analysis, a ground subsample (20 g) was obtained by the quartering sampling method following Codex guidelines. Fumonisins and ZEN determination were performed according to AOAC methods described above (AOAC 2019). All toxins were determined using HPLC connected to a reversed-phase Gemini C18 (4.60 mm × 150 mm, 5 μm, for fumonisins and DON and 4.60 mm × 250 mm, 5 μm, for ZEA and NIV) analytical column (Phenomenex, USA) connected to a pre-column security guard (8 mm × 4 mm i.d., 5 µm particle size; Nucleodur®, Macherey–Nagel, Düren, Germany). The HPLC system consisted of a Waters 1525 pump, Waters 717 Injector, and Waters TCM oven. For fumonisins and ZEN, the HPLC system was coupled to a Waters 2475 Fluorescence detector. The mobile phase and standard solutions used were that described above. Deoxynivalenol and NIV extraction was performed according to the AOAC: 986.17 method. A Waters 2996 photodiode array detector (220 nm) was used. The mobile phase and standard solutions used were that described above. Veracity and precision of the method were evaluated by using maize kernels spiked with each toxin (DON, NIV, ZEN, FB1, or FB2 as appropriate). Replicates of spiked samples were performed, and the relative standard deviation was calculated among them to stipulate the precision of the method. The veracity of the analytical method was evaluated in terms of recovery. Recoveries were determined by spiked maize samples with known levels of standards. Percent method recovery was 93% for DON, 87% for NIV, 76% for FB1, 77% for FB2, and 77% for ZEA. The limits of report quantification (LOQs) were 80 µg/kg (FBs), 30 µg/kg (ZEN), 88 µg/kg (DON), and 50 µg/kg (NIV).
Fungicide sensitivity assay
Sensitivity of F. verticillioides and F. graminearum s. s. isolates was evaluated against two different chemical classes of fungicides, triazoles and benzimidazoles. Fungicides belonging to triazoles were metconazole, tebuconazole, and epoxiconazole, while carbendazim belongs to benzimidazole class. The assay was performed on a total of 20 isolates per species, 10 isolates from each crop season (2018 and 2019). Fungicides were diluted in dimethylsulfoxide 0.1% (DMSO) and incorporated into PDA to achieve concentrations of 0.25, 0.50, 0.75, 1.5, 3, 6, and 10 mg/l. Three replicates of each fungicide concentration per isolate were performed. A mycelial plug (8 mm in diameter) of each isolate was taken from a 5-day-old colony and placed on the center of a PDA plate amended with each fungicide at each concentration. Fusarium verticillioides isolates plates were incubated for 7 days at 25 °C in darkness; F. graminearum s. s. strains were incubated for 5 days under the same conditions. Colony diameters were measured daily in two perpendicular directions. Evaluation was performed based on the radial growth on PDA containing different concentrations of each fungicide, compared to control plates (PDA + DMSO) (Becher et al. 2010; Tateishi et al. 2010). Effective concentration of fungicides leading to a 50% inhibition (EC50) of mycelial growth of each strain was determined. The EC50 was calculated on the basis of linear regression analysis of relative growth inhibition values against the log10-transformed fungicide concentrations based on Probit analysis (Finney 1952). Analysis of variance (ANOVA) of the EC50 values were conducted to determine differences in sensitivity among species (F. verticillioides and F. graminearum s. s.) for each fungicide and among fungicides within each species. Means were compared using Fisher’s least significant difference (LSD) (α = 0.05). Statistical analysis was performed using Infostat (Di Rienzo et al. 2011).
Results
Frequency of Fusarium in maize kernels
According to the 152 field samples analyzed over both years (2018 n = 94, 2019 n = 58), Fusarium species were the most frequent toxigenic contaminants in maize kernels (45%) with incidence values ranging between 7 and 100%, followed by Aspergillus species (15%). Within the Fusarium species, Fusarium verticillioides was the most frequent species isolated, with an average frequency of 44% and 30% in 2018 and 2019, respectively. The second most frequent Fusarium species isolated was the FGSC with 3% average frequency for both years studied. Other Fusarium species identified were F. proliferatum and F. subglutinans, but they were in low frequency over both years (Fig. 2).
To correctly assign the Fusarium species identification, 236 Fusarium isolates from both years were selected as representatives of the Fusarium population isolated from maize kernels and were molecularly identified. This allowed to identify 115 isolates as F. verticillioides, 89 isolates as F. graminearum s.s, 14 isolates as F. subglutinans, 10 isolates as F. proliferatum, 6 isolates as F. meridionale, 1 isolate as F. pseudograminearum, and 1 as F. armeniacum.
Toxigenic capacity of fungal isolates
Fumonisin production was assessed for 84 of the isolates identified molecularly as F. verticillioides. Fumonisin B1 was produced by 69% (n = 33) of the isolates from 2018 and 75% (n = 27) from 2019; the proportion of FB2 producers was 31% and 33% for each year (n = 15 from 2018, n = 12 from 2019) (Fig. 3).
Deoxynivalenol, NIV, and ZEN production was studied in 74 of the F. graminearum s. s. isolates and in 4 of the F. meridionale strains found. The proportion of F. graminearum s. s. mycotoxin producer strains was high during both years of study, with 70.5% (n = 24) and 72.5% (n = 29) ZEN producer strains in 2018 and 2019, respectively, and 85% of DON producer strains for each year of study. On the other hand, NIV producer strains were less frequent with 23.5% (n = 8) and 7.5% (n = 3) in 2018 and 2019, respectively (Fig. 3). Finally, F. meridionale strains showed a different mycotoxin profile compared to F. graminearum s. s. strains, with 2 out of 4 isolates being NIV producers in 2018, while ZEN and DON producer strains were not found.
Trichothecene genotypes
Trichothecene genotype study was performed on 85% (n = 29) and 86% (n = 45) of F. graminearum s. s. isolates from 2018 and 2019, respectively. Moreover, genotype profile was assessed for four F. meridionale isolates. All isolates identified as F. graminearum s. s. belong to genotypes DON-15-AcDON, and all F. meridionale isolates have a genotype NIV-DON.
Natural mycotoxins occurrence on freshly harvested maize grain
The presence of Fusarium mycotoxins (DON, ZEN, NIV, FB1, and FB2) was assessed on 119 maize samples, 61 belonging to 2018 and 58 from 2019. About 97% and 86% of freshly harvested samples from 2018 and 2019, respectively, were contaminated with FB1, which was the main contaminant found in the study (Table 2). Higher concentrations of fumonisins were found on samples from 2018, FB1 averaged 4071 µg/kg and 1529 µg/kg for FB2 (Table 2). Most samples studied had contamination levels of fumonisins lower than 2000 µg/kg, and only 10% or less of the samples showed levels of contamination above 5000 µg/kg (Fig. 4).
The presence of DON was also detected in maize samples, but at lower concentrations during 2018 and 2019 with a mean content of 278 µg/kg and 118 µg/kg, respectively (Table 2). Regarding ZEN, the contamination levels were higher during 2018, with 64% positive freshly harvested samples and a mean value of 302 µg/kg (Table 2). On the other hand, NIV was detected in one sample each year and at low levels.
Sensitivity to fungicides
EC50 values of epoxiconazole for the tested F. verticillioides isolates (n = 20) ranged between 0.002 and 0.107 mg/l with a mean of 0.040 mg/l (Fig. 5). For metconazole, the sensitivity range was between 0.004 and 0.146 mg/l with a mean value of 0.027 mg/l (Fig. 5). EC50 values for tebuconazole ranged from 0.03 to 0.429 mg/l with a mean value of 0.093 mg/l (Fig. 5). Lastly, carbendazim EC50 values ranged between 0.181 and 0.651 mg/l with a mean of 0.410 mg/l (Fig. 5). One of the F. verticillioides isolates (A61) was less sensitive for both tebuconazole and metconazole with EC50 values of 0.499 and 0.186 mg/l, respectively (online resource 1).
In the case of F. graminearum s. s isolates (n = 20), the EC50 values for triazole fungicide class ranged from 0.217 to 3.951 mg/l with a mean of 1.392 mg/l for epoxiconazole, 0.001 to 0.710 mg/l with a mean of 0.155 mg/l for metconazole, and 0.040 to 1.656 mg/l with a mean of 0.763 mg/l for tebuconazole (Fig. 5). Additionally, carbendazim EC50 values ranged between 0.130 and 1.145 mg/l with a mean of 0.649 mg/l (Fig. 5). In the case of epoxiconazole, isolates A539 and A545 were observed to be the least sensitive, with EC50 values of 3.939 and 3.951 mg/l, respectively. Also, isolates A545 and A560 showed low levels of sensitivity for metconazole, with values of 0.710 and 0.403 mg/l (online resource 2).
Considering F. verticillioides, no differences were shown between epoxiconazole and metconazole, although tebuconazole appears to be less effective showing significant differences on EC50 values (p < 0.05) when compared to metconazole and epoxiconazole. Also, isolates were significantly less sensitive to the presence of carbendazim (p < 0.05). On the other hand, F. graminearum s. s. isolates were more sensitive to metconazole (p < 0.05) than other fungicides tested and least sensitive to epoxiconazole.
Overall, F. verticillioides isolates were more sensitive than F. graminearum s. s. strains for all fungicides tested (p < 0.05).
Less sensitive isolates of F. graminearum s.s were observed in the case of all fungicides assessed (Fig. 5). The EC50 for the least sensitive isolates was 3.951 mg/l for epoxiconazole, 1.656 mg/l for tebuconazole, 0.710 mg/l for metconazole, and 1.145 mg/l for carbendazim.
Discussion
This work represents the first survey on Fusarium species associated with maize kernels in Uruguay. Fusarium verticillioides appeared as the predominant species, and to a lesser extent, F. graminearum s. s. was also identified as an important contaminant of this crop. Studies carried out in other regions, in particular, in Brazil and Argentina, showed that F. verticillioides was the most prevalent Fusarium species in freshly harvested maize (Castañares et al. 2019; van der Westhuizen et al. 2003). In our study, F. verticillioides was isolated with a frequency of 37% that is similar to that found in maize samples from Brazil (Almeida et al. 2002), Korea (Choi et al. 2018), Iran (Fallahi et al. 2019), Bélgica (Scauflaire et al. 2011), and China (Zhou et al. 2018). However, our frequency is lower than those observed by other authors. In Brazil, Stumpf et al. (2013) determined that F. verticillioides was present in a frequency of 96% and Barroso et al. (2017) between 54.1 and 91.8% on commercial hybrids of maize. Same results were obtained from Argentina (Castañares et al. 2019), Brazil (Lanza et al. 2014), China (Qin et al. 2020), Nigeria (Bankole and Mabekoje 2004) and Spain (Aguín et al. 2014). These differences could be due to several factors, including climatic conditions, maize genotypes, and cultural practices that influence the occurrence and prevalence of F. verticillioides and other Fusarium species that affect maize (Munkvold 2003).
On the other hand, two more species members of FFSC have been detected with significant frequency: F. subglutinans and F. proliferatum (O'Donnell et al. 1998). Both species often co-occur with F. verticillioides worldwide on maize, making the development of resistant hybrids more difficult for breeders (Fallahi et al. 2019). Considering that this is the first report of some of the species within the FFSC in crops of Uruguay, more studies about this species complex should be carried on in order to develop adequate strategies to control Fusarium ear rot and minimize mycotoxin contamination of maize.
When it comes to FGSC, it is usually suggested that F. meridionale is adapted to maize agroecosystems, as it is the most prevalent contaminant of the FGSC in maize in Brazil and Argentina, despite the prevalence of other FGSC species on wheat and rice in those countries (Alvarez et al. 2009; Del Ponte et al. 2015; Kuhnem et al. 2016; Sampietro et al. 2011). However, our data indicate that F. graminearum s. s. is the main contaminant of the FGSC in maize in Uruguay, suggesting that extended areas of crops such as wheat and barley, usually contaminated with F. graminearum s. s. (Garmendia et al. 2018b; Pan et al. 2016; Umpiérrez-Failache et al. 2013), may be a very important factor in species distribution when these crops coexist in rotation on the same cultivated areas like in our country.
The present study showed that over 70% of F. verticillioides isolated from maize in Uruguay are in vitro fumonisins producers, being FB1 the principal toxin produced by this species. Considering this, and the fact that F. verticillioides was the most prevalent species, the presence of fumonisins in maize grain is a potential threat to human and animal health in our country. In this sense, the presence of F. proliferatum and F. subglutinans of further concern due to the ability of these species to produce fumonisins increases risks of consumption of contaminated maize due to the coexistence of these toxigenic species in maize.
Regarding F. graminearum s. s., in vitro mycotoxin production profile, DON, was the most frequently produced toxin, followed by ZEN and NIV. Previous studies in South America about this species contaminating different crops have also found DON as the main toxin produced (Del Ponte et al. 2022). Nevertheless, our study also showed a very high proportion of isolates being ZEN producers (70.5% and 72.5% from 2018 and 2019, respectively). Although there are very few studies on potential ZEN production by F. graminearum s.s isolates, it is well known that the levels of ZEN are frequently present in grains from different crops, including maize (Salay and Mercadante 2002; Maragos 2010). On the other hand, F. graminearum s. s. isolates evaluated in this study showed DON-15-AcDON genotype, and none of them had NIV genotype. This result is consistent with the previous reports from different crops in Uruguay (Garmendia et al. 2018b; Pan et al. 2013, 2016; Umpiérrez-Failache et al. 2013), where DON-15-AcDON genotype prevails for F. graminearum s. s., showing stability of genotype composition across crops and years in our country. Moreover, in recent years, it has been shown that in Europe, 15-AcDON genotype is the predominant one in wheat and maize, with an overall distribution in cereals up to 83% (Pasquali et al. 2016).
Previous studies that evaluated the toxigenic potential of F. meridionale from Argentina, Brazil, and other parts of the world including Europe, China, and Mexico (Scoz et al. 2009; Astolfi et al. 2012; Sampietro et al. 2011; Desjardins and Proctor 2011; Boutigny et al. 2014) suggested a very strong association of this species to NIV genotype. However, our results showed that all strains of F. meridionale studied were characterized as a DON/NIV genotype and were either non-toxin producers or NIV producers by chemical analysis. Some studies reported the presence of DON/NIV genotype in different species of FGSC. Furthermore, isolates of this genotype showed different chemical profiles (Del Ponte et al. 2022; Lee et al. 2012; Sampietro et al. 2013; Reynoso et al 2011). Moreover, Barros et al. (2012) found that all F. meridionale strains isolated from Argentinian soybean grain showed the DON/NIV genotype. However, these DON/NIV isolates of F. meridionale produced detectable DON and NIV levels and only DON by chemical analysis. Further studies will be necessary in order to characterize the F. meridionale DON/NIV strains.
The fact that F. verticillioides was the most prevalent species found on maize samples and that most of the isolates were able to produce FB1 was to be expected that over 85% of the maize samples were naturally contaminated with fumonisins, being FB1 the predominant toxin. Piñeiro et al. (1997) studied the levels of fumonisins in unprocessed corn kernels form Uruguay; their results showed average contamination levels with FB1 of 963 µg/kg, with a range from ND to 3688 µg/kg, with 50% of contaminated samples with FB1, and no contaminated samples with FB2. Although their results are based only on 22 samples, both surveys show that FB1 contamination of maize grains is frequent in Uruguay, and it is frequently found at high levels. Also, our results are similar to those reported for maize in other parts of the world (Garrido et al. 2012; Scussel et al. 2014; Udovicki et al. 2018; Yli-Mattila and Sundheim 2022) and support the idea that this crop is very prone to fumonisin contamination (Ponce-García et al. 2018).
In Uruguay, maize is principally used for feeding cattle, dairy cattle, poultry, and swine. Considering currents international reglementations, the levels found on 24% (n = 15) of the samples from 2018 and 10% (n = 6) of the samples from 2019 are higher than those recommended by CAC (2019) for food and feed (4000 µg/kg FB1 + FB2), and only one sample exceeded China recommended levels (GB 13078, 2017) in maize grain and maize by-products for animal feed (60,000 µg/kg FB1 + FB2). Despite the similar incidence of F. verticillioides in both years of study (44% and 30% in 2018 and 2019, respectively), the levels of fumonisins were much higher in 2018 compared to 2019. This could be explained by fumonisin production being very variable among strains and that environmental conditions play an important role in its production (Munkvold and Desjardins 1997).
The levels of DON and ZEN found in the present study were similar when compared to the reports from maize of the region (Castañares et al. 2019; Mendes de Souza et al. 2013). Only the DON levels present in 6% (n = 7) of samples exceed the recommended levels in Uruguay for swines and equines (1000 μg/kg). No samples exceed the levels for raw materials destined for the production of animal feed (10,000 μg/kg) (MGAP resolución S/N/001 2001; FDA 2010). Considering levels of ZEN, 11% (n = 13) of the samples exceeded the recommended levels in Uruguay of 250 μg/kg. It could be explained by the low incidence of FGSC (3%) in both years studied.
Chemical applications to control Fusarium ear rot are not a widely used practice, and few studies have demonstrated the efficacy of synthetic fungicide to control Fusarium ear rot in maize, especially against Fusarium fujikuroi species complex (Masiello et al. 2019; Tava et al. 2021). This study provides new information on the sensitivity of the two main toxigenic Fusarium species associated with Fusarium ear rot of maize to different fungicides commonly used in other crops. Sensitivity of F. verticillioides Uruguayan isolates to tebuconazole, metconazole, and carbendazim was similar to those reported by other authors years ago in other geographical regions (Ivić et al. 2011; Marin et al. 2013). No reported data was found on sensitivity of F. verticillioides to epoxiconazole.
Metconazole was the most effective fungicide to inhibit growth of F. graminearum s. s. isolates in this study, followed by carbendazim, despite some reports mentioning the occurrence of resistant field strains to benzimidazole fungicides in other crops (Brent and Hollomon 2007; Chen et al. 2007; Liu et al. 2010). However, our results showed EC50 values higher than 0.362 mg/l and 0.320 mg/l for metconazole, which were the highest values reported by Garmendia et al. (2018b) for isolates of F. graminearum s.s from barley in Uruguay and Spolti et al. (2014) from wheat in Brazil, respectively.
Results showed significant differences in sensitivity of two important Fusarium toxigenic species to four fungicides commonly used, with F. verticillioides being more sensitive to fungicides than F. graminearum s. s. These differences are probably due to long-term exposure of F. graminearum s. s. strains to different classes of fungicides; thus, F. graminearum s. s. is known to be the most frequent pathogen on barley and wheat in Uruguay, causing severe Fusarium head blight (Umpiérrez-Failache et al. 2013; Garmendia et al. 2018b). These crops, especially wheat, are usually treated with fungicides to control this disease. On the other hand, F. verticillioides appears here as the most important contaminant of maize, but generally in Uruguay, farmers produce maize primarily to feed their animals, so no fungicide is applied.
The in vitro assay on fungicides available in the market allowed us to select the most effective chemical against the most occurring pathogenic and toxigenic fungi on maize. Results of fungicide assays should be taken into account to design experiments in the field to confirm in vitro tests.
In conclusion, this is the most extensive study that reports Fusarium species associated with maize kernels in Uruguay and the presence of Fusarium toxins on grain samples. This knowledge is an important step for developing management strategies to minimize Fusarium infection and the presence of their toxins in maize. To this purpose, it was determined that the most effective fungicide for the control of the Fusarium species analyzed is metconazole. However, it is important to continue studying the sensitivity of Uruguayan Fusarium strains to fungicides, considering this an important strategy to control the disease in the field and improve crop sustainability.
References
Aguín O, Cao A, Pintos C, Santiago R, Mansilla P, Butrón A (2014) Occurrence of Fusarium species in maize kernels grown in northwestern Spain. Plant Pathol 63:946–951
Alexander NJ, Proctor RH, McCormick SP (2009) Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev 28:198–215
Almeida AP, Fonseca H, Fancelli AL, Direito GM, Ortega EM, Corrêa B (2002) Mycoflora and fumonisin contamination in Brazilian corn from sowing to harvest. J Agric Food Chem 50:3877–3882
Alvarez CL, Azcarate MP, Pinto VF (2009) Toxigenic potential of Fusarium graminearum sensu stricto isolates from wheat in Argentina. Int J Food Microbiol 135:131–135
Amarasinghe C, Tamburic-Ilincic L, Gilbert J, Brûlé-Babel AL, Fernando WG (2013) Evaluation of different fungicides for control of Fusarium head blight in wheat inoculated with 3ADON and 15ADON chemotypes of Fusarium graminearum in Canada. Can J Plant Pathol 35:200–208
AOAC International Official Methods of Analysis of AOAC International (2019)
Astolfi P, Reynoso MM, Ramirez ML, Chulze SN, Alves TCA, Tessmann DJ, Del Ponte EM (2012) Genetic population structure and trichothecene genotypes of Fusarium graminearum isolated from wheat in southern Braz. J Plant Physiol 61:289–295
Baldwin TT, Zitomer NC, Mitchell TR, Zimeri AM, Bacon CW, Riley RT, Glenn AE (2014) Maize seedling blight induced by Fusarium verticillioides: accumulation of fumonisin B1 in leaves without colonization of the leaves. J Agric Food Chem 62:2118–2125
Bankole S, Mabekoje O (2004) Occurrence of aflatoxins and fumonisins in pre-harvest maize from South-Western Nigeria. Food Addit Contam 21:251–255
Udovicki B, Audenaert K, De Saeger S, Rajkovic A (2018) Overview on the mycotoxins incidence in Serbia in the period 2004–2016. Toxins (basel) 10:279
Barros G, Alaniz Zanon M, Abod A, Oviedo M, Ramirez ML, Reynoso MM, Torres A, Chulze S (2012) Natural deoxynivalenol occurrence and genotype and chemotype determination of a field population of the Fusarium graminearum complex associated with soybean in Argentina. Food Add Cont 29:293–303
Barroso V, Rocha L, Reis T, Reis G, Duarte A, Michelotto M, Correa B (2017) Fusarium verticillioides and fumonisin contamination in Bt and non-Bt maize cultivated in Brazil. Mycotoxin Res 33:121–127
Becher R, Hettwer U, Karlovsky P, Deising HB, Wirsel SG (2010) Adaptation of Fusarium graminearum to tebuconazole yielded descendants diverging for levels of fitness, fungicide resistance, virulence, and mycotoxin production. Phytopathology 100:444–453
Boutigny AL, Ward TJ, Ballois N, Iancu G, Ioos R (2014) Diversity of the Fusarium graminearum species complex on French cereals. Eur J Plant Pathol 138:133–148
Brent KJ, Hollomon DW (2007) Fungicide resistance: the assessment of risk. In Global Crop Protection Federation. (FRAC Monograph No. 2.); The Fungicide Resistance Action Committee Brussels: Brussels, Belgium
Castañares E, Martínez M, Cristos D, Rojas D, Lara B, Stenglein S, Dinolfo M (2019) Fusarium species and mycotoxin contamination in maize in Buenos Aires province, Argentina. Eur J Plant Pathol 155:1265–1275
Chandler EA, Simpson DR, Thomsett MA, Nicholson P (2003) Development of PCR assays to Tri7 and Tril3 trichothecene biosynthetic genes, and characterization of chemotypes of Fusarium. Physiol Mol Plant Pathol 62:355–367
Chen CJ, Wang JX, Luo QQ, Yuan SK, Zhou MG (2007) Characterization and fitness of carbendazim-resistant strains of Fusarium graminearum (wheat scab). Pest Manag Sci 63:1201–1207
Choi J-H, Lee S, Nah J-Y, Kim H-K, Paek J-S, Lee S, Ham H, Kee Hong S, Yunb S-H, Lee T (2018) Species composition of and fumonisin production by the Fusarium fujikuroi species complex isolated from Korean cereals. Int J Food Microbiol 267:62–69
Codex Alimentarius Commission (CAC) (2019). General standard for contaminants and toxins in food and feed. CXS 193–1995. 2019. Available from: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf
Del Ponte EM, Moreira GM, Ward TJ et al (2022) Fusarium graminearum species complex: a bibliographic analysis and web-accessible database for global mapping of species and trichothecene toxin chemotypes. Phytopathology 112:741–751
Del Ponte EM, Spolti P, Ward TJ, Gomes LB, Nicolli CP, Kuhnem PR, Silva CN, Tessmann DJ (2015) Regional and field-specific factors affect the composition of Fusarium head blight pathogens in subtropical no-till wheat agroecosystem of Brazil. Phytopathology 105:246–254
Desjardins AE, Proctor RH (2011) Genetic diversity and trichothecene chemotypes of the Fusarium graminearum clade isolated from maize in Nepal and identification of a putative new lineage. Fungal Biol 115:38–48
Di Rienzo JA, Casanoves F, Balzarini MG, González L, Robledo CW (2011) InfoStat (versión 2011). FCA, Universidad Nacional de Córdoba, Argentina, Grupo InfoStat
Duan Y, Zhang X, Ge C, Wang Y, Cao J, Jia X, Wang J, Zhou M (2014) Development and application of loop-mediated isothermal amplification for detection of the F167Y mutation of carbendazim-resistant isolates in Fusarium graminearum. Sci Rep 4:Article 7094
Fallahi M, Saremi H, Javan-Nikkhah M, Somma S, Haidukowski M, Logrieco AF, Moretti A (2019) Isolation, molecular identification and mycotoxin profile of fusarium species isolated from maize kernels in Iran. Toxins 11:297
FDA – US Food and Drug Administration (2010). Guidance for industry and FDA: advisory levels for deoxynivalenol (DON) in finished wheat products for human consumption and grains and grain by-products used for animal feed. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-and-fda-advisory-levels-deoxynivalenol-don-finished-wheat-products-human
Finney DJ (1952) Probit Analysis (2nd Ed). J Inst Act 783: 388–390
Garrido CE, Hernández Pezzani C, Pacin A (2012) Mycotoxins occurrence in Argentina’s maize (Zea mays L.) from 1999 to 2010. Food Control 25:660–665
Garmendia G, Pattarino L, Negrin C, Martinez-Silveira A, Pereyra S, Ward TJ, Vero S (2018b) Species composition, toxigenic potential and aggressiveness of Fusarium isolates causing Head blight of barley in Uruguay. Food Microbiol 76:426–433
Umpiérrez-Failache M, Garmendia G, Pereyra S, Rodríguez-Haralambides A, Ward T, Vero S (2013) Regional differences in species composition and toxigenic potential among Fusarium head blight isolates from Uruguay indicate a risk of nivalenol contamination in new wheat production areas. Int J Food Microbiol 166:135–140
Garmendia G, Umpierrez-Failache M, Ward TJ, Vero S (2018a) Development of a PCR-RFLP method based on the transcription elongation factor 1-α gene to differentiate Fusarium graminearum from other species within the Fusarium graminearum species complex. Food Microbiol 70:286–332
GB13078-2017 Hygienic Standard for Feeds (2017) https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Hygienic%20Standard%20for%20Feeds_Beijing_China%20-%20Peoples%20Republic%20of_10-24-10.1007/s12550-023-00498-y2017.pdf
Geiser DM, del Mar J-G, Kang S, Makalowska I, Veeraraghavan N, Ward TJ, Zhang N, Kuldau GA, O’donnell K, (2004) FUSARIUM-ID vol 1.0: A DNA sequence database for identifying Fusarium. Euro J Plant Pathol 110:473–479
Gelderblom WC, Jaskiewicz K, Marasas WF, Thiel PG, Horak RM, Vleggaar R, Kriek NP (1988) Fumonisins–novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl Environ Microbiol 54(7):1806–1811
Ivić D, Sever Z, Kumanovska B (2011) In vitro sensitivity of Fusarium graminearum, F. avenaceum and F. verticillioides to carbendazim, tebuconazole, flutriafol, metconazole and prochloraz. Pestic Phytomed (belgrade) 26:35–42
Yli-Mattila T, Sundheim L (2022) Fumonisins in African Countries Toxins 14:419
Kuhnem P, Ward T, Silva C, Spolti P, Ciliato M, Tessmann D, Del Ponte E (2016) Composition and toxigenic potential of the Fusarium graminearum species complex from maize ears, stalks and stubble in Brazil. Plant Pathol 65:1185–1191
Lanza F, Zambolim L, Veras da Costa R, Vieira Queiroz V, Cota L, da Silva D, Coelho de Souza A, Fontes Figueiredo J (2014) Prevalence of fumonisin-producing Fusarium species in Brazilian corn grains. Crop Prot 65:232–237
Lee J, Kim H, Jeon J-J, Kim H-S, Zeller K, Carter L, Leslie J, Lee Y-W (2012) Population Structure of and Mycotoxin Production by Fusarium graminearum from Maize in South Korea. Appl Environ Microbiol 78:2161–2167
Leslie JF, Summerell BA (2006) The Fusarium laboratory manual. Ames, IA, USA: Blackwell Professional
Liu SM, Chen Y, Yu JJ, Chen CJ, Wang JX, Zhou MG (2010) Transfer of the beta-tubulin gene of Botrytis cinerea with resistance to carbendazim into Fusarium graminearum. Pest Manag Sci 66:482–489
Logrieco A, Mule G, Moretti A, Bottalico A (2002) Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur J Plant Pathol 108:597–609
Maragos C (2010) Zearalenone occurrence and human exposure. World Mycotoxin J 3:369–383
Marasas WF, Kellerman TS, Gelderblom WC, Coetzer JA, Thiel PG, van der Lugt JJ (1988) Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J Vet Res 55(4):197–203
Marín P, De Ory A, Cruz A, Magan N, González-Jaén MT (2013) Potential effects of environmental conditions on the efficiency of the antifungal tebuconazole controlling Fusarium verticillioides and Fusarium proliferatum growth rate and fumonisin biosynthesis. Int J Food Microbiol 165:251–258
Masiello M, Somma S, Ghionna V, Logrieco A, Moretti A (2019) In vitro and in field response of different fungicides against Aspergillus flavus and Fusarium species causing ear rot disease of maize. Toxins 11:11
van der Westhuizen L, Shepard G, Scussel V, Costa L, Vismer H, Marasas RJ, W, (2003) Fumonisin contamination and Fusarium incidence in corn from Santa Catarina. Brazil J Agric Food Chem 51:5574–5578
McCormick SP, Stanley AM, Stover NA, Alexander N (2011) Trichothecenes: from simple to complex mycotoxins. Toxins 3:802–814
Mendes de Souza M, Sulyok M, Freitas-Silva O, Soares S, Brabet C, Machinski M, Leiko B, Azevedo E, Krska R, Schuhmache R (2013) Cooccurrence of mycotoxins in maize and poultry feeds from Brazil by liquid chromatography/tandem mass spectrometry. Sci World J 2013:427369
Methol M (2021) Maíz y sorgo: situación y perspectivas. In Anuario de OPYPA, pp193. Available from: https://www.gub.uy/ministerio-ganaderia-agricultura-pesca/comunicacion/publicaciones/anuario-opypa-2021/anuario-opypa-2021
MGAP, Resolución S/N/001 (2001) Límites máximos de DON en alimentos para animales. https://www.gub.uy/ministerio-ganaderia-agricultura-pesca/institucional/normativa/resolucion-sn001-limitesmaximos-don-alimentos-para-animales
Mulè G, Susca A, Stea G, Moretti A (2004) Specific detection of the toxigenic species Fusarium proliferatum and F. oxysporum from asparagus plants using primers based on calmodulin gene sequences. FEMS Microbiol Lett 230:235–240
Munkvold GP, Desjardins AE (1997) Fumonisins in maize: can we reduce their occurrence? Plant Dis 82:556–565
Munkvold GP (2003) Epidemiology of Fusarium diseases and their mycotoxins in maize ears. Eur J Plant Pathol 109:705–713
O’Donnell K, Cigelnick E, Nirenberg HI (1998) Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90:465–493
Pan D, Calero N, Mionetto A, Bettucci L (2013) Trichothecene genotypes of Fusarium graminearum from wheat in Uruguay. Int J Food Microbiol 162:120–123
Pan D, Mionetto A, Calero N, Reynoso MM, Torres A, Bettucci L (2016) Population genetic analysis and trichothecene profiling of Fusarium graminearum from wheat in Uruguay. Genet Mol Res 15:1–11
Pasquali M, Beyer M, Logrieco A et al (2016) A European database of Fusarium graminearum and F. culmorum trichothecene genotypes. Front Microbiol 7:406. https://doi.org/10.3389/fmicb.2016.00406
Piñero S, Silva G, Scott P, Lawrence G, Stack M (1997) Fumonisin levels in Uruguayan corn products.J. Assoc off Anal Chem Int 80:825–829
Ward T, Bielawski JP, Kistler HC, Sullivan E, O’Donnell K (2002) Ancestral polymorphism and adaptive evolution in the trichothecene mycotoxin gene cluster of phytopathogenic Fusarium. Proc Natl Acad Sci USA 99:9278–9283
Pestka JJ (2010) Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol 84:663–679
Picot A, Barreau C, Pinson-Gadais L, Caron D, Lannou C, Richard-Forget F (2010) Factors of the Fusarium verticillioides-maize environment modulating fumonisin production. Crit Rev Microbiol 36:221–231
Pirgozliev SR, Edwards SG, Hare MC, Jenkinson P (2002) Effect of dose rate of azoxystrobin and metconazole on the development of Fusarium head blight and the accumulation of deoxynivalenol (DON) in wheat grain. Eur J Plant Pathol 108:469–478
Zhou D, Wang X, Chen G, Sun S, Yang Y, Zhu Z, Duan C (2018) The major Fusarium species causing maize ear and kernel rot and their toxigenicity in Chongqing. China Toxins 10:90
Ponce-García N, Serna-Saldivar SO, Garcia-Lara S (2018) Fumonisins and their analogues in contaminated corn and its processed foods - a review. Food Addit Contam Part A 35:2183–2203
QinP XuJ, Jiang Y, Hu L, van der Lee T, Waalwijk C, Zhang W, Xu X (2020) Survey for toxigenic Fusarium species on maize kernels in China. World Mycotoxin J 13:213–223
Reddy K, Salleh B, Saad B, Abbas H, Abel C, Shier W (2010) An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev 29:3–26
Reynoso MM, Ramirez ML, Torres A, Chulze S (2011) Trichothecene genotypes and chemotypes in Fusarium graminearum strains isolated from wheat in Argentina. Int J Food Microbiol 145:444–448
Salay E, Mercadante A (2002) Mycotoxins in Brazilian corn for animal feed: occurrence and incentives for the private sector to control the level of contamination. Food Control 13:87–92
Sampietro D, ApudI G, BelizánI M, VattuoneI M, Catalán C (2013) Toxigenic potential of Fusarium graminearum isolated from maize of northwest Argentina. Braz J Microbiol 44:2
Sampietro DA, Díaz CG, González V, Vattuone MA, Ploper LD, Catalán CA, Ward T (2011) Species diversity and toxigenic potential of Fusarium graminearum complex isolates from maize fields in northwest Argentina. Int J Food Microbiol 145:359–364
Scauflaire J, Mahieu O, Louvieaux J, Foucart G, Renard F, Munaut F (2011) Biodiversity of Fusarium species in ears and stalks of maize plants in Belgium. Eur J Plant Pathol 131:59–66
Scoz LB, Astolfi P, Reartes DS, Schmale DG, Moraes MG, Del Ponte EM (2009) Trichothecene mycotoxin genotypes of Fusarium graminearum sensu stricto and Fusarium meridionale in wheat from southern Brazil. Plant Pathol 58:344–351
Scussel V, Savi G, Freitas Costas L, Mendonça J, Manfio D, Bittencourt K, Aguiar K, Stein S (2014) Fumonisins in corn (Zea mays L.) from Southern Brazil. Food Addit Contam: Part B 7:151–155
Spolti P, Del Ponte EM, Dong Y, Cummings JA, Bergstrom GC (2014) Triazole sensitivity in a contemporary population of Fusarium graminearum from New York wheat and competitiveness of a tebuconazole-resistant isolate. Plant Dis 98:607–613
Stumpf R, dos Santos J, Gomes L, Silva C, Tessmann D, Ferreira F, Machinski Junior M, Del Ponte E (2013) Fusarium species and fumonisins associated with maize kernels produced in Rio Grande do Sul State for the 2008/09 and 2009/10 growing seasons. Braz J Microbiol 44:89–95
Sundheim L, Tsehaye H (2015) Fumonisin in Zambia and neighboring countries in a changing climate. Adv Environ Res 39:69–84
Sundlof SF, Strickland C (1986) Zearalenone and zeranol: potential residue problems in livestock. Vet Hum Toxicol 28:242–250
Tateishi H, Miyake T, Mori M, Kimura R, Sakuma Y, Saishoji T (2010) Sensitivity of Japanese Fusarium graminearum species complex isolates to metconazole. J Pestic Sci 35:419–430
Tava V, Prigitano A, Cortesi P, Esposto MC, Pasquali M (2021) Fusarium musae from diseased bananas and human patients: susceptibility to fungicides used in clinical and agricultural settings. J Fungi (basel) 7:784
Funding
This work was financed by the Agencia Nacional de Investigación e Innovación (ANII), project number FSA-I-2017–1-139531.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
del Palacio, A., Corallo, B., Simoens, M. et al. Major Fusarium species and mycotoxins associated with freshly harvested maize grain in Uruguay. Mycotoxin Res 39, 379–391 (2023). https://doi.org/10.1007/s12550-023-00498-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-023-00498-y