Abstract
Fusarium species causing maize kernel rot are major threats to maize production, due to reduction in yield as well as contamination of kernels by mycotoxins that poses a health risk to humans and animals. Two-hundred maize kernel samples, collected from 20 major maize growing areas in Ethiopia were analyzed for the identity, species composition and prevalence of Fusarium species and fumonisin contamination. On average, 38 % (range: 16 to 68 %) of maize kernels were found to be contaminated by different fungal species. Total of eleven Fusarium spp. were identified based on morphological characteristics and by sequencing the partial region of translation elongation factor 1-alpha (EF-1α) gene. Fusarium verticillioides was the dominant species associated with maize kernels (42 %), followed by F. graminearum species complex (22.5 %) and F. pseudoanthophilium (13.4 %). The species composition and prevalence of Fusarium species differed among the areas investigated. Fusarium species composition was as many as eight and as few as four in some growing area. The majority of the maize samples (77 %) were found positive for fumonisin, with concentrations ranging from 25 μg kg−1 to 4500 μg kg−1 (mean: 348 μg kg−1 and median: 258 μg kg−1). Slight variation in fumonisin concentration was also observed among areas. Overall results indicate widespread occurrence of several Fusarium species and contamination by fumonisin mycotoxins. These findings are useful for intervention measures to reduce the impact of the main fungal species and their associated mycotoxins, by creating awareness and implementation of good agricultural practices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maize (Zea mays L.) is the most important crop in Ethiopia, cultivated in all parts of the country and under different environmental conditions (Central Statistical Agency of Ethiopia, CSA 2012; Geleti et al. 2011). In 2013/14, the total maize production in the country was 6.67 million tons harvested from nearly 2 million hectares of land, and this is 26.3 % of the total grain production in the country (Central Statistical Agency of Ethiopia, CSA 2012). Almost all maize grains produced in Ethiopia are used for direct human food, while the crop residues play an important role as animal feed (Geleti et al. 2011). Fusarium species are the most common fungal pathogens of maize, and the fungi are responsible for various diseases including seedling blight, stalk rot and ear rot (Logrieco et al. 2002). Ear rot disease, caused by many Fusarium species, is a major production constraint of maize throughout the world, including Ethiopia (Ayalew 2010). In maize Fusarium spp. cause two distinct types of ear rots, which are recognized as Gibberella ear rot and Fusarium ear rot (Mesterházy et al. 2012). Fusarium ear rot is caused primarily by F. verticillioides (Sacc.) Nirenberg, F. proliferatum (Matsush.) Nirenberg, and F. subglutinans (Wollenw. & Reinking) P.E. Nelson, Toussoun and Marasas; while F. graminearum (Schwabe), F. culmorum (Wm. G. Sm.) Sacc., F. cerealis (Cooke) Sacc., and F. avenaceum (Fr.) Sacc. are the main species causing Gibberella ear rot in maize (Logrieco et al. 2002; Mesterházy et al. 2012; Munkvold 2003). Infection of maize by Fusarium spp. may occasionally cause considerable losses in grain yield and quality deterioration (Logrieco et al. 2002; Vigier et al. 1997), but more importantly grains harvested from infected ears may be contaminated with mycotoxins (Mesterházy et al. 2012; Munkvold 2003). Some Fusarium spp. can also grow as endophytes in the plant tissue without causing any visible symptom of infection, but kernels may contain trace amounts of mycotoxins (Mesterházy et al. 2012; Munkvold 2003). Mycotoxins are poisonous secondary metabolites, which are harmful to both human and animal health (Reddy et al. 2010). Gibberella ear rot disease frequently leads to contamination with deoxynivalenol, nivalenol and zearalenone mycotoxins, whereas Fusarium ear rot leads to accumulation of fumonisins (Logrieco et al. 2002; Mesterházy et al. 2012). Mycotoxin contaminated commodities may be rejected in the market and contribute to economic losses for growers (Waśkiewicz et al. 2012).
The fumonisins are the most common contaminants of maize and maize based products throughout the world (Picot et al. 2010; Reddy et al. 2010; Sundheim and Tsehaye 2015). Maize is contaminated more frequently with high amounts of fumonisin than any other crop (Sundheim and Tsehaye 2015; Waśkiewicz et al. 2012). Although several Fusarium species can produce fumonisins, F. verticillioides is considered the primary cause of Fusarium ear rot and fumonisin contamination of maize in the tropical and sub-tropical environments (Logrieco et al. 2002; Picot et al. 2010; Sundheim and Tsehaye 2015). The presence of fumonisins in grains is a serious threat, because it can cause several health disorders in humans and domestic animals (Waśkiewicz et al. 2012). Consumption of fumonisin contaminated feed causes leucoencephalomalacia in horses (Kellerman et al. 1990), and pulmonary edema and hydrothorax in pigs (Harrison et al. 1990). Fumonisins are also nephrotoxic, hepatotoxic and hepatocarcinogenic in laboratory animals such as rats (Gelderblom et al. 1996). Furthermore, dietary exposure to fumonisin B1 has been associated with elevated human esophageal cancer incidence (Sydenham et al. 1990) and neural tube defect in humans (Missmer et al. 2006).
High levels of maize ear and kernel infection by several Fusarium species have been reported in different parts of the globe (Goertz et al. 2010; Ncube et al. 2011; Reyes-Velázquez et al. 2011; Vigier et al. 1997). The species composition and frequency of occurrence of different Fusarium spp. and fumonisin contamination varies greatly between different years and maize growing areas (Dorn et al. 2011; Goertz et al. 2010; Ncube et al. 2011). This may largely be caused by unusual or stressful environmental conditions during growth, and at the time of harvest, primarily temperature and precipitations are important factors for mycotoxin contamination (Doohan et al. 2003; Picot et al. 2010). Climatic situations such as reduced precipitation (drought) and the prevalence of ear infecting insect pests often have a major influence on fumonisin contamination of maize kernels (Munkvold 2003). Fusarium ear rot is favored by warm and dry conditions, while Gibberella ear rot has been associated with cooler and wetter weather situations (Logrieco et al. 2002; Munkvold 2003; Vigier et al. 1997). Agricultural practices, such as crop rotation and tillage systems, can also influence the occurrence and prevalence of Fusarium species, as infected crop debris on the soil surface helps the survival of Fusarium spp. and serves as a source of inoculum for infection of the next generation of maize plants (Munkvold 2003).
Growing resistant maize cultivars and implementation of good agricultural practices, including insect pest management, may reduce Fusarium infection and subsequent mycotoxin contamination (Mesterházy et al. 2012; Munkvold 2003). Thus, monitoring the composition and abundance of Fusarium species causing ear rots of maize and comparing that with the climatic conditions is vital to design management strategies including breeding programs for resistance to the pathogens predominant in the target environment. Despite the importance of maize in Ethiopian agriculture and the well-known threat of mycotoxins to human and animal health, and the legislated regulation of maximum acceptable levels of mycotoxins (EU commission 2006; Food and Drug Administration, FDA 2001), very little is known about the species composition and prevalence of Fusarium spp. as well as fumonisin contamination levels on maize kernels produced in Ethiopia. The aim of the present study was to identify Fusarium species associated with maize kernels from different major growing areas of Ethiopia, to assess their fumonisin contamination levels, and to try to elucidate the effect of different climatic conditions on the fungal infection and fumonisin contamination level.
Materials and methods
Sample collection areas and agro-ecological zones
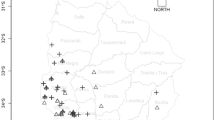
Twenty major maize growing areas were selected randomly for collection of maize kernel samples. According to the agro-ecological classification of Ethiopia, the sample collection areas (Fig. 1) were in seven major agro-ecological zones; namely: tepid humid mid-highlands (H3), warm moist lowlands (M2), tepid moist mid-highlands (M3), warm sub-moist lowlands (SM2), cool sub-moist mid-highlands (SM4), warm sub-humid lowlands (SH2) and tepid sub-humid mid-highlands (SH3). The general characteristics of these agro-ecological zones in terms of elevation, annual rainfall, average temperature and major annual crops grown as described by the Ministry of Agriculture and Rural Development (Ministry of Agriculture and Rural Development, MoARD 2005) are summarized below (Table 1).
Collection of maize samples and related additional data
During July to August of 2012, a total of 200 maize kernel samples were collected from 20 different major maize growing areas (districts) in Ethiopia, 10 samples from each area (Fig. 1). Maize samples were collected from smallholder farmers. Sampling sites within each area were separated by at least 1 km and at most 5 km from each other. Nearly 1 kg of maize kernel samples were collected randomly from each sampling site within each area, and these samples were in storage for 6–7 months. Daily maximum and minimum temperature, relative humidity as well as rainfall data for the weather stations closest to the sampling sites were obtained from the Ethiopian Metrological Agency. Thus, climatic data stretching from seeding to harvesting (May to December 2011), as well as for the storage period (January to June 2012) were considered to include both the field and storage situation, respectively. The duration from harvest to sampling (months of storage time), the seasonal average daily temperature, relative humidity and seasonal total rainfall for each area were as presented in Table 1. Samples were labeled with proper identification codes and placed in cloth bags to prevent condensation that might promote fungal growth. Global positioning system (GPS) co-ordinates and elevation were recorded at each sampling site.
Assessment of kernel infection level, isolation and identification of Fusarium spp.
A total of 500 kernels per area (50 kernels per sampling site) were used for determination of fungal infection level and isolation of Fusarium species. Maize kernels were surface sterilized by soaking in 1 % sodium hypochlorite (NaOCl) solution for 2 min, rinsed twice in sterile distilled water and dried briefly with sterile paper towels. Then kernels were transferred to CZPD agar plates (a modified Czapek-Dox Iprodione Dichloran Agar) containing propiconazole (0.375 mg L−1) and fenpropimorph (1.125 mg L−1) instead of iprodione (Halstensen et al. 2006). Five kernels were plated on each petri dish, and incubated for 7 to 10 days at 25 °C in the dark. The percentage of kernels contaminated with fungi was recorded by counting the number of kernels from which internal mold contaminants grew (Leslie and Summerell 2006). Colonies that appeared to be Fusarium based on shape and color of the mycelium were transferred to Spezieller Närstoffarmer Agar (SNA) (Nirenberg 1976), and the identity of Fusarium species were confirmed by growing for 7 to 10 days at 20 °C under alternating near UV/white fluorescent light (12 h) and dark (12 h) (Leslie and Summerell 2006). Subsequently, single spore isolates were obtained by spreading serial dilutions of spore suspension on water agar plates. After incubation of plates at room temperature (22 °C) for 16–20 h, a single germinating conidium was transferred to new SNA plates (Nirenberg 1976). Afterwards 6 mm mycelial disc of the cultures were transferred to potato dextrose agar (PDA, Difco, Madison, USA) and carnation leaf agar (CLA) (Leslie and Summerell 2006). All these single spore cultures were incubated for 2–6 weeks at 20 °C under alternating near UV/white fluorescent light (12 h) and dark (12 h), before identification. The identification of Fusarium isolates to species level was primarily achieved using morphological and cultural characters as described by Leslie and Summerell (2006).
Sequencing of the translation elongation factor 1-alpha (EF-1α) gene region was performed on representative isolates (Table 3) to support the morphological identification. The EF-1α gene has been commonly used for molecular identification of Fusarium to species level. The EF-1α gene occurs consistently as a single-copy in the genus Fusarium, and it shows a high level of sequence polymorphism among closely related species, compared to other genes such as calmodulin, β-tubulin and histone H3 (Geiser et al. 2004). For DNA extraction, pure cultures from single spores were grown on PDA (Difco, Madison, USA) at 22 °C under white light for 7 to 10 days, and mycelium was scraped from the surface and ground in liquid nitrogen using a mortar and pestle. DNA was extracted using DNeasy Plant Mini Kit (Qiagen) according to manufacturer’s instructions. The EF-1α gene was amplified in PCR assay using the primer pairs EF1 (5′-ATGGGTAAGGAGGACAAGAC-3) and EF2 (5′-GGAGGTACCAGTCATCATGTT-3′) as described by O’Donnell et al. (1998). Amplification reactions were done in volumes of 25 μl containing 2.5 μl 10× PCR buffer (10 mM Tris-HCl; 50 mM ClK; 15 mM MgCl2, pH 8.3), 2 μl dNTPs (2.5 mM), 0.5 μl of each primer (50 μM), 0.125 μl AmpliTaq DNA Polymerase (5 U μl−1) (Applied Biosystems, Foster City, CA, USA) and 20 ng of template DNA. The amplification conditions consisted of one cycle of initial denaturation at 95 °C for 5 min, 35 cycles of denaturing at 94 °C for 50 s, annealing at 53 °C for 50 s, extension at 72 °C for 1 min, final extension at 72 °C for 7 min, followed by cooling at 4 °C. Amplified products were submitted for sequencing by GATC Biotech (Cologne, Germany). Sequence data were assembled and analyzed using the CLC Main Workbench software 6.9 (Aarhus, Denmark), and consensus sequences were used to search the most related sequences at the GenBank (NCBI-National Centre for Biotechnology Information) (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and the Fusarium-ID database (http://isolate.fusariumdb.org/index.php).
Fumonisin analysis
From each sample, representative kernel sub-samples (100 g kernels) were milled to fine powder using the Micro plant grinding machine (Tianjin Taisite instrument Co., Ltd., China, mesh size 1 mm) and stored at −20 °C. Samples were thawed at room temperature (22 °C) for 13–14 h before fumonisin extraction. Fumonisin was extracted from 10 g samples with 50 ml 70 % methanol on a shaker (1000 rpm) for 3 min. The extract was filtered through a Whatman no. 1 filter paper, and the filtrate was collected for evaluation. The concentration of total fumonisin in each sample was quantified using a competitive enzyme linked immunosorbent assay (ELISA) kits (RIDASCREEN®Fumonisin, R-Biopharm AG, Darmstadt, Germany) according to the manufacturer’s instructions. ELISA test kits were validated with maize samples of known fumonisin content. Samples with fumonisin concentration exceeding the highest detection limit for the kit were diluted with the extraction solvent, and the obtained results were multiplied by the dilution factor. All samples were run in duplicate wells, and the lowest detection limit of the kit was 0.025 ppm.
Statistical analyses
Differences in occurrence of Fusarium spp., fumonisin concentrations and proportion of fungal contaminated kernels per area were compared using the non-parametric Kruskal-Wallis one-way ANOVA. Fusarium spp. recorded on maize kernels per area and agro-ecological zone were calculated as a percentage of the total number of Fusarium isolates per area and agro-ecology. Data on incidence of Fusarium spp. on maize samples and relative prevalence in the 20 maize growing areas were pooled to illustrate a countrywide prevalence of the different species. Pearson’s correlation coefficients (Araṅkacāmi and Rangaswamy 1995) were calculated to determine the relationship between kernel fungal contamination levels, fumonisin concentrations and the occurrence of Fusarium spp. as well as seasonal mean daily temperature, relative humidity and seasonal total rainfall data for the areas. For fumonisin concentrations, all samples were included in the analysis by replacing half value of the minimum detection limit for samples that were below the detection limit. Statistical analysis was performed using SPSS version 22 (IBM SPSS statistics 22, Armonk, New York); and all test were performed at a probability level of P = 0.05.
Results
Fungal contamination in maize kernel samples
Fungal contamination level, determination of the species composition and prevalence of Fusarium species were analyzed in a total of 10,000 maize kernels, 50 kernels from each of the 200 samples. On average, 38 % (range 16 to 68 %) of the maize kernels were contaminated with several fungal species (Table 2). Among the fungal contaminated kernels, about 33 % were contaminated only by Fusarium spp. while the rest was contaminated by one or more of other fungi including Aspergillus (29.9 %), Penicillium (17.4 %), Stonecarpella (12.7 %), Acremonium (4 %), Mucor and Rhizopous species (3.9 %). In some cases, more than one fungus were observed on the kernels. The proportion of kernels contaminated by fungi varied significantly (p ≤ 0.039) among maize growing areas. The highest mean fungal contamination of kernels were observed in samples collected from Dedesa (45.2 %), followed by Agaro (43.6 %) and Jimma area (42.6 %). Significantly lower fungal contamination were recorded from areas with higher elevation and lower temperature (SM4), such as Maichew (31.2 %) and Korem (31.4 %) (Tables 1 and 2). The proportion of kernels infected with fungi varied substantially among samples within areas. For example, kernel infection in samples collected from the Agaro area varied from 30 % to 68 % (Table 2). When data on fungal contamination of kernels were combined and analyzed according to agroecological conditions (Fig. 2), the highest kernel contamination was recorded in areas categorized as warm moist lowlands (M2) (43 %), followed by the tepid humid mid-highlands (H3) (42 %). Kernel contamination was lowest (31.3 %) in the cool sub-moist mid-highlands (SM4) compared to the other agro-ecological zones.
Fungal infection levels of maize kernels from different agroecological zones of Ethiopia. aAgroecological zones- H3: Tepid humid mid-highlands; M2: Warm moist lowlands; M3: Tepid moist mid-highlands; SH2: Warm sub-humid lowlands; SH3: Tepid sub-humid mid-highlands; SM2: Warm sub-moist lowlands; and SM4: Cool sub-moist mid-highlands (Source: Ministry of Agriculture and Rural Development, MoARD 2005); Vertical bars indicate standard error of the mean
Identification of Fusarium species associated with maize kernels
A total of 1254 Fusarium isolates were recovered and identified from the maize kernel samples assessed. Microscopic analysis of morphological fungal structures and EF-1α gene sequencing revealed the presence of eleven different Fusarium species associated with maize kernels grown in different major maize growing areas of Ethiopia. Fusarium verticillioides was the most abundant species, representing 42 % of the total number of Fusarium isolates recovered from the maize kernels, followed by the F. graminearum species complex (22.5 %), F. pseudoanthophilum (13.4 %) and F. oxysporum (7.5 %) (Fig. 3). Fusarium incarnatum, F. brevicatenulatum and F. temperatum were less frequent, constituting about 2.8 to 4.8 % of the Fusarium species isolated (Fig. 3). Fusarium equiseti, F. subglutinans and F. lacertarum were among the rarely isolated species. For isolates categorized as unidentified Fusarium sp. based on morphology, no good match was found in the NCBI and Fusarium ID databases. The EF-1α gene sequences for this Fusarium sp. was most similar to isolates of the Gibberella fujikuroi species complex with 93 % resemblance (Table 3).
Species composition and prevalence of Fusarium species in the major maize growing areas and different agro-ecological zones of Ethiopia
The species composition and prevalence of Fusarium species isolated in different areas varied, as presented in Fig. 4. Eight different Fusarium species were isolated from Korem and Dessie areas, seven Fusarium species were isolated from Agaro, Bedele, Bako, Gibe, Dedesa and Kemissie areas; while in Nekemte, Ziway, Melkassa and Alamata four species were isolated (Fig. 4). The total count of each Fusarium species varied significantly (p ≤ 0.05) among areas investigated except for F. pseudoanthophilum. The most prevalent Fusarium species on maize kernels in several maize growing areas was F. verticillioides followed by the F. graminearum species complex. F. verticillioides occurred in all areas investigated, representing more than two-thirds of the Fusarium species isolated in some areas (Fig. 4). The relative prevalence of F. verticillioides was 81 % in Ziway and 77 % in Alamata, while in Korem, Bako and Ambo, it was only from 19 to 21 %. The F. graminearum species complex was recorded in 90 % of the areas assessed, but it was predominant in areas with higher elevation and lower temperature (Fig. 4 and Table 2). According to the molecular identification results, F. boothii appeared to be the dominant member of the F. graminearum species complex associated with maize kernels in Ethiopia (Table 3). Fusarium oxysporum, F. incarnatum, F. brevicatenulatum and F. temperatum were among the less prevalent Fusarium species, but occurred in several areas up to 20 % (Fig. 4).
Species composition and prevalence of Fusarium spp. on maize kernels in different growing areas in Ethiopia, during 2012. *sc: Fusarium graminearum species complex mainly F. boothii. Maize grain sample collection areas: Aga, Agaro; Ala, Alaba; Alm, Alamata; Amb, Ambo; Bak, Bako; Bed, Bedele; Ded, Dedessa; Dis, Dissie; Ged, Gedeo; Gib, Gibe; Haw, Hawassa; Jim, Jimma; Kem, Kemmisse; Kor, Korem; Mai, Maichew; Mel, Melkassa; Nek, Nekemte; Sir, Sire; Wes, Welaita-sedo and Ziw, Ziway
The data on Fusarium species occurrence in the different maize growing areas were grouped into the respective agro-ecological zones of Ethiopia, and the results were as presented in Fig. 5. Fusarium verticillioides was recorded in all agro-ecological zones assessed, but the species was most prevalent in the SM2-zone (64.5 %), the SH2-zone (55 %) and the M2-zones (53 %), which are characterized by lower elevation, warm and dry conditions. The F. graminearum species complex was less prevalent in these zones and more abundant in zones generally characterized by low temperature and wetter conditions, such as the M3-zone, SM4-zone and the SH3-zone (Fig. 5). The prevalence of F. pseudoanthophilum was similar in several agroecological zones investigated except the SH2-zone. The highest relative prevalence of both F. incarnatum and F. oxysporum was in the SM4-zone. Fusarium temperatum, F. equiseti, F. subglutinans, F. lacertarum and Fusarium sp. were detected in only some agro-ecological zones and with less than 5 % prevalence (Fig. 5).
Prevalence of Fusarium spp. on maizes kernels from different agroecological zone of Ethiopia, in 2012. Fusarium species a: F. verti, F. verticillioides; F. grami sc, Fusarium graminearum species complex mainly F. boothii; F. pseud, F. pseudoanthiphilium; F. oxys, F. oxysporum; F. brevi, F. brevicatenulatum; F. incar, F. incarnatum; F. tempe, F. temperatum; F. subgl, F. subglutinans; F. equis, F. equiseti and F. lacer, F. lacertarum
Fumonisin contamination of maize samples
Fumonisin was detected in maize samples collected from all but one of the maize growing areas sampled (Table 4). The majority of the 200 samples (77 %) contained fumonisin at concentrations ranging from 25 μg kg−1 to 4500 μg kg−1. The overall mean and median fumonisin concentration in the samples were 348 μg kg−1 and 258 μg kg−1, respectively. Fumonisin levels were higher than the overall mean in samples from Jimma (mean: 918.6 μg kg−1, median: 501.5 μg kg−1), Ziway (mean: 577 μg kg−1, median: 392 μg kg−1), Alamata (mean: 533 μg kg−1, median: 392.5 μg kg−1) and Hawassa (mean: 523 μg kg−1, median: 455 μg kg−1). The fumonisin level in one sample from Jimma exceeded the maximum tolerable limit of 2000 μg kg−1 set by the US Food and Drug Administration in food intended for direct human consumption (Food and Drug Administration, FDA 2001). In total, about 7 % of the maize samples exceeded the maximum tolerable limit set by the European Union in maize flour (>1000 μg kg−1) (EU Commission 2006). These were three samples from Ziway, two from Kemmissie, two from Alamata, two from Jimma, and one each from Alaba, Hawasa, Nekemte, Dissie and Korem. Bako was the only area where none of the samples were contaminated with detectable level of fumonisin (Table 4). The Sire area had also fewer incidents of fumonisin-contaminated samples, with a mean concentration far below the mean for all the samples. When fumonisin data was grouped into the agro-ecological zones of Ethiopia, the highest mean fumonisin concentration was recorded in the H3-zone (568.8 μg kg−1) followed by the SH2-zone (422 μg kg−1) and the M2-zone (420.5 μg kg−1). The lowest mean fumonisin concentrations were observed in samples from the M3 and SH3 zones (Fig. 6).
Mean fumonisin level in maize kernels from different agroecological zones of Ethiopia. aAgroecological zones- H3: Tepid humid mid-highlands; M2: Warm moist lowlands; M3: Tepid moist mid-highlands; SH2: Warm sub-humid lowlands; SH3: Tepid sub-humid mid-highlands; SM2: Warm sub-moist lowlands; SM4: Cool sub-moist mid-highlands (Source: Ministry of Agriculture and Rural Development, MoARD 2005); Vertical bars indicate standard error of the mean
Correlation between occurrence of Fusarium species, kernel contamination, fumonisin and climatic data
The total contamination of kernels with fungi was significantly correlated with the recorded temperature (r = 0.794, p ≤ 0.001) and rainfall (r = 0.500, p ≤ 0.029) for the growing season (2011). Similar positive correlation of kernel contamination with the recorded temperature (r = 0.791, p ≤ 0.001) and relative humidity (r = 0.761, p ≤ 0.001) for the storage period was observed. Positive correlation was also observed between the occurrence of some of the Fusarium spp. and kernel contamination levels (Table 5). Significantly strong positive correlation (r = 0.862, p ≤ 0.001) was observed between the incidence of F. verticillioides, the primary producer of fumonisin, and fumonisin concentrations in maize samples. There was a significant positive correlation between fumonisin concentration with temperature recorded in the growing season (r = 0.533, p ≤ 0.016), relative humidity (r = 0.521, p ≤ 0.018) and temperature recorded for the storage period (r = 0.518, p ≤ 0.019). However, there was no or poor correlation between rainfall data recorded for the growing season and fumonisin contamination (r = 0.276, p ≥ 0.238) (Table 5).
Discussion
The results reported in the present study show that maize grown in different ecological conditions in Ethiopia contains a wide range of Fusarium species. Several Fusarium spp., both common and less frequent pathogens of maize, were isolated from maize kernels. Fusarium verticillioides was the predominant one, found in all areas investigated and representing 42 % of the total number of isolates recovered. It was, however, most prevalent in the low altitude and high temperature areas. The consistent recovery of F. verticillioides throughout all maize growing areas and Ethiopian agro-ecological zones indicates its intimate association with the plant and its adaptation to the tropical climate. This is in line with previous observations of Ayalew (2010), who concluded that this Fusarium species is dominant in maize grain produced in Ethiopia, although predominance levels differ. In some parts of eastern and central Ethiopia, Ayalew (2010) has reported that 99 % of the Fusarium spp. on the internal and external surface of maize kernels was F. verticillioides. The high prevalence of F. verticillioides on maize kernels is also in agreement with observations made in other countries such as South Africa (Ncube et al. 2011), Mexico (Reyes-Velázquez et al. 2011) and Kenya (Bii et al. 2012).
Among the Fusarium spp. reported in this study, the F. graminearum species complex is the second major contaminant of maize kernels in Ethiopia, and it is most important in areas characterized by high elevation, low temperature and wet conditions. Observations of F. graminearum thriving in cooler temperature and wetter conditions than F. verticillioides, have also been reported from other maize-growing areas in the world (Vigier et al. 1997; Dorn et al. 2011; Scauflaire et al. 2011). Fusarium pseudoanthophilum and F. oxysporum were also among the common Fusarium spp. on maize kernels isolated in the current study. Leslie and Summerell (2006) indicated that F. pseudoanthophilum could be found in hot dry areas and wet tropical regions, while species from the F. oxysporum complex are cosmopolitans that can be recovered from different climatic conditions. In addition to the above-mentioned Fusarium spp., a large number of other species such as F. incarnatum, F. brevicatenulatum, F. temperatum, F. equiseti, F. subglutinans, F. lacertarum and Fusarium sp. were detected on maize kernels. Many of these species occurred in low frequency in several areas and agroecological zones in Ethiopia. Some of these species could be saprophytes, saprobes or opportunistic colonizers of maize plants; however, they may still cause yield losses and mycotoxin contamination of the kernels. To our knowledge, this is the first report of the occurrence of F. temperatum, F. brevicatenulatu, F. pseudoanthophilum, F. incarnatum, F. equiseti and F. lacertarum in maize in Ethiopia.
The current study shows considerable variation in species composition and relative prevalence of Fusarium spp., as well as fumonisin contamination levels among samples collected from different maize growing areas and agro-ecological zones in Ethiopia. The reason for this could partly be due to the variation in climatic factors in the maize growing areas, mainly temperature, humidity and precipitation. The influence of climatic conditions on the species composition and prevalence of Fusarium spp. may be due to a direct effect on growth, production and dispersal of inoculum, but also an indirect effect on soil and vegetation type, which may influence saprophytic survival (Doohan et al. 2003; Munkvold 2003). The positive correlations of fungal kernel contamination with temperature and rainfall data, as well as fumonisin concentration with relative humidity and temperature data found in this study, show the strong effect of environmental factors. Weather conditions characterized by high temperature and low rainfall in the growing season, particularly after silking, favor colonization of maize ears by pathogenic Fusarium species and subsequent fumonisin contamination (Goertz et al. 2010; Vigier et al. 1997). Contamination of kernels with Fusarium species and fumonisin may also increase considerably with wet or humid weather condition in the later growing season, especially at the time of crop harvest and during drying (Munkvold 2003).
In addition to climatic factors, agricultural practices such as crop rotation (previous crop), insect pest management, choice of cultivars, tillage systems and crop residue management, post-harvest drying and handling practices applied by different maize growing small-scale farmers, may have great impact on kernel contamination, the prevalence of Fusarium species on maize kernels and subsequent fumonisin contamination. The pronounced differences recorded for percentage of kernels contaminated by fungal agents and fumonisin concentration among samples collected from the same areas points to the importance of different on-farm agricultural practices and postharvest handling activities (Fandohan et al. 2005) employed by different maize growers. Some farmers store the unshelled cobs on tree branches and the grains in underground pits before transfer into the house. Such practices may expose maize grains to late rain showers and hence high fungal infection. In some parts of Ethiopia, maize is often grown in short rotations with preceding small grain cereals, such as wheat and sorghum or maize mono-cropping. Such repeated planting of maize and other cereals in the same field may lead to the high incidence of Fusarium spp. and other fungal contaminants by increasing the amount of inoculums. Logistics regression modeling of cropping systems employed to predict fumonisin contamination level in maize showed that preceding crop, maturity class of hybrids, grain moisture and harvesting week significantly affects the level of fumonisin contamination (Battilani et al. 2008).
The results obtained in the present study indicate that about two-third (77 %) of the maize samples were contaminated with fumonisin, which indicate a widespread occurrence of the toxin in maize grown in Ethiopia. These results are in accordance with those reported by Ayalew (2010), with detected fumonisin concentrations ranging from 300 to 2400 μg kg−1 in 17 samples collected from areas in eastern and central Ethiopia. However, the fumonisin concentrations obtained in the maize samples in the present study (mean 348 and median 258 μg kg−1) were lower than reported from other neighboring countries in eastern and southern Africa (Bii et al. 2012; Ncube et al. 2011; Sundheim and Tsehaye 2015). Under good growing condition the fungus is a commensal, causing small damage to kernels and little fumonisin formation (Pitt et al. 2013). In the Bako and Sire areas, no or relatively few samples contaminated with fumonisins were detected (Table 4). A reason for this could be that these areas are located nearby the national center for maize research where farmers can get access to improved maize hybrids that are resistance to insect pests, fungal infection and fumonisin contamination.
In general, higher fumonisin concentration was found in samples collected in the humid and warmest areas, and fumonisin concentration was positively correlated with temperature and relative humidity, while there was poor correlation between total seasonal rainfall and fumonisin concentration. This is in agreement with previous reports from different countries (Goertz et al. 2010; Ncube et al. 2011).
The highest mean total fumonisin was recorded in the H3 zone (Fig. 6), although the prevalence of F. verticillioides, the primary producer of fumonisin, was highest in the SM2 zone (Fig 5). This indicates an important role of humidity in fumonisin contamination, as the H3 zone is more humid than the SM2 zone. Meteorological data recorded for the year 2011 indicate the presence of late season rainfall during the time of harvesting in the areas included in the H3 zone (Jimma and Bedele) but not in areas included in the SM2 zone (Alamata and Melkassa). The exceptionally highest fumonisin concentration (4500 μm kg−1) recorded in the current study was also from the H3 zone and this may exaggerate the mean fumonisin concentration in this zone.
From the present study, it becomes clear that a great diversity of Fusarium species infects maize kernels in Ethiopia but species composition and relative prevalence differs depending on area and agro-ecological conditions. Pooled results over all areas investigated indicated that F. verticillioides is the predominant species on maize kernels in Ethiopia followed by the F. graminearum species complex. Overall results indicate widespread occurrence of fumonisin mycotoxins on maize kernels in Ethiopia. These findings will serve as an important foundation for any study on Fusarium and fumonisins in maize in Ethiopia, and points to the most important Fusarium spp. for designing control strategies. Further assessment need to be continued in the future to analyze the prevalence of Fusarium spp. and fumonisin contamination in relation to agronomic practices over different years, to observe trends in fumonisin contamination and food safety. The observed widespread prevalence of different toxigenic fungal species such as Aspergillus spp., F. graminearum species complex, F. subglutinans and others may indicate the possibility of contamination of maize kernels by several mycotoxins other than fumonisin. Thus, the occurrence of other frequent contaminants of maize and harmful mycotoxins such as aflatoxins, deoxynivalenol, nivalenol and zearalenone should also be consider in future assessments.
References
Araṅkacāmi, I., & Rangaswamy, R. (1995). A text book of agricultural statistics. New Delhi: New Age International Publisher Ltd..
Ayalew, A. (2010). Mycotoxins and surface and internal fungi of maize from Ethiopia. African Journal of Food, Agriculture, Nutrition and Development, 10, 4109–4123.
Battilani, P., Pietri, A., Barbano, C., Scandolara, A., Bertuzzi, T., & Marocco, A. (2008). Logistic regression modeling of cropping systems to predict fumonisin contamination in maize. Journal of Agricultural and Food Chemistry, 56, 10433–10438.
Bii, F., Wanyoike, W., Nyende, A., Gituru, R., & Bii, C. (2012). Fumonisin contamination of maize (Zea mays) in aflatoxin ‘hot’zones in Eastern Province of Kenya. African Journal of Health Sciences, 20, 28–36.
Central Statistical Agency of Ethiopia (CSA) (2012). Reports on area and crop production forecasts for major grain crops (for private peasant holding, Meher season). Statistical Bulletin. http://www.csa.gov.et/images/general/news/2006%20forecast.pdf. Accessed 11 July 2014.
Doohan, F., Brennan, J., & Cooke, B. (2003). Influence of climatic factors on Fusarium species pathogenic to cereals. European Journal of Plant Pathology, 109, 755–768.
Dorn, B., Forrer, H. R., Jenny, E., Wettstein, F. E., Bucheli, T. D., & Vogelgsang, S. (2011). Fusarium species complex and mycotoxins in grain maize from maize hybrid trials and from grower's fields. Journal of Applied Microbiology, 111, 693–706.
EU Commission (2006). Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union, L364/5- L364/24.
Fandohan, P., Zoumenou, D., Hounhouigan, D., Marasas, W., Wingfield, M., & Hell, K. (2005). Fate of aflatoxins and fumonisins during the processing of maize into food products in Benin. International Journal of Food Microbiology, 98, 249–259.
Food and Drug Administration (FDA) (2001). Guidance for industry fumonisin levels in human foods and animal feeds, final guidance. http://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/ucm109231.htm. Accessed 02 June 2013.
Geiser, D. M., del Mar Jimenez-Gasco, M., Kang, S., Makalowska, I., Veeraraghavan, N., Ward, T. J., et al. (2004). FUSARIUM-ID v. 1.0: a DNA sequence database for identifying Fusarium. European Journal of Plant Pathology, 110, 473–479.
Gelderblom, W., Snyman, S., Abel, S., Lebepe-Mazur, S., Smuts, C., Van der Westhuizen, L., et al. (1996). Hepatotoxicity and-carcinogenicity of the fumonisins in rats. Advances in Experimental Medicine and Biology, 392, 279–296.
Geleti, D., Tolera, A., Mengistu, S., Demisse, K., & Esatu, W. (2011). Improving the fodder contribution of maize-based farming systems in Ethiopia: approaches and some achievements. In W. Mossisa et al. (Eds.), Meeting the challenges of global climate change and food security through innovative maize research (pp. 272–281). Addis Ababa: CIMMYT.
Goertz, A., Zuehlke, S., Spiteller, M., Steiner, U., Dehne, H. W., Waalwijk, C., et al. (2010). Fusarium species and mycotoxin profiles on commercial maize hybrids in Germany. European Journal of Plant Pathology, 128, 101–111.
Halstensen, A. S., Nordby, K.-C., Klemsdal, S. S., Elen, O., Clasen, P.-E., & Eduard, W. (2006). Toxigenic Fusarium spp. as determinants of trichothecene mycotoxins in settled grain dust. Journal of Occupational and Environmental Hygiene, 3, 651–659.
Harrison, L. R., Colvin, B. M., Greene, J. T., Newman, L. E., & Cole, J. R. (1990). Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. Journal of Veterinary Diagnostic Investigation, 2, 217–221.
Kellerman, T. S., Marasas, W., Thiel, P., Gelderblom, W., Cawood, M., & Coetzer, J. A. (1990). Leukoencephalomalacia in two horses induced by oral dosing of fumonisin B1. Onderstepoort Journal of Veterinary Research, 57, 269–275.
Leslie, J. F., & Summerell, B. A. (2006). The Fusarium laboratory manual. Ames: Blackwell publishing.
Logrieco, A., Mule, G., Moretti, A., & Bottalico, A. (2002). Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. European Journal of Plant Pathology, 108, 597–609.
Mesterházy, Á. K., Lemmens, M., & Reid, L. M. (2012). Breeding for resistance to ear rots caused by Fusarium spp. in maize–a review. Plant Breeding, 131, 1–19.
Ministry of Agriculture and Rural Development (MoARD) (2005). Major Agro-ecological Zones of Ethiopia. Addis Ababa: Forestry, Land Use and Soil Conservation Department.
Missmer, S. A., Suarez, L., Felkner, M., Wang, E., Merrill Jr., A. H., Rothman, K. J., et al. (2006). Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environmental Health Perspectives, 114, 237–241.
Munkvold, G. P. (2003). Epidemiology of Fusarium diseases and their mycotoxins in maize ears. European Journal of Plant Pathology, 109, 705–713.
Ncube, E., Flett, B. C., Waalwijk, C., & Viljoen, A. (2011). Fusarium spp. and levels of fumonisins in maize produced by subsistence farmers in South Africa. South African Journal of Science, 107, 1–7.
Nirenberg, H.I. (1976). Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt Fur Land- und Forstwirtschaft, Berlin-Dahlem, 169, 1–117.
O’Donnell, K., Kistler, H. C., Cigelnik, E., & Ploetz, R. C. (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences, 95, 2044–2049.
Picot, A., Barreau, C., Pinson-Gadais, L., Caron, D., Lannou, C., & Richard-Forget, F. (2010). Factors of the Fusarium verticillioides-maize environment modulating fumonisin production. Critical Reviews in Microbiology, 36, 221–231.
Pitt, J., Taniwaki, M. H., & Cole, M. (2013). Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of food safety objectives. Food Control, 32, 205–215.
Reddy, K., Salleh, B., Saad, B., Abbas, H., Abel, C., & Shier, W. (2010). An overview of mycotoxin contamination in foods and its implications for human health. Toxin Reviews, 29, 3–26.
Reyes-Velázquez, W. P., Figueroa-Gómez, R. M., Barberis, M., Reynoso, M. M., Rojo, F. G., Chulze, S. N., et al. (2011). Fusarium species (section Liseola) occurrence and natural incidence of beauvericin, fusaproliferin and fumonisins in maize hybrids harvested in Mexico. Mycotoxin Research, 27, 187–194.
Scauflaire, J., Mahieu, O., Louvieaux, J., Foucart, G., Renard, F. & Munaut, F. (2011). Biodiversity of Fusarium species in ears and stalks of maize plants in Belgium. European Journal of Plant Pathology, 131, 59–66.
Sundheim, L., & Tsehaye, H. (2015). Fumonisin in Zambia and neighboring countries in a changing climate. Advances in Environmental Research, 39, 69–84.
Sydenham, E. W., Thiel, P. G., Marasas, W. F., Shephard, G. S., Van Schalkwyk, D. J., & Koch, K. R. (1990). Natural occurrence of some Fusarium mycotoxins in corn from low and high esophageal cancer prevalence areas of the Transkei, southern Africa. Journal of Agricultural and Food Chemistry, 38, 1900–1903.
Vigier, B., Reid, L. M., Seifert, K. A., Stewart, D. W., & Hamilton, R. I. (1997). Distribution and prediction of Fusarium species associated with maize ear rot in Ontario. Canadian Journal of Plant Pathology, 19, 60–65.
Waśkiewicz, A., Beszterda, M., & Goliński, P. (2012). Occurrence of fumonisins in food-an interdisciplinary approach to the problem. Food Control, 26, 491–499.
Acknowledgments
This research was financially supported by the Norwegian Agency for Development Cooperation (NORAD) via the inter University collaboration between the Norwegian University of Life Sciences (UMB) and Mekelle University (MU) through the MU-UMB (NORAD-phase III) project. The authors also thank the Ethiopian Ministry of Agriculture and Rural Development for supplying agroecological GIS data and the metrological service agency for supplying the metrological data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsehaye, H., Brurberg, M.B., Sundheim, L. et al. Natural occurrence of Fusarium species and fumonisin on maize grains in Ethiopia. Eur J Plant Pathol 147, 141–155 (2017). https://doi.org/10.1007/s10658-016-0987-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-0987-6