Abstract
An enzyme-linked immunosorbent assay (ELISA) for the Alternaria mycotoxin tenuazonic acid (TeA) was evaluated by comparative analysis of naturally contaminated sorghum grains and sorghum-based infant food, using a stable isotope dilution LC-MS assay (SIDA; limit of detection (LOD) 1.0 μg/kg) as the reference method. LODs of the ELISA were 30 μg/kg in sorghum grains and 220 μg/kg in sorghum-based infant cereals. With SIDA, 100% of the samples (n = 28) had been positive for TeA in a concentration range of 6–584 μg/kg (mean 113 μg/kg). The ELISA consistently detected TeA in all naturally contaminated samples at cut-off levels of 30–60 μg/kg (sorghum) and 200–300 μg/kg (infant cereals), as based on corresponding to SIDA values. Although the ELISA was much less sensitive than the SIDA method, it may be useful as a screening method for sorghum and sorghum-based infant foods and can be employed to identify samples containing elevated concentrations of TeA in food, well below the proposed level of concern (500 μg/kg).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
L-tenuazonic acid ((5S)-3-acetyl-1, 5-dihydro-4-hydroxy-5-[(1S)-1-methylpropyl]-2H–pyrrol-2-one) is one of the major mycotoxins produced by fungi of the genus Alternaria. A primary mode of action of TeA is the inhibition of protein synthesis (Shigeura and Gordon 1963). TeA and other Alternaria toxins have attracted increasing attention during the last years.
Recently, a survey for Alternaria toxins reported tenuazonic acid (TeA) levels for several types of foods from the German market, including tomato products, bakery products, juices, vegetable oils, and sunflower seeds; concentrations were between 11 ± 0.4 μg/kg in vegetable oils and 490 ± 24 μg/kg in sunflower seeds (Hickert et al. 2016). A provisional mean daily intake of TeA and its isomer allo-TeA by the German population of 0.2 μg kg body weight has been reported recently (Hoevelmann et al. 2016). The European Food Safety Authority (EFSA) evaluated the toxicological potential of TeA by following the threshold of toxicological concern (TTC) approach, yielding a TTC value of 1.5 μg TeA/kg body weight per day (EFSA 2011). Recently, high levels of TeA were found in sorghum grains and in sorghum-based infant food containing either millet or sorghum (Asam and Rychlik 2013). In order to protect infants from the risk of consuming highly contaminated infant food, a first action limit for TeA of 500 μg/kg has been deduced (Rychlik et al. 2016) and installed by the Bavarian Health Protection Authority.
This indicates a need to intensely control such cereals for the presence of TeA. Several liquid chromatographic methods with mass spectrometric detection have been developed, some using either precolumn derivatization (Siegel et al. 2009) or stable isotope dilution assay (Asam et al. 2011), to overcome the difficulties in TeA chromatography arising from its strong acidic and metal complexing properties (Lebrun et al. 1985). The development of antibodies against TeA has been described previously (Gross et al. 2011) and the developed enzyme-linked immunosorbent assay (ELISA) was suggested as a complementary analytical technique for rapid screening purposes of food (Gross et al. 2011). Here, we report the evaluation of the TeA-ELISA for the analysis of sorghum grains and sorghum-based infant food. The ELISA was evaluated by comparison of the results with those obtained by SIDA-LC-MS/MS as the reference method, using a range of naturally contaminated food products.
Materials and methods
Chemicals
TeA (copper (II) salt, purity ≥98%), 2,4 dinitrophenylhydrazine, and undecyclic acid were obtained from Sigma-Aldrich (Steinheim and Deisenhofen, Germany). The UV absorbance maxima for TeA copper salt were determined in acetonitrile and found at 291, 225, 240, and 194 nm which is in agreement with published UV spectra of TeA (Scott and Kanhere 1980; Combina et al. 1998). All other solvents were obtained from Merck (Darmstadt, Germany) or Sigma-Aldrich and were at least of reagent grade. Water for HPLC was purified by a Milli-Q-system (Millipore, Schwalbach, Germany). The internal standard [13C6,15N]-TeA has been described previously (Asam et al. 2011). For ELISA analysis, an acetyl-TeA stock solution was prepared using a protocol described earlier for deoxynivalenol (Usleber and Märtlbauer 1998; Gross et al. 2011).
Naturally contaminated sample materials
The sorghum grains of different origin were used as obtained previously (Asam and Rychlik 2013). All samples of infant food had been purchased from retail stores in Germany (Asam and Rychlik 2013). A total of 28 selected sorghum-based food samples (sorghum grains, n = 12; infant cereals, n = 16) were analyzed in this study. All samples had been first analyzed by stable isotope dilution LC-MS assay (SIDA) (Asam and Rychlik 2013) using [13C6,15N]-TeA as internal standard as described earlier (Asam et al. 2011; Asam and Rychlik 2013). The SIDA method for TeA, which has been extensively evaluated (Asam et al. 2011; Asam et al. 2012; Asam and Rychlik 2013) yielded a limit of detection (LOD) of 1 μg/kg and a limit of quantification (LOQ) of 3 μg/kg. Subsequently, samples covering a wide concentration range were subjected to ELISA analysis. With this method, TeA had been detected in a range of 2–896 μg/kg (sorghum) and 6–584 μg/kg (infant cereals), respectively. All ELISA analyses were performed without prior knowledge about the SIDA results.
ELISA analysis
Samples were analyzed by a competitive direct ELISA method as described previously (Gross et al. 2011). The sample preparation procedure includes acetylation of TeA, because the ELISA is most specific for acetyl-TeA. In brief, sample material (5 g) was mixed with 15 mL (for sorghum grains) or 70 mL (for sorghum-based infant food) sodium acetate buffer (0.1 mol/L, pH 4.5); then, the pH was adjusted to 2.5 with HCl (2 mol/L). Extraction was achieved by magnetic stirring for 30 min, followed by centrifugation (1500×g). The supernatant was filtered through a paper filter; subsequently, 5 mL of the filtrate was extracted three times by liquid-liquid partitioning with ethyl acetate (8 mL). The combined organic phase was transferred to an evaporation flask, and the solvent was evaporated in a rotary evaporator at 40 °C under reduced pressure. The residue was redissolved with 1 mL of acetylation reagent consisting of 20 μL of acetic anhydride and 4 mg of 4-methylaminopyridine per milliliter of acetonitrile. After 1 h of incubation, the acetylation reaction was stopped by addition of 19 mL phosphate-buffered saline (PBS; 0.01 mol/L phosphate buffer, pH 7.3, containing 0.1 mol/L NaCl). This solution was directly used for ELISA analysis; further dilutions (1:2, 1:4) were prepared in 5% acetonitrile/PBS. At least three dilutions per sample extract and four replicate wells for each dilution were analyzed by ELISA.
ELISA absorbance values were measured at 450 nm with a model Sunrise plate reader (Tecan, Crailsheim, Germany) and evaluated by Magellan ELISA calculation software (Tecan) as described earlier (Gross et al. 2011; Bauer et al. 2016).
Recovery of the ELISA sample preparation procedure was checked by addition of toxin standard solution to sorghum and infant cereals before extraction. For each sample matrix, three to four samples were spiked with toxin concentration levels of 200–600 μg/kg (sorghum) and 400–600 μg/kg (infant cereals). Repeatability was determined by analyzing one naturally contaminated sample for each matrix on at least three different days.
Results and discussion
ELISA test performance
For spiked sorghum grains, recovery of TeA in the ELISA was between 51.5 and 37.9% in a concentration range of 200–600 μg/kg, with relative standard deviations of 32–34% (Table 1). Similar low recoveries of this ELISA procedure had been reported for tomato products (Gross et al. 2011). So far, no clear cause for these low recoveries could be identified. Losses of the toxin could have occurred during primary extraction, liquid-liquid partitioning, or at the acetylation step. However, losses during extraction of sorghum grains seem to be more likely, because the recovery results obtained for sorghum-based infant cereal were much better, with the liquid-liquid partitioning and acetylation steps being the same for both matrices. Recoveries for TeA in sorghum-based infant cereals were between 90.7 and 103% in a concentration range of 400–600 μg/kg, which was regarded as well sufficient for a semi-quantitative method (Table 1). The calculated LOD of the ELISA method, estimated from the standard curve LOD multiplied with the minimum sample dilution factor, was 30 μg/kg for sorghum grains and 220 μg/kg for infant cereals. Considering the low recoveries for sorghum grains, and the relative standard deviations in a range of 20–30%, a cut-off level of the ELISA for positive samples in a range of 30–60 μg/kg for sorghum grains and 200–300 μg/kg for infant cereals seems to be realistic. The relatively high LOD of the ELISA for infant cereals can be attributed to the strong water-soaking properties of these materials, which required a high solvent-sample ratio (5 g sample plus 70 mL extraction solvent). For repeated independent analyses of two naturally contaminated materials for both types of sample matrix, relative standard deviations of 22% (170 ± 37 μg/kg; n = 4) for sorghum grain (sample 20) and 27% (523 ± 139 μg/kg; n = 3) for sorghum-based infant food (sample 13) were obtained.
Comparison of ELISA and SIDA results for naturally contaminated samples
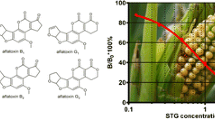
Within the method parameters determined for spiked samples, the TeA concentrations measured by ELISA principally agreed with those obtained by SIDA. All samples that after SIDA analyses contained TeA at levels below the ELISA cut-off level were consistently negative in the TeA-ELISA. Vice versa, all samples with SIDA results for TeA higher than about 1.5 times of the LOD of the ELISA were positive in the screening method (Tables 2 and 3). After SIDA analysis, one sample of sorghum (sample 22) and four samples of infant food (samples 5–8) revealed TeA levels which were close to the calculated LOD of the ELISA. Two of these samples yielded positive results, (samples 5 and 6) three samples were negative (samples 7, 8, 22) in the ELISA. However, at the LOD of a method, such discrepancies have to be expected. The total number of available TeA-positive samples was not sufficient to allow for a robust quantitative comparison of the results obtained by both methods. Considering the results for samples which were positive by both methods, a correlation coefficient of r 2 = 0.9774 was obtained for sorghum grains, while r 2 = 0.1692 was obtained for infant cereals. In most cases, the ELISA underestimated the TeA content determined by SIDA; therefore, sample heterogeneity as a reason for these discrepancies seems to be unlikely. Thus, the ELISA is best suited for testing raw material before processing or preparation.
Overall, taking the concentration range tested in this study into account, the TeA-ELISA has to be regarded as a semi-quantitative test, having cut-off values of 30–60 μg/kg in sorghum grains and 200–300 μg/kg in infant foods. Considering that TeA is still a relatively difficult analyte for all mycotoxin methods because of its highly polar, chelate-forming properties, a relatively easy and cost-efficient method such as the TeA-ELISA may be useful in routine analysis, but certainly can improve food safety by rapid testing of incoming sorghum grains before further processing by the food manufacturer. Frequent contaminations of sorghum and grain with high levels of TeA surely are a food safety concern. According to the results obtained by SIDA and ELISA, a daily sorghum intake of 30 g per infant food, which may be consumed at an age of around 1 year (∼10 kg body weight), would in case of the most highly contaminated samples result in an excess of the TTC (1.5 μg/kg body weight). In agreement with the aforementioned Bavarian regulations (Rychlik et al. 2016), sorghum grains used as raw materials should not contain more than 500 μg/kg of TeA. The ELISA described here seems to be a suitable method to control TeA at such levels. However, further work will aim on improving the recovery of TeA in the ELISA method.
References
Asam S, Rychlik M (2013) Potential hazards due to the occurrence of the mycotoxin tenuazonic acid in infant food. Eur Food Res Techno 236:491–497
Asam S, Liu Y, Konitzer K, Rychlik M (2011) Development of a stable isotope dilution assay for tenuazonic acid. J Agric Food Chem 59:2980–2987
Asam S, Lichtenegger M, Liu Y, Rychlik M (2012) Content of the Alternaria mycotoxin tenuazonic acid in food commodities determined by a stable isotope dilution assay. Mycotoxin Res 28:9–15
Bauer JI, Gross M, Gottschalk C, Usleber E (2016) Investigations on the occurrence of mycotoxins in beer. Food Control 63:135–139
Combina M, Dalcero AM, Torres A (1998) Spectrometric studies on stability of teniazinic acid (TeA) solution in organic solvent. Mycotoxin Res 14:54–59
EFSA Panel on Contaminants in the Food Chain (CONTAM) (2011) Scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J 9:1–97
Gross M, Curtui V, Ackermann Y, Latif H, Usleber E (2011) Enzyme immunoassay for tenuazonic acid in apple and tomato products. J Agric Food Chem 59:12317–12322
Hickert S, Bergmann M, Ersen S, Cramer B, Humpf HU (2016) Survey of Alternaria toxin contamination from the German market, using a rapid HPLC-MS/MS approach. Mycotoxin Res 32:7–18
Hoevelmann Y, Hickert S, Cramer B, Humpf HU (2016) Determination of exposure to the Alternaria mycotoxin tenuazonic acid and its isomer allo-tenuazonic acid in a German population by stable isotope dilution HPLC-MS3. J Agric Food Chem 64:6641–6647
Lebrun MH, Duvert P, Gaudener A, Deballon A (1985) Complexation of the fungal metabolite tenuazonic acid with copper (II), iron (III), nickel (II) and magnesium (II) ions. J Inorg Biochem 24:167–181
Rychlik M, Lepper H, Weidner C, Asam S (2016) Risk evaluation of the Alternaria mycotoxin tenuazonic acid in foods for adults and infants and subsequent risk management. Food Control 68:181–185
Scott PM, Kanhere SR (1980) Liquid chromatographic detection of tenuazonic acid in tomato paste. J Assoc Off Anal Chem 63:612–621
Shigeura HT, Gordon CN (1963) The biological activity of tenuazonic acid. Biochemist 2:1132–1137
Siegel D, Rasenko T, Koch M, Nehls I (2009) Determination of the Alternaria mycotoxin tenuazonic acid in cereals by high-performance liquid chromatography-electrospray ionization ion-trap mulitstage mass-spectrometry after derivatization with 2,4-dinitrophenylhydrazine. J Chrom A 1216:4582–4588
Usleber E, Märtlbauer E (1998) A limited survey of cereal foods from the German market for Fusarium toxins (deoxynivalenol, zearalenone, fumonisins). Arch Leb 49:43–45
Acknowledgements
The authors thank Margit Kessler and Renate Stumpf for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gross, M., Asam, S. & Rychlik, M. Evaluation of an enzyme immunoassay for the detection of the mycotoxin tenuazonic acid in sorghum grains and sorghum-based infant food. Mycotoxin Res 33, 75–78 (2017). https://doi.org/10.1007/s12550-016-0266-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-016-0266-6