Abstract
Sterigmatocystin (STG) is a highly toxic secondary fungal metabolite structurally closely related to the well-known carcinogenic aflatoxins. Its presence has been reported in grains and grain-based products as well as in other foodstuffs like nuts, green coffee beans, spices, beer and cheese. Due to the lack of suitable data on the occurrence of STG, in 2013, the European Food Safety Authority (EFSA) could not characterise its risk for human health and recommended that more data on STG in food and feed needed to be collected. In order to provide a new tool for the specific detection of STG, a competitive enzyme-linked immunosorbent assay (ELISA) was developed, optimised and validated in this study based on a sensitive monoclonal antibody specific to STG with no cross-reactivity with aflatoxins. The sample preparation method for rice, wheat and maize was based on a modified QuEChERS (quick, easy, cheap, effective, rugged and safe) approach. The assay was validated for the detection of STG in rice, wheat and maize in accordance with the guidelines for validation of semi-quantitative screening methods included in Commission Regulation (EU) 519/2014. The screening target concentration (STC) was set at 1.5 μg/kg. The cutoffs for rice, wheat and maize were 1.2, 1.2 and 1.3 μg/kg and the false suspected rates were 0.34, 1.15 and 0.78%, respectively. Good correlation was found between the results obtained by the STG ELISA and LC-MS/MS method for naturally contaminated rice samples. This validated method can be applied as a sensitive and high-throughput screening for the presence of STG in a range of agricultural commodities.

A new enzyme-linked immunosorbent assay based on an antibody specific to sterigmatocystin for the detection of this mycotoxin in corn, wheat and rice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
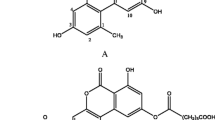
Mycotoxins are toxic secondary metabolites produced by fungi belonging to Aspergillus, Fusarium and Penicillium species. They can contaminate agricultural commodities during production, storage, processing and transport. The ingestion of contaminated products can cause a variety of adverse effects in both humans and animals. There are between 300 and 400 mycotoxins known to humans [1]. Regulatory bodies in many countries worldwide have set limits for the most toxic mycotoxins in food in order to protect human health. In the European Union (EU), there are maximum, indicative or guidance limits for well-known and harmful mycotoxins such as aflatoxins, ochratoxin A, fumonisins, zearalenone, deoxynivalenol, T-2/HT-2 toxins, patulin and citrinin in food and feed commodities (Commission Regulation (EC) 1881/2006 [2]; Commission Recommendation 2006/576/EC [3]; Commission Recommendation 2013/165/EU [4]). Among these toxins, aflatoxins are the most potent carcinogens found in nature, classified as group 1 carcinogens (carcinogenic to humans) by the International Agency for Research on Cancer [5]. They occur in corn, peanuts, rice, soybean, nuts and other crops. Structurally related sterigmatocystin (STG) (Fig. 1) is a toxin that shares the biosynthetic pathway with aflatoxins [6]. It is produced mainly by Aspergillus species such as A. nidulans and A. versicolor, which can infect crops post-harvest. STG accumulation has been reported in grains but also nuts, green coffee beans, spices, beer and cheese. STG is considered to be a potential carcinogen, mutagen and teratogen [7] and it was included in group 2B agents (possibly carcinogenic to humans) by IARC [8]. The data on its occurrence and toxicity are limited; therefore, in most countries, there are no regulatory limits concerning this toxin in contrast to aflatoxins. In the past, only the Czech Republic and Slovakia prior to entering the EU had maximum limits for STG of 5 and 20 μg/kg in different agricultural commodities [9]. According to EFSA, STG concentration from 1.5 to 8 μg/kg in grains and grain-based products constitutes a low health concern [6]. EFSA has also recommended to collect more data on the occurrence of STG in food and feed using analytical methods with an LOQ at least 1.5 μg/kg. In response to this call, the survey on STG in food was performed [10]. A total of 1259 samples of cereal grains, cereal products, beer and nuts collected in Europe between 2013 and 2014 were analysed by LC-MS/MS methods. The authors found low levels of STG contamination mainly in all unprocessed rice, 21% of the processed rice and 22% of oat samples analysed. In a previous survey including Latvian grains—wheat, barley, oat, buckwheat and rye—13 out of 95 sample and 42 out of 120 samples, from 2006 and 2007 harvests, respectively, were found to contain STG [11].
STG can be detected using thin layer chromatography [9] or HPLC [12]. LC-MS/MS methods have also been developed for the detection of STG in food and feed such as wheat, rice, oat, rye, maize and barley [13]; white rice and sorghum [14]; beer and cheese [12]; feed [15]. STG is also commonly included in many LC-MS/MS multi-mycotoxin methods [16,17,18,19,20]. As a lower cost alternative, immunochemical methods can be used for screening purposes. Enzyme-linked immunosorbent assay (ELISA) is still the most popular in the field due to its simplicity, high-throughput and cost-effectiveness. However, for STG detection only a few ELISA tests have been developed so far (Table 1). Kong et al. [21] obtained a sensitive monoclonal antibody (mAb) and used it to develop an indirect competitive ELISA with an IC50 (50% inhibitory concentration) of 0.092 ng/ml for the detection of 1.2 μg/kg of STG in cereals. Li et al. [22] developed an indirect competitive ELISA based on a mAb with an IC50 of 0.36 ng/ml for the analysis of wheat, maize and peanuts. The method was rather time-consuming and expensive as it required the application of an immunoaffinity chromatography step during the sample preparation procedure. It was also based on matrix-matched calibration in order to reduce matrix effect, so the standards for calibration had to be prepared in blank matrix for each experiment. Li et al. [23] presented an indirect competitive ELISA based on an antibody with an IC50 of 2.5 ng/ml. The assay was validated only for wheat at relatively high concentrations. Another immunochemical method—lateral flow immunochromatographic strip—was also developed by Kong et al. [21] with a visual limit of detection (LOD) of 3, 1.2 and 3 μg/kg for wheat, maize and rice, respectively.
In order to provide a new tool for the fast and sensitive detection of STG in cereals a direct competitive ELISA was developed in the presented research based on a new mAb. While there are currently no legal limits for STG, EFSA recommends to use analytical methods for STG with an LOQ of at least 1.5 μg/kg [6]. The developed ELISA aimed to be able to detect the STG in different cereals at this level. Another goal of this study was also to validate the developed method in accordance with the guidance for validation of semi-quantitative screening methods for mycotoxins included in Commission Regulation (EU) 519/2014 [24].
Materials and methods
Chemicals, consumables and apparatus
STG, aflatoxins (B1, B2, G1, G2 and M1), acetonitrile (ACN), methanol (MeOH), acetone, chloroform, n-heptane, sulphuric acid, disodium hydrogen phosphate, sodium dihydrogen phosphate, potassium dihydrogen phosphate, sodium carbonate, sodium bicarbonate, sodium chloride, magnesium sulphate, Tween 20, bovine serum albumin (BSA), keyhole limpet hemocyanin (KLH), horseradish peroxidase (HRP), sodium borohydride and dimethylformamide (DMF) were purchased from Sigma-Aldrich (Dorset, UK & Zwijndrecht, the Netherlands). Primary-secondary amine (PSA) bulk sorbent was purchased from Agilent (Amstelveen, the Netherlands). Substrate 3,3′,5,5′-tetramethylbenzidine (TMB) for HRP enzyme was obtained from Neogen (Lansing, USA).
Samples were blended using IKA A11 Basic laboratory mill (IKA, Staufen, Germany) and centrifuged in Sigma 4K10 centrifuge (Sigma, Osterode am Harz, Germany). Microtiter plates were read on BioTek EL808 type ELISA plate reader (BioTek, Bad Friedrichshall, Germany).
Production of STG conjugates
The immunogen (STG-BSA) and the coating antigen (STG-KLH) were produced in accordance with the method published by Li et al. [23] and Kononenko et al. [25]. STG was first converted to hemiacetal by heating with acid and then conjugated to proteins by reductive alkylation with sodium borohydride. In short, 2.5 mg of STG was dissolved in 1.25 ml of acetone and 0.03 ml of 10% sulphuric acid was added. The solution was refluxed for 4 h at 56 °C and then the solvent was evaporated to dryness under a stream of nitrogen. The residue was dissolved in 10 ml of water and extracted three times with 5 ml of chloroform. Combined chloroform was then washed with 5 ml of water and dried above sodium sulphate for 30 min. Next, sodium sulphate was filtered and the solvent was evaporated to dryness. The yellow residue was dissolved in 500 μl of DMF. Half of the STG hemiacetal solution was added to 5 mg of BSA dissolved in 1.5 ml of water and half to 5 mg of KLH solution. The solutions were mixed for 1 h at room temperature. Then, 50 μl of sodium borohydride solution at the concentration of 1 mg/ml was added and the reaction mixture was refrigerated for 1 h. Finally, the conjugates were purified by dialysis against 0.1 M phosphate buffer pH 8.0 with three changes of the dialyzing solution. The conjugates were stored at − 20 °C. STG-HRP was produced in a similar way, first by converting 2.5 mg of STG to hemiacetal and then conjugating it to 10 mg of HRP.
Monoclonal antibodies production
Fifteen micrograms of STG-BSA conjugate per injection was used to immunise two mice in accordance with the procedure described in details in [26]. The blood samples collected from animals 10 days after each injection were screened in antigen-coated ELISA [26]. STG-KLH conjugate at the concentration of 1 μg/ml was used as a coating antigen. The animal showing the best response in terms of antibody titre and sensitivity after 3 immunisations was euthanised. Its spleen was removed and the splenocytes were fused with SP2 myeloma cells in accordance with the procedure described by Köhler and Milstein for the first time in 1975 [27]. The detailed procedures for fusion, screening, large scale antibody production in flasks and antibody purification are described in [26].
STG ELISA
The microtiter ELISA plates were first coated with polyclonal rabbit anti-mouse antibody. Hundred microliters of 10 μg/ml solution of this antibody in phosphate buffered saline (PBS) pH 7.4 was added to each well of the plate and incubated overnight at room temperature. Then, the solution was discarded and the plate was used for the STG ELISA. For calibration, a seven-point standard curve for STG in PBS pH 7.4 containing 1% BSA (assay buffer) was prepared. The concentrations of standards were 0, 0.125, 0.25, 0.5, 1, 2 and 4 ng/ml. Fifty microliters of standards or extracted samples were added in duplicate to the wells of the plate. Then, 25 μl of the monoclonal anti-STG antibody (2 mg/ml stock diluted 1/4000 in the assay buffer) and 25 μl of the STG-HRP conjugate (1 mg/ml stock diluted 1/1000 in the assay buffer) were added to each well. The plate was incubated at 20–25 °C in the dark for 90 min. Then, the solution was removed and the plate was washed 3 times with rinsing buffer (PBS containing 0.05% Tween 20). Hundred microliters of TMB substrate was added to each well and then the colour was developed for 30 min at 20–25 °C in the dark. The absorbance was recorded at 450 nm on a microtiter plate reader after stopping the enzymatic reaction with 100 μl of sulphuric acid.
Samples
For the STG ELISA validation cereal samples: wheat, maize and rice (5 different types of each, 4 samples of each type) were obtained from local stores. Seven naturally contaminated rice samples and one sample not containing STG were obtained from CIRAD, France and analysed by a multi-mycotoxins LC-MS/MS method [20]. The concentrations of STG found to be present in these samples were as follows: 1.20, 1.25, 1.56, 1.70, 4.83 and 7.8 μg/kg.
Sample preparation
The extraction procedure was based on the method developed by Zhao et al. [13]. Two grams of a homogenised sample was weighed in a propylene tube. Then, 6 ml of 95% ACN in water and 2 ml of n-heptane were added. The solution was mixed for 5 min followed by the addition of 0.5 g NaCl and 0.5 g MgSO4 and then it was shaken for 30 s. After 5 min centrifugation at 4000×g, 30 mg of PSA SPE bulk sorbent was added to 3 ml of the bottom layer and mixed for 5 min. After centrifugation for 1 min at 4000×g, 1 ml of the supernatant was evaporated under steam of nitrogen gas. The sample was reconstituted by adding 100 μl of MeOH and 0.9 ml of PBS with 1% BSA. The solution was centrifuged for 5 min at 4000×g and 50 μl of non-diluted sample was analysed by the STG ELISA.
ELISA validation
The validation of the developed STG ELISA was based on the guidance for validation of semi-quantitative screening methods such as ELISA or quantitative LFD for mycotoxins included in Commission Regulation (EU) 519/2014 [28]. The screening target concentration (STC), which is the concentration of interest for detection of the mycotoxin, was set to 1.5 μg/kg. The aim of the validation was to demonstrate the applicability of the STG ELISA for the detection of mycotoxin at the set STC level and higher in 3 types of cereal grains: rice, wheat and maize. This was accomplished by analysing sets of 20 blank samples and 20 samples spiked at STC for each commodity type. The analysis was performed under intermediate precision conditions by spreading the analysis of these samples over 5 different days. The results were then used to determine 2 parameters: cut-off and false suspected rate. Cut-off is the concentration measured in a sample above which it is classified as “suspect” and it is calculated using the following equation that allows for 5% rate of false negative result:
where RSTC:mean concentration of the positive control samples at STC ; t_value0.05 one tailed t_value for a rate of false negative results of 5%, which is 1.729 for 20 samples set (19 degrees of freedom) ; SDSTC:standard deviation at STC. The second parameter, false suspected rate, can be found from a table for t-distribution using a t_value calculated in the following equation:
where meanblank:mean concentration of 20 blank samples and SDblank: standard deviation calculated for 20 blank samples.
During the validation study, sets of 20 samples for each matrix were also spiked at two other concentrations: 3 and 6 μg/kg and analysed over 5 different days to determine recovery and repeatability. The accuracy of the method was tested by analysing naturally contaminated rice samples and comparing the results to these obtained by LC-MS/MS method.
Results and discussion
Production of the anti-STG mAb
A spleen from the mouse showing the best titre was used in a fusion experiment resulting in approximately 1800 hybridomas. Three hybridomas were found to produce antibodies with the highest sensitivity to STG and they were selected for further work. Two rounds of cloning were performed before the final monoclonal cell lines were established for each hybridoma selected. They were used to produce mAbs in flask cultures. The antibodies were then concentrated and purified. The stock of each antibody at the concentration of 2 mg/ml was prepared and characterized using a competitive antigen-coated ELISA. The standard curves for STG were prepared in the range 0.001–1000 ng/ml. The antibody 2F3 showed the highest sensitivity and it was selected for the STG ELISA development (Table 2).
Sensitivity and cross-reactivity
The mAb specific to STG was used to develop a direct competitive ELISA. A seven-point standard curve was prepared in buffer for STG at 0, 0.125, 0.25, 0.5, 1, 2 and 4 ng/ml. A typical standard curve is presented in Fig. 2. The mean IC50 was 0.64 ± 0.02 ng/ml. The cross-reactivity with aflatoxins B1, B2, G1, G2 and M1 was below 1% indicating suitability of the developed assay for the specific detection of STG. Therefore, the occurrence of aflatoxins in samples analysed by STG ELISA should not lead to any false positive results.
ELISA validation
For validation, sets of blank samples and blank samples spiked at 1.5 μg/kg for each matrix were analysed over 5 different days. For each matrix, there was a complete separation between blank and spiked samples (Fig. 3.). The calculated false suspected rates were 0.34, 1.15 and 0.78% (Table 3), meaning that on average, less than 1 sample in 100 might be wrongly classified as containing more than 1.5 μg/kg of STG, requiring unnecessary analysis by a confirmatory method. The results of the recovery and repeatability study for the STG ELISA are presented in Table 4. The recovery was determined in samples spiked at 1.5, 3 and 6 μg/kg. The mean recovery was in the range 88 and 127% and the CV was lower than 20.3%.
The ELISA presented within this communication demonstrates important improvements when compared to the limited number of published methods for STG (Table 1). The developed method, a direct competitive ELISA, requires fewer steps than the indirect competitive ELISAs developed so far; therefore, it is much easier to perform. In addition, the sample preparation method does not involve an expensive and time-consuming immunoaffinity chromatography step. The developed sample extraction procedure was adapted from the method developed by Zhao et al. [13] and is based on a modified QuEChERS approach. In this method, magnesium sulphate and sodium chloride are first added to a sample extracted with 95% ACN to reduce water content and then primary-secondary amine (PSA) is used to remove interfering compounds. A small amount of PSA sorbent is added directly to the sample extract, mixed and then removed by centrifugation. This procedure is much faster and easier to perform compared to methods involving the use of columns packed with PSA sorbent. PSA was shown to reduce matrix effects as it is a weak anion exchanger and can bind fatty acids, some pigments, carbohydrate and organic acids. The method was successfully applied for the extraction of STG from rice, wheat and maize matrices. Due to the application of this sample preparation procedure, the matrix effects were reduced allowing for the sensitive detection of STG.
Analysis of naturally contaminated samples
In order to further characterize the developed method, seven naturally contaminated rice samples were analysed by the STG ELISA and the results were compared to these obtained by an LC-MS/MS method [20]. The correlation coefficient was found to be 0.9851, indicating a very good accuracy of the STG ELISA (Fig. 4).
Conclusions
A new mAb against STG toxin was prepared and used to develop a direct competitive ELISA with an IC50 of 0.64 ng/ml and no cross-reactivity with aflatoxins. A sample preparation method for efficient STG extraction from cereals: wheat, maize and rice was developed and optimised without the need for any time-consuming sample purification strategies such as solid phase extraction or immunoaffinity chromatography. The required sensitivity was achieved by using a modified QuEChERS approach with PSA sorbent applied to reduce matrix effects. The assay was validated in accordance with the guidelines for validation of semi-quantitative screening methods for mycotoxins included in Commission Regulation (EU) 519/2014 [24]. The STC was set at 1.5 μg/kg and the method was validated for the detection of STG at a level of STC and above with low false suspected rate. The recovery was also determined at different levels and it was between 88 and 127% with the CV lower than 20.3%. Good accuracy of the STG ELISA was demonstrated by comparing the results obtained by ELISA and LC-MS/MS method for naturally contaminated samples. The new ELISA can be applied for sensitive and high-throughput screening for the presence of STG in cereals.
References
Bhat R, Rai RV, Karim AA. Mycotoxins in food and feed: present status and future concerns. Compr Rev Food Sci F. 2010;9:57–81.
European Commission. Commission regulation (EC) no 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union. 2006;L364:5–23.
European Commission. Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (2006/576/EC). Off J Eur Union. 2006;L229:7–9.
European Commission. Commission Recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products (2013/165/EU). Off J Eur Union. 2013;L91:12–5.
International Agency for Research on Cancer (IARC). Chemical agents and related occupations. A review of human carciongens. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2012;100F:225–244.
European Food Safety Authority (EFSA). Scientific opinion on the risk for public and animal health related to the presence of sterigmatocystin in food and feed. EFSA J. 2013;11(6):3254.
Veršilovskis A, De Saeger S. Sterigmatocysin: occurrence in foodstuffs and analytical methods—an overview. Mol Nutr Food Res. 2010;54:136–47.
International Agency for Research on Cancer (IARC). Some naturally occurring substances. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Summaries and Evaluations. 1987;10:72.
Stroka J, Dasko L, Spangenberg B, Anklam E. Determination of the mycotoxin, sterigmatocystin, by thin-layer chromatography and reagent-free derivatisation. J Liq Chromatogr Relat Techno. 2004;27:2101–11.
Mol HGJ, Pietri A, MacDonald SJ, Anagnostopoulos C, Spanjer M. Survey on sterigmatocystin in food. EFSA Supporting Publication. 2015;EN-774:56.
Veršilovskis A, Bartkevičs V, Miķelsone V. Sterigmatocystin presence in typical Latvian grains. Food Chem. 2008;109:243–8.
Marley E, Brown P, Mackie J, Donnelly C, Wilcox J, Pietri A, et al. Analysis of sterigmatocystin in cereals, animal feed, seeds, beer and cheese by immunoaffinity column clean-up and HPLC and LC-MS/MS quantification. Food Addit Contam A. 2015;32:2131–7.
Zhao Y, Huang J, Ma L, Wang F. Development and validation of a simple and fast method for simultaneous determination of aflatoxin B1 and sterigmatocystin in grains. Food Chem. 2017;221:11–7.
Ok HE, Tian F, Hong EY, Paek O, Kim S-H, Kim D, et al. Harmonized collaborative validation of aflatoxins and sterigmatocystin in white rice and sorghum by liquid chromatography coupled to tandem mass spectrometry. Toxins. 2016;8:371.
Biancardi A, Dall’Asta C. Determination of sterigmatocystin in feed by LC-MS/MS. Food Addit Contam. 2015;32:2093–100.
Sulyok M, Krska R, Schuhmacher RA. A liquid chromatography/tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples. Anal Bioanal Chem. 2007;389:1505–23.
Monbaliu S, Van Poucke C, Detavernier C, Dumoultn F, Van Velde MDE, Schoeters E, et al. Occurrence of mycotoxins in feed as analysed by a multi-mycotoxin LC-MS/MS method. J Agr Food Chem. 2010;58:66–71.
Jackson LC, Kudupoje MB, Yiannikouris A. Simultaneous multiple mycotoxin quantification in feed samples using three isotopically labeled internal standards applied for isotopic dilution and data normalization through ultra-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Sp. 2012;26:2697–713.
Malachová A, Sulyok M, Beltrán E, Berthiller F, Krska R. Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J Chromatogr A. 2014;1362:145–56.
Oplatowska-Stachowiak M, Haughey SA, Chevallier OP, Galvin-King P, Campbell K, Magowan E, et al. Determination of the mycotoxin content in distiller’s dried grain with solubles using a multianalyte UHPLC–MS/MS method. J Agr Food Chem. 2015;63:9441–51.
Kong D, Xie Z, Liu L, Song S, Kuang H, Cui G, et al. Development of indirect competitive ELISA and lateral-flow immunochromatographic assay strip for the detection of sterigmatocystin in cereal products. Food Agr Immunol. 2017;28:260–73.
Li M, Li P, Wu H, Zhang Q, Ma F, Zhang Z, et al. An ultra-sensitive monoclonal antibody-based competitive enzyme immunoassay for sterigmatocystin in cereal and oil products. PLoS One. 2014;9:e106415.
Li S, Chen PY, Marquardt RR, Han Z, Clarke JR. Production of a sensitive monoclonal antibody to sterigmatocystin and its application to ELISA of wheat. J Agr Food Chem. 1996;44:372–5.
European Commission. Commission regulation (EU) 519/2014 of 16 May 2014 amending regulation (EC) no 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T-2, HT-2 toxin and citrinin and screening methods of analysis. Off J Eur Union. 2014;L147:29–43.
Kononenko GP, Burkin AA, Soboleva NA. Comparative characterization of immune reagents based on hemiacetals of aflatoxin B1 and sterigmatocystine. Appl Biochem Micro. 2002;38:487–92.
Oplatowska-Stachowiak M, Sajic N, Xu Y, Haughey SA, Mooney MH, Gong YY, et al. Fast and sensitive aflatoxin B1 and total aflatoxins ELISAs for analysis of peanuts, maize and feed ingredients. Food Control. 2016;63:239–45.
Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7.
Oplatowska-Stachowiak M, Kleintjens T, Sajic N, Haasnoot W, Campbell K, Elliott CT, et al. T-2 toxin/HT-2 toxin and ochratoxin A ELISAs development and in-house validation in food in accordance with commission regulation (EU) no 519/2014. Toxins. 2017;9:388.
Acknowledgements
The authors would like to thank Lucia Streppel and Piet van Wichen from EuroProxima for their support of this research.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 655119.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving animals
Animal experiments were performed in accordance with the UK Animals Scientific Procedures Act 1986 under the license PPL2756 issued on the 12/02/2014 by the Department of Health, Social Services and Public Safety for Northern Ireland. The study received approval from the Queens University Belfast Animal Welfare and Ethical Review Body on 09/01/2014.
Rights and permissions
About this article
Cite this article
Oplatowska-Stachowiak, M., Reiring, C., Sajic, N. et al. Development and in-house validation of a rapid and simple to use ELISA for the detection and measurement of the mycotoxin sterigmatocystin. Anal Bioanal Chem 410, 3017–3023 (2018). https://doi.org/10.1007/s00216-018-0988-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0988-8