Abstract
The ultimate objective of this study is to find a way to replace toxic lead-based solder with a non-toxic replacement that retains all of the desirable characteristics of the conventional solder. In this work, the integral and partial enthalpy of mixing for Sn–Ga–In ternary alloy systems were measured by the help of drop calorimeter along six of the cross sections at different temperatures of 673 K, 723 K and 773 K. Pieces of pure tin were dropped into molten Ga0.25In0.75, Ga0.50In0.50, Ga0.75In0.25 alloys and pieces of pure Indium into Ga0.25Sn0.75, Ga0.50Sn0.50, Ga0.75Sn0.25. In order to calculate the interaction parameter, Redlich–Kister–Muggianu (RKM) model was used which considers the substitutional solution mechanism. Geometric models i.e. Kohler, Muggianu, Chou, Toop, and Hillert have been used to determine the integral mixing enthalpies and compared with experimental data. It has been seen a good agreement between the theoretical models and results of this study.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent times, the harmful consequences that lead-containing materials have had on the environment and health have led to a rising demand for the development of lead-free alternatives that are friendlier to the environment. Conventional solders containing lead and tin, that are used in a wide range of electronic applications are an example of material that raises serious concerns. It is now absolutely necessary to look for lead-free solder alternatives that have characteristics that are comparable to those of Pb–Sn solder to solve this problem. As a possible replacement for traditional solders that contains lead, the alloy system consisting of tin (Sn), gallium (Ga), and indium (In) is the main objective of this research. Because of its superior ability to transmit electricity and its good resistance to corrosion, gallium stands out as a potentially useful choice. In addition, gallium's melting point of 29.76 °C, which is substantially lower than most other metals, making it a potential candidate for our study. This lowers the melting point of the alloy system as a whole. Notably, preceding studies have shown the positive effects of adding gallium into lead-free solders, improving mechanical characteristics and better wettability [1,2,3]. Aside from gallium, indium is an essential component in this alloy system. Having a melting point that is just 156.6 °C lower than gallium's, indium helps to lower the alloy's total melting point and provides excellent resistance to corrosion. Despite the fact that the value of electrical conductivity of indium is not even close to that of copper [4,5,6,7], the addition of this element considerably improves the wetting behaviors of the alloy. This results in improvement in the strength of solder bond and hence making the solder more reliable. Tin, which is another crucial component of the system, has a melting point of just 213.93 °C and a boiling temperature that is 2270 °C. Tin’s melting point is lower than lead's melting point, but it has a higher boiling point. The alloy's overall performance is further enhanced by the fact that it has high electrical conductivity and is resistant to corrosion [8,9,10]. A lead-free solder replacement that has characteristics that are equivalent to those of typical lead–tin solder may be developed by using gallium, indium, and tin. This is a fascinating path to investigate. The outcomes of this study will, eventually, add to the continuous efforts that are being put forth in the development of environmentally friendly and sustainable materials for the electronics sector. In order to build an alloy comprising Sn, Ga, and In that may replace conventional Sn based solder, the first step is to determine the thermodynamic parameters of the alloy, which is then followed by the construction of phase diagram. It is challenging to discover any alloy that is capable of satisfying all of the criteria for a lead-free solder. According to the research that has been done, there is no calorimetric data that can be used for making direct measurements of enthalpies of mixing for this ternary system. Because of this, the determination of the enthalpy of mixing for this ternary system is aimed at providing an alternative to applications using lead-based solder.
1.1 Ga–In System
Researchers from a variety of fields have looked at the phase diagram of the gallium–indium (Ga–In) system in an effort to better comprehend its thermodynamic characteristics. Ansara et al. [11] used a Tian-calvet micro calorimeter in their research. Their thermodynamic analysis results in determination of heat capacities and these heat capacities help in giving a clear picture about the system's distinct phases, which were previously unknown. The Ga–In system was also investigated by Anderson et al. [12], who found that the trend of molar enthalpy is very closely resembles with the data that was already present in the literature. Ansara et al. [13] continued their inquiry into the enthalpies of mixing by measuring the molar excess enthalpies for the liquid Ga–In system at a temperature of 995 K. The enthalpy data were expanded upon by Handzlik et al. [14, 15] who investigated the enthalpy of mixing values at different temperatures (1123 K, 1273 K, and 1423 K). These data are very necessary to have a complete understanding of the system’s thermodynamic behavior across a wider temperature range. The mixing enthalpy for liquid Ga–In alloy systems were calculated by Predel et al. [16], who found that the value of the mixing enthalpy reached its maximum when xIn was roughly less than 0.5. Singh et al. [17] studied the given binary system using drop calorimetric technique at temperature range of 673 K to 773 K.
1.2 Ga–Sn System
Numerous thermodynamic investigations of the Ga–Sn system have been carried out in an attempt to get a better understanding of the eutectic composition of the system as well as its mixing behavior. At a temperature of 693 K, Anderson et al. [18] investigated the Ga–Sn system at eutectic composition by using several approaches from the field of thermal analysis. The partial and total mixing enthalpies of the Ga–Sn system were determined by Li et al. [19] using a calvet type calorimeter at a fixed temperature of 803 K. However, these measurements were only performed for compositions in which xSn was less than 0.35. Kulawik et al. [20] presented data on the enthalpy of mixing and compared it to data from other published sources. They most likely did an overall study and comparison of the enthalpy data that came from a variety of sources in order to identify any discrepancies or unusual occurrences that could have occurred. Zivkovic et al. [21] conducted research on the thermodynamics of the Ga–Sn binary system at three different temperatures: 1000 K, 1073 K, and 1200 K. This experiment at many temperatures may have led to complete understanding of the system’s thermodynamic behavior across a wider temperature range. Singh et al. [17] studied the given binary system using drop calorimetric technique at temperature range of 673 K to 773 K.
1.3 In–Sn System
Several thermodynamic studies have been conducted on the In–Sn binary alloy with the purpose of better comprehending the material’s behavior as well as its characteristics. Using a calvet type micro calorimeter, Rechchach et al. [22] investigated the binary In–Sn alloy at a temperature of 500 °C. The researchers kept the temperature constant throughout the experiment. The objective of Korhonen et al. [23] was to estimate the thermodynamic behavior of the In–Sn binary system by making the best use of the data that is currently available from the published research. The In–Sn binary system was subjected to a thermodynamic analysis by Lee et al. [24], who used thermodynamic models in their investigation. They most likely validated their model by comparing its predictions to the outcomes of the experiments that were actually conducted using the thermodynamic parameters that were obtained from those experiments. Using a calvet type micro calorimeter, Luef et al. [25] determined the partial and integral mixing enthalpies of the In–Sn binary system at a higher temperature of 900 °C. These experiments were carried out at a higher temperature. Oelsen calorimetry was used by Zivkovic et al. [26] to determine the thermodynamic characteristics for the liquid In–Sn system when it was heated to 600 K. Singh et al. [17] studied the given binary system using drop calorimetric technique at temperature range of 673 K to 773 K.
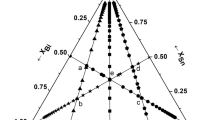
The literature review suggests that there is no calorimetric data available for the Sn–Ga–In system. Therefore, we have undertaken to investigate this system. In this work, we used a drop calorimeter to determine the partial and integral enthalpies of mixing for Sn–Ga–In ternary alloy system along six of the cross sections (see Fig. 1 for more information) at different temperatures of 673 K, 723 K, and 773 K. Pieces of pure tin were dropped into molten Ga0.25In0.75, Ga0.50In0.50, Ga0.75In0.25 alloys and pieces of pure Indium were dropped into Ga0.25Sn0.75, Ga0.50Sn0.50, Ga0.75Sn0.25. Molar enthalpies of mixing were calculated from the partial molar enthalpies. Plotting iso-enthalpy curves was then accomplished using integral mixing enthalpy. It was discovered that the temperature affected the mixing enthalpies. Based on ternary enthalpy values, the substitutional solution RKM model was used to determine the interaction parameter; to get these parameters, least square fitting was employed. Additionally, the enthalpies of mixing of the given ternary have been calculated by using the Kohler, Muggianu, Chou, Toop and Hillert geometric models and compared with the experimental ones. These models use the binary interaction parameters and these parameters are deduced from the literature [17]. When the values getting from RKM model and measured experimental values are compared, it is found that there is a good agreement between them.
Measured cross-sections (intersections a to h indicated, see Table 3) and alloy compositions in the Sn–Ga–In ternary system
2 Experimental Details
2.1 Materials
To investigate the thermodynamic behavior, a calorimetric analysis of a ternary alloy was conducted, employing high-purity metals—specifically, Ga, In, and Sn. Calibration was performed using α-Al2O3 needles sourced from the National Institute of Standards and Technology (NIST) in Gaithersburg, Maryland, USA. To ensure cleanliness, the metals underwent a thorough cleaning with n-hexane in a supersonic bath. Subsequently, the metals were vacuum-dried within an antechamber glove box to eliminate any residual solvent. Following this, the metals were segmented into smaller pieces and accurately weighed to the precision of 10–4 g. The specifications for the pure metals and the use of protective Argon gas are detailed in Table 1.
2.2 Calorimetric Measurements
Throughout this experimental investigation, a drop calorimeter (model number-MHTC 96 Line Evo) manufactured by SETARAM Instruments in France was employed to gather enthalpy data related to the studied system. Ensuring constant cooling, a graphite tube type resistance furnace with a water-flowing arrangement around its exterior was utilized, and temperature monitoring inside was achieved through a thermopile equipped with 56 pairs of S-type thermocouples. The furnace's maximum operating temperature is 1593 K. To automate the introduction of metals into the crucible, a motorized device specifically designed for dropping was utilized. Calibration reference for the heat flow curve was established by dropping four pieces of α-Al2O3 needles at the conclusion of each sample series. The CALISTO software comprehensively monitors the calorimeter. Before the experiment commenced, specific amounts of two of the three metals were placed in the crucible with an outer diameter of 12 mm and a length of 60 mm. The CALISTO software regulated both the gas circulation and the water cooling system. Pre-programming included parameters such as the rate of heating and cooling, holding duration, and the rate of gas flow. Throughout the experiment, a continuous supply of argon at a rate of approximately 30 mL/min was maintained to prevent oxidation of the alloys. Argon served dual roles as a carrier and protective gas during the entire experiment. Upon reaching the designated holding temperature, the automated motorized dropping system introduced the first sample into the crucible. The dropping device, equipped with a timer set to automatically release the sample every half an hour, ensured a systematic approach. The data acquisition software continuously recorded heat signals at 673 K, 723 K, and 773 K. Following the completion of all sample drops, α-Al2O3 standard samples were introduced to calibrate the heat signals towards the experiment's conclusion. Calorimetric investigation of the Sn–Ga–In ternary alloy system was executed employing the methodology outlined above. Pure tin specimens were introduced into molten Ga0.25In0.75, Ga0.50In0.50, Ga0.75In0.25 alloys, while pure indium pieces were dropped into Ga0.25Sn0.75, Ga0.50Sn0.50, Ga0.75Sn0.25 alloys. This study encompassed a total of six isopleths. A thermal equilibration process lasting approximately 16 h was implemented to establish a stable baseline before introducing the sample material. To assess the negligible weight loss due to evaporation, the combined mass of both the crucible and samples was measured before and after the experiments. To ensure reproducibility, each experiment was conducted twice with identical variables. The acquired heat signals underwent integration using the CALISTO Data Processing software, which facilitated the determination of enthalpy values. Using the CALISTO data processing programme, we were able to successfully integrate the peaks that appeared in the heat flow curve. The value of the heat signal that was obtained from the α-Al2O3 needle drops served as the basis for the calculation of the calibration constant (K). Calculating the enthalpy, or heat effect, for each given heat signal requires the multiplication of the integral values of that signal by a known value (K).
Here, the reaction enthalpy (\({\Delta H}_{Reaction,X,i}\)) which is a function of heat effect (\(\Delta {H}_{Signal,X,i}\cdot K\)) and the enthalpy (\({\Delta H}_{X,i}^{{T}_{D} \to {T}_{M}}\)) increment of the sample to be dropped, is calculated by Eq. 1 when species X is dropped from drop temperature (TD) to bath temperature (TM).
where \({n}_{X,i}\) (no. of moles) is the number of species X that was dropped into the liquid bath with the assistance of an automated dropping system, and each peak is associated with a distinct \(\Delta {H}_{Signal,X,i}\) value, which is the integrated area (in µVs). It was computed using baseline integration methods for every peak. The change in the molar enthalpy of species X is represented by \({\Delta H}_{X,i}^{{T}_{D} \to {T}_{M}}\) and may be evaluated with the use of enthalpy data found in the literature [27] for each species within the appropriate temperature range.
Although a very small amount of species X was added in with the liquid metal in the crucible, Eq. (2) may be used to calculate the partial enthalpy \(\Delta {\overline{H} }_{X,i}\):
For each measurement, two of the three metals were added to the crucible in precise proportions before the third was added at the designated temperature. Pieces of pure tin were dropped into molten Ga0.25In0.75, Ga0.50In0.50, Ga0.75In0.25 alloys and pieces of pure Indium into Ga0.25Sn0.75, Ga0.50Sn0.50, Ga0.75Sn0.25. The integral molar mixing enthalpy (\(\Delta {H}_{mix}\)) of ternary system can be calculated by using Eq. (3).
Here \({n}_{binary}\) = total no. of moles of two base metals in crucible.
\({\Delta }_{mix}{H}_{respective binary}\) = enthalpy of mixing value for the initial composition of binary in the crucible.
The literature [27] was referred to get In and Sn’s enthalpy increase from room temperature to drop temperature, which was then included into Eq. (1). It’s possible for calorimetric readings to be inaccurate for a variety of reasons, including the kind of calorimeter that was used, the techniques for calibrating it, the integration of the heat flow curve baseline, the solubility of the solute in the solvent, and the concentration of impurities. The experimental error of the calorimeter is anywhere from ten to twelve percent. It was discovered that the mistake in calibration brought on by dropping α-Al2O3 needles fell within a region that was no more than 1.5%.
3 Results and Discussion
3.1 Experimental Results
The partial and integral enthalpy of mixing values for the given ternary system at temperature of 673 K, 723 K and 773 K were shown in the Tables S1 and S2. These experimental values are used to find the ternary interaction parameter values as given in Table 2.
3.2 Theoretical Models
Using different theoretical models, one may extrapolate the thermodynamic features of ternary or higher order systems from data on binary systems. Ansara and Dupin’s theoretical model is a major contribution [28]. Luef et al. [25] have also used the Redlich–Kister–Muggianu polynomial for substitutional solutions. To determine the ternary interaction parameters, we performed a least-squares fit to the available data using Eq. 4.
where \({L}_{i : j}^{(\upsilon )}\) (ν = 0, 1, 2, …) are the interaction parameters(binary) of the binary systems and \({L}_{i:j:k}^{\nu }\) (ν = 0, 1, 2,…) are the interaction parameters(ternary). The experimentally determined mixing enthalpy is then compared with the estimated values, with just the binary contributions being taken into consideration. Table 2 contains the results of the binary interaction parameter that was derived from the data that was accessible in the literature for all binary systems [17]. The difference between the two values shows the contribution of the ternary interaction to this system, which may then be optimized using Eq. 4.
The enthalpies of mixing along six of the cross sections depicted in Fig. 1 for Sn–Ga–In Ternary alloy systems were determined at different temperatures of 673 K, 723 K, and 773 K. Pieces of pure tin were dropped into molten Ga0.25In0.75, Ga0.50In0.50, Ga0.75In0.25 alloys and pieces of pure Indium was dropped into Ga0.25Sn0.75, Ga0.50Sn0.50, Ga0.75Sn0.25 alloys. The closeness of readings near the intersection points on the six cross-sections indicates the quality of our experimental data (see Table 3 and Fig. 1). However, systemic errors, such as those resulting from insufficient mixing reactions, cannot be completely ruled out.
As shown in Fig. 2, the mixing enthalpy values for the two sections (Ga0.50In0.50)1−xSnx and (Ga0.75In0.25)1−xSnx are endothermic in nature but for section (Ga0.25In0.75)1−xSnx it is showing exothermic nature for the composition of Sn ranging from 0.75 to 1. Exothermic or negative value of mixing enthalpy in this range tells about the region where the alloy is supposed to be more stable. Also it suggests that there is finite amount of interatomic interaction among three metals in this range as there is good chance of Gibb’s energy to be negative as enthalpy is negative although it also depends on sign of entropy as well.
Plot representing the enthalpies of mixing of the given ternary alloy system corresponding to cross-section of (Ga0.25In0.75)1−xSnx; circle, (Ga0.50In0.50)1−xSnx; triangle and (Ga0.75In0.25)1−xSnx; star at 673 K, In–Sn binary data from the literature [17]; square, Ga–Sn binary data from the literature [17]; half-filled circle and solid line represents RKM fitted polynomial curve
Referring to Fig. 3, the integral mixing enthalpy values for the section (Ga0.25In0.75)1−xSnx, (Ga0.50In0.50)1−xSnx are getting negative or exothermic for the composition range of xSn between 0.5 and 1 but remains endothermic for the section (Ga0.75In0.25)1−xSnx. Exothermic or negative value of mixing enthalpy in this range tells about the region where the alloy is supposed to be more stable. Also it suggests that there is finite amount of interatomic interaction among three metal atoms in this range as there is good chance of Gibb’s energy to be negative as enthalpy is negative although it also depends on sign of entropy as well. As mixing enthalpy values are small as compared to positive enthalpy values in this system, the stability of the alloys will mostly depend on the entropy of mixing.
Plot representing the enthalpies of mixing of the given ternary alloy system corresponding to cross-section of (Ga0.25In0.75)1−xSnx; circle, (Ga0.50In0.50)1−xSnx; triangle and (Ga0.75In0.25)1−xSnx; star at 723 K, In–Sn binary data from the literature [17]; square, Ga–Sn binary data from the literature [17]; half-filled circle and solid line represents RKM fitted polynomial curve
Referring to Fig. 4, the integral mixing enthalpy values are positive for the section (Ga0.75In0.25)1−xSnx throughout the composition of xSn but for two sections (Ga0.25In0.75)1−xSnx, (Ga0.50In0.50)1−xSnx, the values of integral mixing enthalpy are found to be negative or exothermic for the composition range of xSn between 0.3 to 1 and between 0.5 to 1 respectively. Exothermic or negative value of mixing enthalpy in this range tells about the region where the alloy is supposed to be more stable. Also it suggests that there is finite amount of interatomic interaction among three metal atoms in this range as there is good chance of Gibb’s energy to be negative as enthalpy is negative although it also depends on sign of entropy as well. This is a non-ideal system departing very little from ideal enthalpy of mixing.
Plot representing the enthalpies of mixing of the given ternary alloy system corresponding to cross-section of (Ga0.25In0.75)1−xSnx; circle, (Ga0.50In0.50)1−xSnx; triangle and (Ga0.75In0.25)1−xSnx; star at 773 K, In–Sn binary data from the literature [17]; square, Ga–Sn binary data from the literature [17]; half filled circle and solid line represents RKM fitted polynomial curve
From Figs. 2, 3 and 4, it can be inferred that as the temperature increases from 673 to 773 K the two sections (Ga0.25In0.75)1−xSnx and (Ga0.50In0.50)1−xSnx are getting exothermic for large range of xSn. Addition of Sn makes the alloy more stable and it is also desired for the solder applications.
Figure 5 illustrates that all three isopleths are endothermic in nature with respect to composition of In(xIn) and as the xGa/xSn value increases the enthalpy of mixing values are getting more positive (endothermic) with respect to the composition of In(xIn). So, there may be a chance of less tendency for the atoms to be mixed in this system at 673 K.
Plot representing the enthalpies of mixing of the given ternary alloy system corresponding to cross-section of (Ga0.25Sn0.75)1−xInx; circle, (Ga0.50Sn0.50)1−xInx; triangle and (Ga0.75Sn0.25)1−xInx; star, In–Sn binary data from the literature [17]; square, Ga–In binary data from the literature [17]; half-filled circle at 673 K and solid line represents RKM fitted polynomial curve
Figure 6 illustrates the variation in mixing enthalpies with the indium composition for three different isopleths (Ga0.25Sn0.75)1−xInx, (Ga0.50Sn0.50)1−xInx and (Ga0.75Sn0.25)1−xInx and two binaries at 723 K. For three isopleths and the Ga–In binary, the mixing enthalpies are endothermic for the entire composition of In except for the isopleth (Ga0.25Sn0.75)1−xInx, where values are slightly negative in the range of xIn = 0.2–0.4. Also it suggests that there is finite amount of interatomic interaction among three metal atoms in this range as there is good chance of Gibb’s energy to be negative as enthalpy is negative although it also depends on sign of entropy as well.
Plot representing the enthalpies of mixing of the given ternary alloy system corresponding to cross-section of (Ga0.25Sn0.75)1−xInx; circle, (Ga0.50Sn0.50)1−xInx; triangle and (Ga0.75Sn0.25)1−xInx; star at 723 K, In–Sn binary data from the literature [17]; square, Ga–In binary data from the literature [17]; half-filled circle and solid line represents RKM fitted polynomial curve
Figure 7 shows the nature of plots of mixing enthalpies for three isopleths (Ga0.25Sn0.75)1−xInx, (Ga0.50Sn0.50)1−xInx and (Ga0.75Sn0.25)1−xInx and two binaries In–Sn and Ga–In at 773 K. For two isopleths (Ga0.50Sn0.50)1−xInx and (Ga0.75Sn0.25)1−xInx the mixing enthalpies are endothermic for entire In composition, but for isopleth (Ga0.25Sn0.75)1−xInx the values of mixing enthalpies are negative or exothermic in nature in the range of xIn = 0.15–1. Also it suggests that there is finite amount of interatomic interaction among three metal atoms in this range as there is good chance of Gibb’s energy to be negative as enthalpy is negative although it also depends on sign of entropy as well.
Plot representing the enthalpies of mixing of the given ternary alloy system corresponding to cross-section of (Ga0.25Sn0.75)1−xInx; circle, (Ga0.50Sn0.50)1−xInx; triangle and (Ga0.75Sn0.25)1−xInx; star at 773 K, In–Sn binary data from the literature [17]; square, Ga–In binary data from the literature [17]; half-filled circle and solid line represents RKM fitted polynomial curve
From Figs. 5, 6 and 7, it can be inferred that as the temperature increases from 673 to 773 K, the enthalpies of mixing become more exothermic for (Ga0.25Sn0.75)1−xInx isopleth.
Figure 8 illustrates the effect of temperature on the enthalpy of mixing for isopleth (Ga0.25In0.75)1−xSnx at different mole fraction of Sn. Also, it can be inferred from above plot that enthalpy curves at 673 K, 723 K, and 773 K are not close to one another. This confirms that the enthalpies of mixing of ternary Sn–Ga–In system is not independent of temperature. So, temperature has a very important role to play on mixing of Ga, Sn and In atoms. The degree of mixing is better for the given isopleth at 773 K as the enthalpy of mixing values are most negative at 773 K.
The influence of temperature on the enthalpy of mixing for isopleth (Ga0.25Sn0.75)1−xInx at various mole fractions of In is shown in Fig. 9. The above figure also suggests that the enthalpy curves at 673 K, 723 K, and 773 K are not close to one other. This demonstrates that the ternary Sn–Ga–In system's enthalpies of mixing are not temperature-independent. Therefore, temperature has a significant impact on how Ga, Sn, and In atoms combine. The degree of mixing is better for the given isopleth at 773 K as the enthalpy of mixing values are most negative at 773 K.
There are six iso-enthalpies curves i.e. − 0.2 kJ/mol, 0 kJ/mol, 0.4 kJ/mol, 0.8 kJ/mol, 1 kJ/mol, 1.2 kJ/mol as shown in the Fig. 10. Along each curve enthalpy value is constant. To generate these plots, the values of molar mixing enthalpy with respect to amount of the species which is to be dropped are used. The behavior of both the binaries Ga–Sn and Ga–In affects the majority of the iso-enthalpy curves because In–Sn system is slightly negative or exothermic in nature. It is seen that enthalpy of mixing becomes positive from In–Sn binary towards Gallium corner. As gallium composition increases the enthalpy of mixing increases. All the iso-enthalpy curves are oriented towards In–Sn binary. Therefore, the stability of the alloy is maximum for the composition close to In–Sn binary.
Five extrapolation geometric models (Kohler, Muggianu, Chou, Toop and Hillert) were used to predict the enthalpy of mixing values of ternary Sn–Ga–In system. Binary interaction parameter of all binaries were required to predict the thermodynamic properties of the given ternary system. Binary interaction parameter of all binary systems In–Sn, Ga–In and Ga–Sn were taken from the data available in literature [17] and given in the Table 2.
The various predictive extensions (referring to equations 5, 6, 7, 8, and 9) from the binary to ternary Sn–Ga–In System are shown below:
Kohler model [29]
Muggianu model [29]
Chou model [29]
Toop model [29]
Hillert model [29]
A comparison of experimental data with all geometric model data along with RKM modelled fitted data at 773 K is shown in Fig. 11 for the isopleth (Ga0.50In0.50)1−xSnx. RKM modelled polynomial fitted data fits well to the experimental data. Binary and ternary interaction parameter values are shown in Table 2. Large value of ternary interaction parameters show that there is strong interaction among three elements, that’s why we are getting large values of three L parameters. Therefore, we could infer that ternary contribution is quite significant in this ternary system and also referring to Figs. 8 and 9, the experimental values are not close to each other so temperature dependence is there. A comparison of experimental data and the data predicted from CALPHAD technique [30,31,32,33,34,35], with all geometric model data along with RKM modelled fitted data at 773 K is shown in Fig. 12 for the isopleth (Ga0.75Sn0.25)1−xInx. RKM modelled polynomial fitted data fits well to the experimental data. Also the data predicted from CALPHAD technique [30] by using THERMOCALC software is very close to the experimental data for most of the composition range.
Experimental and calculated integral enthalpy of mixing for the isopleth (Ga0.75Sn0.25)1−xInx by using the five geometric models (Kohler, Muggianu, Chou, Toop and Hillert) at 773 K along with RKM Polynomial fitted Curve and data predicted from CALPHAD technique [30]
4 Summary and Conclusions
Partial mixing enthalpy and integral mixing enthalpy values were derived from this study on Sn–Ga–In Ternary alloy systems by using drop calorimeter along six of the cross sections (see Fig. 1) at temperatures ranging from 673 to 773 K. It was found that mixing enthalpies were temperature dependent. The substitutional solution RKM model was used to derive the interaction parameter based on ternary enthalpy values and to get these parameters least square fitting is used. Additionally, the enthalpies of mixing of the given ternary have been calculated by using the Kohler, Muggianu, Chou, Toop and Hillert geometric models and compared with the experimental ones. It is also observed that the data predicted from CALPHAD technique [30] is very close to the experimental data for most of the composition range. These models use the binary interaction parameters and these parameters are deduced by using a Redlich–Kister polynomial fitting to accurately describe the binary interactions within each binary alloy system. When the values getting from RKM model and measured experimental values are compared, it is found that there is a good agreement between them.
Data Availability
The data substantiating the conclusions of this study are presented within the article. For supplementary data that enhance the study, interested parties may contact the corresponding author and request access.
References
P.D. Sonawane, V.K. Bupesh Raja, K. Palanikumar, E. Ananda Kumar, N. Aditya, V. Rohit, Effects of gallium, phosphorus and nickel addition in lead-free solders: a review. Mater. Today Proc. 46, 3578–3581 (2020). https://doi.org/10.1016/j.matpr.2021.01.335
M.N. Ervina Efzan, M.N. Nur Faziera, Review on the effect of Gallium in solder alloy. IOP Conf. Ser. Mater. Sci. Eng. 957, 012054 (2020). https://doi.org/10.1088/1757-899X/957/1/012054
V. Singh, D. Jaiswal, D. Pathote, C.K. Behera, Drop calorimetric measurement of In–Zn system for lead-free solder applications. Mater. Today Proc. 57, 285–288 (2022). https://doi.org/10.1016/j.matpr.2022.02.601
M.S. Yeh, Effects of indium on the mechanical properties of ternary Sn–In–Ag solders. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 34(2), 361–365 (2003). https://doi.org/10.1007/s11661-003-0337-0
C.K. Behera, A. Sonaye, Measurement of zinc activity in the ternary In–Zn–Sn alloys by EMF method. Thermochim. Acta 568, 196–203 (2013). https://doi.org/10.1016/j.tca.2013.06.039
M.R. Kumar, S. Mohan, C.K. Behera, Measurements of mixing enthalpy for a lead-free solder Bi–In–Sn system. J. Electron. Mater. 48(12), 8096–8106 (2019). https://doi.org/10.1007/s11664-019-07646-0
C.K. Behera, M. Shamsuddin, Thermodynamic investigations of Sn–Zn–Ga liquid solutions. Thermochim. Acta 487(1–2), 18–25 (2009). https://doi.org/10.1016/j.tca.2009.01.004
F.M. Azizan, H. Purwanto, M.Y. Mustafa, Effect of Sn addition on mechanical properties of zinc-based alloy. Adv. Mater. Res. 576, 378–381 (2012). https://doi.org/10.4028/www.scientific.net/AMR.576.378
A.V. Khvan, T. Babkina, A.T. Dinsdale, I.A. Uspenskaya, I.V. Fartushna, A.I. Druzhinina, A.B. Syzdykova, M.P. Belov, I.A. Abrikosov, Thermodynamic properties of tin: part I experimental investigation, ab-initio modelling of α-, β-phase and a thermodynamic description for pure metal in solid and liquid state from 0 K. Calphad 65, 50–72 (2019). https://doi.org/10.1016/j.calphad.2019.02.003
V. Singh, D. Pathote, D. Jaiswal, M.R. Kumar, K.K. Singh, C.K. Behera, Measurement of mixing enthalpies for Sn–Bi–Sb lead-free solder system. J. Electron. Mater. 52, 6316–6334 (2023). https://doi.org/10.1007/s11664-023-10579-4
I. Ansara, J.P. Bros, C. Girard, Thermodynamic analysis of the GaIn, AlGa, AlIn and the AlGaIn systems. Calphad 2(3), 187–196 (1978). https://doi.org/10.1016/0364-5916(78)90008-1
T.J. Anderson, I. Ansara, The Ga–In (Gallium–Indium) system. J. Phase Equilib. 12(1), 64–72 (1991). https://doi.org/10.1007/BF02663677
I. Ansara, M. Gambino, J.P. Bros, Study of thermodynamics of the ternary system gallium–indium–antimony. J. Cryst. Growth 32(1), 101–110 (1976). https://doi.org/10.1016/0022-0248(76)90016-6
D. Jendrzejczyk-Handzlik, P. Handzlik, Enthalpies of mixing of liquid Ga–In and Cu–Ga–In alloys. J. Mol. Liq. 293, 111543 (2019). https://doi.org/10.1016/j.molliq.2019.111543
D. Jendrzejczyk-Handzlik, P. Handzlik, Mixing enthalpies of liquid Au–Ga–In alloys. J. Mol. Liq. 301, 112439 (2020). https://doi.org/10.1016/j.molliq.2019.112439
B. Predel, D.W. Stein, Thermodynamic properties of the gallium-indium systems. J. Less-Common Met. 18(1), 49–57 (1969). https://doi.org/10.1016/0022-5088(69)90119-2
V. Singh, D. Pathote, D. Jaiswal, K.K. Singh, C.K. Behera, Calorimetric measurements of Ga–In, Ga–Sn, and In–Sn binary alloy systems as sustainable lead-free solder alternatives. J. Mater. Sci. Mater. Electron. (2023). https://doi.org/10.1007/s10854-023-11521-4
T.J. Anderson, I. Ansara, The Ga–Sn (Gallium–Tin) system. J. Phase Equilib. 13(2), 181–189 (1992). https://doi.org/10.1007/BF02667485
D. Li, S. Delsante, W. Gong, G. Borzone, Partial and integral enthalpies of mixing of Ag–Ga–Sn liquid alloys. Thermochim. Acta 523(1–2), 51–62 (2011). https://doi.org/10.1016/j.tca.2011.04.032
S. Kulawik, W. Gierlotka, A. Dębski, W. Gasior, A. Zajaczkowski, Thermodynamic assessment of the Ga–Sn–Zn system. Calphad, 69, 101765 (2020). https://doi.org/10.1016/j.calphad.2020.101765
D. Zivkovic, D. Manasijevic, Z. Zivkovic, Thermodynamic study of Ga–Sn and Ga–Zn systems using quantitative differential thermal analysis. J. Therm. Anal. Calorim. 74(1), 85–96 (2003). https://doi.org/10.1023/A:1026373602352
M. Rechchach, A. Sabbar, H. Flandorfer, H. Ipser, Enthalpies of mixing of liquid In–Sn and In–Sn–Zn alloys. Thermochim. Acta 502(1–2), 66–72 (2010). https://doi.org/10.1016/j.tca.2010.02.008
T.M. Korhonen, J.K. Kivilahti, Thermodynamics of the Sn–In–Ag solder system. J. Electron. Mater. 27(3), 149–158 (1998). https://doi.org/10.1007/s11664-998-0205-1
B.J. Lee, C.S. Oh, J.H. Shim, Thermodynamic assessments of the Sn–In and Sn–Bi binary systems. J. Electron. Mater. 25, 983 (1996). https://doi.org/10.1007/BF02666734
C. Luef, H. Flandorfer, H. Ipser, Enthalpies of mixing of liquid alloys in the In–Pd–Sn system and the limiting binary systems. Thermochim. Acta 417(1), 47–57 (2004). https://doi.org/10.1016/j.tca.2004.01.019
D. Zivkovic, A. Mitovski, L. Balanovic, D. Manasijevic, Z. Zivkovic, Thermodynamic analysis of liquid In–Sn alloys using Oelsen calorimetry. J. Therm. Anal. Calorim. 102(3), 827–830 (2010). https://doi.org/10.1007/s10973-010-0785-x
R. Hultgrcn, R.L. Orr, P.D. Anderson, K.K. Kelley, Selected Values of Thermodynamic Properties of Metals and Alloys (Wiley, New York, 1963)
I. Ansara, N. Dupin, Cost 507 Thermo Chemical Database for Light Metal Alloys. European Commission DG X11 (European Commission, Luxembourg, 1998). https://www.opencalphad.com/databases/CGNA18499ENC_001.pdf
M. El Maniani, A. Sabbar, Partial and integral enthalpies of mixing in the liquid Ag–In–Sn–Zn quaternary alloys. Thermochim. Acta 592, 1–9 (2014). https://doi.org/10.1016/j.tca.2014.07.028
B. Sundman, B. Jansson, J.O. Andersson, The thermo-calc databank system. Calphad 9(2), 153–190 (1985). https://doi.org/10.1016/0364-5916(85)90021-5
V. Singh, D. Jaiswal, D. Pathote, K.K. Singh, C.K. Behera, Measurement of mixing enthalpies for Bi -Zn lead-free solder system. Mater. Today Proc. 57, 285–288 (2022).https://doi.org/10.1016/j.matpr.2022.02.601
D. Jaiswal, D. Pathote, V. Singh, C.K. Behera, Electrochemical behaviour of lead-free Sn-In-Al solders alloys in 3.5 wt.% NaCl solution. Mater. Today Proc. 57, 187–193 (2022). https://doi.org/10.1016/j.matpr.2022.02.315
D. Jaiswal, V. Singh, D. Pathote, C.K. Behera, Electrochemical behaviour of lead-free Sn–0.7Cu–xIn solders alloys in 3.5 wt% NaCl solution. J. Mater. Sci. Mater. Electron. 32, 23371–23384 (2021). https://doi.org/10.1007/s10854-021-06824-3
D. Jaiswal, D. Pathote, V. Singh, C.K. Behera, Effect of Al addition on electrochemical behavior of Sn-0.7Cu-xAl lead-free solders alloys in 3.5 wt. % NaCl solution. J. Mater. Eng. Perform. 31, 7550–7560 (2022). https://doi.org/10.1007/s11665-022-06771-y
M.R. Kumar, V. Singh, V.K. Rai, D. Jaiswal, C.K. Behera, Investigation on mixing heat effect of Bi-In and In-Sn system at 730 K. Mater. Today Proc. 18, 2917–2923 (2019). https://doi.org/10.1016/j.matpr.2019.07.161
Acknowledgements
The authors extend their thanks to the Head of the Department of Metallurgical Engineering at the Indian Institute of Technology (Banaras Hindu University), Varanasi, India, for providing essential support during the execution of this research.
Funding
No financial support was received for this study.
Author information
Authors and Affiliations
Contributions
Vikrant Singh—Conceptualization, methodology, formal analysis, writing of the original draft. Dileep Pathote—Visualization, Dheeraj Jaiswal—Visualization. Kamalesh K. Singh and C.K. Behera—supervision and writing of the original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm the absence of any conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, V., Pathote, D., Jaiswal, D. et al. Measurements of Enthalpies of Mixing of Sn–Ga–In Ternary Alloy System by Calorimetric Technique. Met. Mater. Int. (2024). https://doi.org/10.1007/s12540-024-01726-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12540-024-01726-5