Abstract
The current paper reviews the research conducted on wrought Cu–Sn, Cu–Sn–Ti and Cu–Sn–Zn alloys intending to reveal the details on morphological and mechanical behaviour during various manufacturing operations. The motivational force of employing copper-based alloys in automotive and marine industries is its mechanical properties and corrosion resistance. One of the important factors that affect mechanical properties is micrograph. The grain size, grain distribution, heat treatment, morphological behaviour and the intermediate phase formation highly influence the properties of the copper-based alloys. Moreover, the change in microstructure through different manufacturing process (wrought process), heat treatment and addition of alloying element also improve the mechanical and thermal properties. This paper aims to review the major conclusions on improving the microstructure and mechanical behaviour of wrought Cu–Sn, Cu–Sn–Zn and Cu–Sn–Ti alloys. Analysis and observations of each investigation were prepared by incorporating the morphological results obtained through Optical Microscope, Transmission Electron Microscope, Scanning Electron Microscope, X-ray Diffraction Technique and Electron Dispersive Spectroscopy. This review paper also attempts to give the areas of future researches and developments that can be made for these wrought alloys.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Wrought Cu-based alloys are employed in the manufacturing sector of bearings, bushings and other components that are used in machinery and plant constructions. On one hand, due to its high electrical conductivity, the wrought Cu-based alloys are utilized as electrical and electronic units in automobile vehicles and machines. On the other hand, owing to their high mechanical, tribological and thermal stresses, these wrought alloys are used in the form of bearings, sliding elements, bushings to ensure drive energy conversion or transmission in machines and internal combustion engines. As these wrought alloys used in marine or automobile sectors are reaching their stability under load for increasing mechanical, tribological and thermal stresses, wrought special alloys like Cu–Sn–Ti are given the special focus of interest. Understanding the properties of an alloy is a crucial state for extending its service under varying working conditions. The property of an alloy mainly depends on its composition, microstructure and manufacturing operations. As bronze is the second known alloy to the mankind that happened to be the combination of Cu and Sn. Amount of Sn in the bronze alloy can vary from 2 to 20 wt%. In Cu–Sn alloys amount of Sn has a major role which influence its strength and usability. This review paper lists out the major conclusions from the previous investigation conducted on wrought Cu–Sn, Cu–Sn–Zn and Cu–Sn–Ti alloys to affirm its applications in the field of microelectronics for making electronic interconnections [1, 2], industrial applications [3] and automobile industries [4]. Bonding [5,6,7] and diffusion [9,10,11,12,13,14,15] characteristics, the effect of elevated temperature [4, 8] and electric current [16, 17] on Cu–Sn and its alloys are also discussed.
To achieve desired properties and to be cost-effective, other alloying elements like zinc, lead, phosphorous, nickel, iron, titanium and aluminium are introduced. Among these alloying elements, Ti is found to possess outstanding strength, corrosion resistance and low density thereby making Cu–Sn–Ti alloys useful in many applications [18,19,20]. Phase diagrams and isothermal sections for both Cu–Sn [21,22,23,24] and Cu–Sn–Ti [25,26,27,28,29,30,31] alloys are also discussed. But, limited studies on wrought Cu–Sn–Ti alloys made them less noticeable in for manufacturing industries. Therefore, this work also focuses on future developments and researches that can be conducted on this ternary system to make the best use of these wrought alloys.
2 General Characteristics of Wrought Cu–Sn Alloy
Cu–Sn is non-ferrous alloys which are found to be ductile, heat conductive and possess good corrosion-resistant than the main metal making the alloy. An alloy mixture is stronger because it contains atoms from different elements that are different in sizes. The binary Cu–Sn system was modelled thermodynamically and the phases were calculated using the CALPHAD method [1]. Mainly, two intermetallic compounds (IMCs) were present in the Cu–Sn system (Cu3Sn and Cu6Sn5). The homogenous ranges in ε (Cu3Sn) and η (Cu6Sn5) phases were utilized to determine the Cu–Sn phase diagram. The experimental results were found to be in terms with the calculated thermodynamic properties. Even though the formation of IMCs at the interface made electronic connectors strong, presence of voids deteriorated the bond strength. Hence, the formation of kirkendall vacancy was proposed [2] and the development of IMCs in the Cu–Sn binary system established a criterion for determining the maximum concentration of vacancy in the Cu–Sn system. It was observed that the maximum kirkendall vacancy fraction was found in Cu3Sn, near the Cu/Cu3Sn interface, which implied that the kirkendall voids were mainly formed in Cu3Sn. Whereas, phase separation [32] was noticed during the sedimentation process in Cu–Sn alloys. Sedimentation process at 180 °C (at 3500RPM and radius in a range of 88–93 mm) for 2 h had resulted in the deposition of lighter pure Sn atoms at the centre of the axis of rotation and heavier atoms near the outer boundary. The Bi-velocity model [32] was used to determine the diffusion process in the multicomponent system under external force fields and normalized stress distribution during processing. Thus it was concluded that the sedimentation process affected the morphology of Cu–Sn sample, and stress influenced the fraction of phases under the centrifuge process.
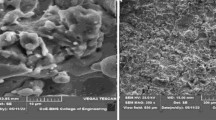
The features of γ decomposition [33] were investigated through thin foil electron microscopy. During the γ decomposition through a thin foil, it was found that the reactions were comparable to those that occurred in the transformation of Cu–16%Sn alloy on the isothermal treatment of quenched bulk specimens. Pearlite mode of decomposition (γ → α+δ) was found to occur at 450–520 °C which is isothermally heat treated. While in thin films decomposition, the transformation occurred when slowly cooled from γ region and the specimens was isothermally heated below 450 °C. When the thin film was cooled faster, δ precipitation occurred with a subsequent bainitic reaction. The aspects of δ-precipitates depend on the rate of cooling despite the size of the specimen (massive or thin). The bainitic reaction involved the formation of two phases (α’ and δ), both with the same structure. Further, the precipitation and decomposition behaviour [34] of Cu–15%Sn (Quenched) was characterized using TEM. From Fig. 1a, b, it can be inferred that ζ-phase of cellular microstructure with the α-matrix discontinuously emerged near the grain boundary region at 300 °C. Moreover, Back Scattered Electron (BSE) images for annealing at 320 °C as shown in Fig. 1c confirmed the presence of δ-phase at the grain boundary regions, without exhibiting a cellular morphology. ε-phase was seen developing in the form of platelets inside the grains.
Experiments were conducted to investigate the formation of voids and its effect on IMCs [35, 36]. It was noted that the void density increased spontaneously, but tended to saturate at a fixed density, whereas the size of voids grew proportionally as a function of thermal annealing time. The above research conducted would permit the developers and manufactures to understand the reliability of joints and to determine the key parameters associated with void formation. Since the void formation is the main parameter that governs the reliability of a bond, several experiments on the bonding process in the Cu–Sn system was conducted. A fluxless Solid–Liquid Inter Diffusion (SLID) bonding process [5] was observed using Cu3Sn as the oxidation barrier for Cu interconnects, and the shear strength of the bond was noted. By ageing Cu and Cu/Cu3Sn (multilayer) film at a higher temperature in air, improvement in the oxidation behaviour of Cu3Sn (IMC) was confirmed. Shear strength of the bonded interconnect was 10 MPa, on average. It was found that, with the addition of Cu3Sn layer, the solid–liquid diffusion did not decrease in the bonding region.
An investigation was conducted on the evolution of microstructure in Cu–Sn coatings and by varying wt% of Sn content [3]. It was observed that the variation in wt% of Sn showed no effect in the surface roughness of Cu–Sn coating. Cu3Sn precipitates were observed all-over the Cu matrix of Cu–Sn coating. Moreover, the volume fraction of Cu3Sn precipitates increased with a rise in Sn content. Maximum adhesion strength was acquired at 5 wt%Sn that developed thick Cu–sulphide layer at the interface of rubber and coating. Panchenko et al. [6] investigated the grain distribution of IMCs (Cu6Sn and Cu3Sn) and orientation of crystals in miniaturized SLID interconnects for 3D stacking. Bonding temperature was set to 240–260 °C, subsequent thermal storage at different times were also maintained to determine the grain orientation of the phases. Initially, Cu3Sn texture was not visible during phase growth but with longer storage time and higher temperature Cu3Sn surface became more noticeable. Lin et al. [7] proposed the wetting behaviour of Cu and Cu–Sn IMCs coated with a thin Au layer. The main factor that enhanced the wettability in the bimetallic system was the reaction of oxide film on the surface of the metallic substrate. Driving force for spreading was due to the precipitation reaction of IMC. But, in the absence of the reaction of oxide film on a substrate, precipitation of the IMC layer doesn’t serve as the driving force for spreading.
2.1 Effect of Elevated Temperature
The thermal treatment of Cu–Sn alloy has a significant effect on the mechanical properties of the system and the resulting IMC layer formed after ageing. The morphological behaviour of Cu–Sn alloy at high-temperature deformation conditions was examined [4]. It was noticed that the Dynamic Recrystallization (DRX) was accountable for the flow behaviour of Cu–Sn alloy and the work hardening effect was neutralized at the deformation stage. Continuous grain generation was observed with small miss-orientation. The reaction of the Coincident Site Lattice (CSL) boundaries with normal grain boundaries generated Σ3 High Angle Boundaries (HABs). The thermal compression greatly varied with the distribution of CSL boundaries, which affected the mechanical properties, leading to good plasticity of the material. Moreover, the growth behaviour of Cu–Sn IMCs in Sn/Cu/Sn composite preform during ageing at 150 °C for different times was determined [8]. Amount of Cu3Sn increased with a rise in ageing temperature. Subsequently, Sn and Cu reacted and decreased gradually. Sn was fully depleted by the growth of Cu6Sn5. Cu3Sn phase grew at the expense of Cu6Sn5 that lead to a decrease in the thickness of Cu6Sn5. The total thickness of the IMC layer in the composite was determined by the square root of ageing temperature.
2.2 Diffusion Characteristics
The microstructure modifications due to solid-state reactions are largely based on diffusion processes at elevated temperatures. Long-term stability and ageing of materials are found to be important in high-temperature applications, which are controlled by diffusion. The diffusion coefficient of ε and η phases was studied by annealing Cu–Sn diffusion couples at 190–200 °C, to measure Kirkendall effect of the system [9] and the movement of Kirkendall markers towards the Sn side confirmed that the preferential diffusion of Sn atoms occurred by vacancy mechanism. On annealing the couples (Cu–η, α–η, δ–η) only one ε-phase layer was seen in the diffusion zone of each couple. Compared to other couples, ε-phase in the δ-η couple grew at the highest rate, though it had the smallest diffusion coefficient. Active growth of the ε-phase layer occurred at the concentration gap between the ε-phase and its adjoining phases. Moreover, the intrinsic and inter-diffusion coefficient of Cu and Sn in ε and η phases at 200 °C. Instability at the interfaces (Cu3Sn/Cu6Sn5 and Cu6Sn5/Sn) was because of high anisotropy in the diffusion behaviour of the phases [10]. This also resulted in an uncertainty in the position of the marker, causing variation in the intrinsic diffusivity of Cu and Sn. Hence, Cu dispersed readily in the Cu5Sn6 and Cu3Sn phase.
The evolution of the intermetallic layer at the interface of electrodeposited Sn–Pb coatings on Cu alloys [11]. Annealing time and temperature were the factors that influenced the degree of formation of Cu–Sn IMC layers. Grain boundary diffusion and defect diffusion controlled the rate of Cu mass transport in the Sn coating at a lower temperature. Growth mechanism of different phases in the Cu–Sn system was identified and the activation energies for IMCs were found out [12]. The results had confirmed that Sn and Cu diffused faster in Cu6Sn5 and Cu3Sn phases respectively. Moreover, Bader et al. [13] inspected the phenomenon of inter-diffusion in Cu–Sn systems. Inter-diffusion coefficients for the IMCs were calculated for Cu–Sn diffusion couples at 130–200 °C [14]. The self-diffusion characteristics of Cu and Sn in single-crystal Cu3Sn ordered alloy. The Cu–Sn system had a composition of 15 at% in the β-phase region and of 16.7 at% in the γ-phase region. It was also inferred that the diffusivity of Cu was larger than Sn in both β and γ phase alloys [15].
2.3 Effect of Electric Current
The energy fields such as the electric and magnetic fields, as an effective external field, can affect the phase transformations, grain refinement, the formation and growth of the intermetallic compound by interfacial diffusion reactions, recrystallization, constituent phase dissolution and transformation during homogenization, phase precipitation during ageing, phase stability, and phase morphology in metals and alloys The orientation of grains and mechanical properties of Cu–Sn IMC joints by varying electric current was studied [16] and the Cu/Sn/Cu joints were subjected to an electric current density of 2 × 102A/cm2 at 200 °C. It was confirmed that the growth and orientation of Cu3Sn IMC were least affected by electric current, with the completely formed Cu3Sn IMC after 360 min. While the time required for the complete formation of Cu6Sn5 IMC was 60 min. Moreover, yield strength (YS) and microhardness values for each intermetallic layers were found out and concluded that the joints formed under 2 × 102A/cm2 possessed the best shear strength (44.87 MPa). Furthermore, the mechanism for the development of Cu–Sn alloy coatings was processed by Pulsed Current (PC) electrodeposition method [17], this experiment was to affix the use of bronze alloy in automobile and aerospace industries. Cu–Sn alloy coatings were composed of Cu–Sn electrodeposition and η (IMC) dual-layer. When compared to Direct Current (DC) electrodeposition, PC electrodeposition was advantageous for electrodeposition of Cu. Amount of Cu present in Cu–Sn alloy coatings was found to be inversely proportional to electrodeposition current density. This increase of Cu content in Cu–Sn coatings was due to the metal displacement reaction between Cu and Sn during relaxation time.
3 Different Forms of Bronze Alloy and Their Properties
Bronze in wrought condition are of different types and these types of Cu–Sn bronze possess different properties. The types and properties of these wrought Cu-based alloys are described below.
3.1 Cu–Sn Thin Films
The properties and kinetics of IMC formed between Cu–Sn thin films are being discussed below since mechanical characterization of thin films has become more crucial for a wide range of applications such of these films in microelectronic packaging and MEMS technology. The phenomenon of inter-diffusion and IMC formation in Cu–Sn thin films was investigated [37]. It was noted that during annealing at − 2–100 °C, Cu6Sn (η) phases was present at all temperatures while the Cu3Sn (ε) phase was formed only when the temperature was greater than 60 °C. Inter-diffusion of Cu in Sn enhanced the order at the growth front of Cu6Sn5. Sn whiskers grew on the surface of Sn films with Cu as under layer, and the driving force for the evolution of Sn whiskers was due to the reaction in Cu–Sn films and inter-diffusion. The interfacial reactions that took place in bimetallic Sn/Cu thin films were noted and the study [38] confirmed the presence of ƞ-phase at room temperature and combination of both η and ε phases at 150 °C, along with a discussion of sputtering effects and film composition. The kinetics for the evolution of Cu6Sn5 between Cu/Sn thin films and Cu3Sn formation between Cu/Cu6Sn5 was noted [39]. At room temperature, growth of Cu6Sn5 took place with the diffusion of Cu atoms. Growth of Cu3Sn was observed with a parabolic reduction of Cu6Sn5 at 115–150 °C. Moreover, this study [39] proved that the high-temperature phase (Cu4Sn) can be made metastable by annealing at room temperature. Furthermore, the spontaneous interfacial reaction in Cu–Sn thin films to demonstrate the effects of mobility in irreversible processes was determined [40]. Mechanical stresses and chemical affinity were the forces that drove the fluxes of Cu and Sn, though the outcome of the irreversible process can be changed by atomic mobility. Hillocks grew when the reaction temperature was kept at 150 °C. Stress relaxations were affected by the oxides formed on the surface of Sn film. There were certain weak spots on Sn film surface where the oxides were broken and the whiskers grew upon them, which means, inhomogeneous relaxation took place. The kinetics of compound formation in bimetallic Cu–Sn thin films was investigated and the study [41] confirmed that the interstitial transport of Cu in Sn and grain boundary diffusion of Sn in Cu was responsible for the evolution of IMCs. The microstructure of Cu3Sn phase in the Cu layer was found to be non-planar, while linear Cu6Sn5 compound growth kinetics was determined for short diffusion at 86 °C.
Auger electron spectroscopy was incorporated in a study conducted by Chopra et al. [42] to explain about the shift in marker position for Cu–Sn thin-film diffusion couples, which was attributed to compound interface drag effect. It was confirmed that 1 at% of Sn in Cu alloy films possessed the best resistance to electromigration, though bulk cases were of growing concern as it can be used for the application of solder bumps as interconnects for packaging high-power devices. Hence, a review on the interfacial reactions taking place in thin-film case and the bulk case was studied [43].
3.2 Powder Cu–Sn Alloys
Copper and copper alloy powders have been used extensively in industrial applications for many years. Probably the best known is the self-lubricating bearing which was the first major application and still accounts for about 70% of the granular copper powder used. This application takes advantage of the ability to produce a component with controlled interconnected and surface-connected porosity. The effects of temperature and pressure (processing parameters) on the physical and microstructural characteristics of porous Cu–Sn alloys were produced by pressure sintering [44]. Variation of hardness was explained along with the secondary phase determination and its morphological behaviour. Figure 2 depicts the microstructure of the processed alloy at T = 250 °C, P = 75 MPa for 30 min. This confirms the non-homogeneous structure for the resultant alloy with a generalized porosity. Furthermore, Fig. 3 shows the linear variation of hardness with a rise in processing parameters (pressure and temperature) for three different holding times.
Optical micrographs of the processed alloy at T = 250 °C, P = 75 MPa, t = 30 min [44]
Variation of hardness for different holding time with respect to a temperature, and b pressure [44]
The harmonic-structured Cu–Sn alloy was fabricated by mechanical milling (MM) and the spark plasma sintering (SPS) process. After the SPS process on the taken composite MM powders, a harmonic-structure comprising of a network of bronze and a dispersed area with pure copper was resulted (Fig. 4). The dotted line in Fig. 5 depicts the upper limit of the rule of mixture between pure copper and only bronze. Hence, it was also confirmed that the hardness of the harmonic-structured Cu–Sn alloy was found to improve.
OM micrograph of the harmonic structured Cu–Sn alloy with pure copper and bronze [45]
Vickers hardness of harmonic-structured Cu–Sn alloy with various bronze volume fraction [45]
The variation in mechanical properties during spray forming and cold rolling of Cu–13.5 wt%Sn alloy was compared with cast ingots [46] and Fig. 6 confirms that the sprayed powders had a regular surface with fine dendritic structure. Whereas, Fig. 7 depicts a fine equiaxed morphology of the spray formed microstructure with a grain size of 32 μm, whereas permanent mould cast samples possessed dendritic structure with Sn-rich δ phase in between the dendrites. After cold rolling of the spray formed specimens, there was a decrease in pores. This resulted in homogeneous single-phase as shown in Fig. 8 with excellent workability. Elastic modulus and flow stress for the cold-rolled specimens was found to be 88GPa and 800 MPa, respectively. These combinations of properties made them usable in spring field technology.
Microstructure of the powders of a 10 μm, and b 50 μm in diameter [44]
Microstructure of Cu–15 wt%Sn alloy a as-deposited, and b permanent mold cast [44]
Microstructure of cold-rolled Cu–15 wt%Sn alloy [44]
The monodisperse Cu–Sn alloy powders [47] were synthesized through the reduction of mixed metal oxides (CuO–SnO2 powders) coupled with in situ de-wetting of liquid–solid interface. Liquid Cu–10%Sn alloy de-wet graphite surface was annealed at 1273 K. It was noted that the Cu–Sn particles were seen smooth and well rounded, though Cu and Sn in the alloy were evenly distributed. Meanwhile, Nassef et al. [48] manufactured four Cu–Sn metal matrix composites of different composition (Cu–10%Sn–5%C, Cu–10%Sn, Cu–10%Pb and Cu–10%Sn–10%Pb) using hot pressed powder alloying process. From the investigation conducted, the effect of Carbon (C) and lead (Pb) on the mechanical properties of composites were determined as shown in Table 1. Hot pressed Cu–Sn alloy specimens contained homogeneous α and δ IMC phases. From Table 2, it is evident that the addition of carbon improved the wear resistance of Cu–Sn alloy but lowered the compressive properties. Cu–Pb alloy possessed higher mechanical properties while Cu–Sn alloy had higher wear resistance when compared to other alloys.
The liquid-phase sintering (LPS) on multilayered Cu–Sn particles were processed by mechanical alloying [49] in order to examine the phase formation. Wettability of liquid Sn on solid Cu particles was enhanced by the multilayer Cu–Sn particles during LPS at 400 °C for 15 min. An amorphous Cu–Sn layer was developed at the initial stages of sintering. Composition of the amorphous layer was Cu2.48Sn with small Cu3Sn particles that existed between Cu and Cu6Sn5 layer.
3.3 Shape Memory Effect of Cu–Sn Alloy
The shape memory alloys (SMAs) and shape memory technology have been developed for high-temperature applications due to their ability to return to pre-deformed shape after heating above the transformation temperature, as well as these alloys have a small hysteresis and high transformation temperature comparing with other shape memory alloys. The shape memory effect on Cu–Sn wrought alloys were studied and an investigation was conducted on the characteristic transformation temperatures (Ms, Mf, As and Af) and shape memory effect of Cu–15%Sn alloy [50]. It was noted that the extent of reverse martensitic transformation and shape recovery was reduced on martensitic ageing. While complete reverse transformation and shape recovery was possible with high heating rate from the martensitic condition. Specimens were moderately aged in the parent phase and had no drop in their As and Af temperature when the martensitic heating was increased. Parent phase ageing increased the extent of reverse transformation and shape recovery and was improved with longer ageing durations. Moreover, Mf temperature was dependent on the parent phase ageing duration and temperature. The shape memory effects of Cu–40%Sn alloys processed by melt-spinning technique reveals the spontaneous solidification was achieved by melt-spinning, and the melt had a noticeable influence on the pseudo-elastic properties of the martensitic transformation of the alloy. Melt-spun Cu–40%Sn alloy exhibited the shape memory effect upon heating [51].
Similarly, the shape memory properties and oxidation behaviour of Cu–Sn ribbons, which were rapidly liquid quenched existed the shape memory behaviour when the deformed martensite region was heated at room temperature [52]. Total oxidation percentage was decreased with a rise in heating rate. Austenite phase samples (ribbons) transformed to martensite phase when the samples were kept in liquid nitrogen. Austenite starting temperature (As) and austenite finishing temperature (Af) was determined as − 16.4 °C and − 8.4 °C, respectively. The morphological changes were observed for Cu–15%Sn SMA by ageing at 323–373 K [53] and it also gave the behaviour of Cu–15%Sn SMA, which was quenched from β-region and aged at a lower temperature [54]. From the observations made by a dark field imaging technique, the presence of two phases in the DO3 matrix was confirmed. Annealing at 373 K indicated the presence of L-phase, and the phase possessed four variants in BCC matrix. When the alloy was annealed at 323 K for a longer period of time presence of an unidentified phase was confirmed, which produced extra diffraction spots of unusual shapes.
3.4 Anisotropic Behaviour of Cu–Sn Wrought Alloys
When the properties of a material vary with different crystallographic orientations, the material is said to be anisotropic. When a material is formed, the grains are usually distorted and are found to be elongated in one or more directions which makes the material anisotropic, Selective laser melting (SLM) on Cu–15Sn high-tin bronze alloys were conducted for determining its microstructural evolution, anisotropic properties and mechanical properties. Statistical analysis was adopted to obtain a relationship between the processing parameter and the density of the fabricated alloys. The fabricated wrought bronze alloys were compared with the QSn15-1-1 (GB/T 5231-2012) drawing specimens. The morphological behaviour of the developed wrought Cu–Sn revealed an ultra-fine grain structure as shown in Fig. 9.
Observations under SEM of Cu–15Sn samples a–d before annealing, and e, f after annealing for 4 h [55]
The ultra-fine grain structure observed in the wrought Cu15Sn bronze is attributed to the high cooling rate happened during the processing of Cu15Sn wrought bronze alloy through the SLM process. Figure 10a confirms that a variation in the tensile properties greatly affected the structure of the wrought alloy. These changes in the structure in the developed wrought alloy were owed to grain coarsening and decomposition of the intermetallic phase. It is likely that anisotropy characteristics in mechanical properties would be formed in the building direction of the crystallographic structures. Strengthening behaviour has changed from fine grain to solution strengthening mechanism. Fractured SEM images of the wrought alloy developed at various annealing conditions are depicted in Fig. 10b–e. This shows that the developed wrought alloy possessed rough surfaces lacking obvious cleavage steps. Furthermore, shallow dimples and partial deep dimples like features can be observed from the fracture images.
a Stress–strain plot for wrought specimens b–e fractured surface of wrought specimens [55]
3.5 Molten Cu–Sn Alloy
Different grades of wetting are required depending on the application. The surface tension and contact angle are characteristic of the wetting behaviour of molten metals on the solid surface The wetting behaviour and surface tension of molten Cu–Sn alloy was studied [56] and the surface tension was measured for 11-different alloy compositions as a function of temperature, and it was found that the surface tension was noted to rise with increasing Cu content. Analysis of the wetting behaviour of Cu–Sn alloys proved that with an increase in Sn content there observed a significant drop in contact angle. On the other hand, substrate erosion took place with a decrease in Sn-content. The development of medium-range order (MRO) in Cu–Sn alloy by melting the alloy at superheated temperature was initiated [21]. It was found that the evolution of MRO was nearly related to the Cu–Sn binary phase diagram. Compound formation ability was most effective at Cu5Sn in the binary phase diagram. Moreover, MRO disappeared from the structure of Cu–Sn melts at 35.3–60 wt% of Sn.
3.5.1 Solidification Characteristics
Alloy solidification rate controls the fineness of the structure. The form and distribution of the phases are controlled by the concentration of the main alloying elements and impurities present. The enthalpies of fusion and liquids temperature of Cu–Sn alloys were studied using the Differential Scanning Calorimetry (DSC) [57]. It was evident that the enthalpy of fusion for Cu–Sn alloys was linked to the primary solid phase of peritectic Cu–Sn alloys. Transformation of coarse grain dendrites to fine grains (primary α phase) occurred when the rate of undercooling was increased in Cu22 wt%Sn. The monotectic transformation (γ → α+L) was found to be an exothermic process on undercooling Cu42.5 wt%Sn to 70 K at a rate of 5 K−1. A two-phase microstructure comprising ε and ƞ-phases were observed in the solidified monotectic alloys, whereas ƞ-phase was found at the boundaries of ε and smaller particles of ƞ inside the ε-phase. The peritectic solidification process of Cu–70%Sn alloy under an ultrasonic field of 20 kHz, based on morphology versus ultrasound power was noted [58] and the nucleation in primary ε phase was enhanced by the effect of ultrasonic fields, where its grain size was found to be refined. Further increment in the power of ultrasonic fields turned these refined grains into equiaxed shape (as shown in Figs. 11 and 12). Improvement in the mechanical properties confirmed that the ultrasonic field enhanced the performance of the peritectic alloys to a great extent.
Microstructure of solidified Cu–70%Sn alloy for top and bottom of samples processed under a, b static condition, and c, d 110 W ultrasound [58]
Microstructure of solidified Cu–70%Sn alloy for top and bottom of samples processed under a–c 220 W ultrasound, and d–f 440 W ultrasound [58]
The isothermal Peritectic Coupled Growth (PCG) in directionally solidified Cu–20 wt%Sn alloy (temperature gradient = 45 K/mm, pulling rate = 1 μm/s) was investigated [59]. Composition of the liquid phase at PCG front was analyzed and confirmed that there was a negative growth undercooling and diffusion coupling at the PCG interface front. Lu et al. [60] examined microstructures of the directional solidified Cu55Sn hypoperitectic alloy, and confirmed the presence of peritectic ƞ phase, primary ε phase and eutectic (ƞ + Sn) as phase constituents when directionally solidified at a rate of 0.5–100 μm. Solidification criteria was important for getting the complete characteristics of electronic interconnections, hence Xian et al. [61] put forward an experiment to examine the crystal growth mechanisms of Cu6Sn5 during solidification of electronic interconnections.
3.5.2 Phase Diagram
A phase diagram shows the limiting conditions for solid, liquid, and gaseous phases of a single or a mixture of substances while changing pressure and temperature or some other combination of variables, such as solubility and temperature. They are invaluable when designing industrial equipment and seeking optimum conditions for manufacturing processes, and in determining the purity of substances. The evolution of the MRO in Cu–Sn alloy which relates to the Cu–Sn binary phase diagram was investigated [21] and the compound formation ability was most effective at Cu5Sn in the binary phase diagram. It was also observed that for different Cu–Sn melts, the MRO was not stable. Hence, Furtauer et al. [22] developed a phase diagram for the Cu–Sn system and the purpose was to determine two high-temperature phases (β andγ) and their phase-relations. From XRD analysis and previous literature [23], it was concluded that the β-phase transformed into γ-phase by a higher-order reaction. Figure 13 depicts the proposed Cu–Sn phase by Furtauer et al. [22].
Cu–Sn binary phase diagram as suggested by Furtauer et al. [22]
But, Li et al. [24] investigated and developed a new thermodynamic description for Cu–Sn binary system. The Thermodynamic description proposed helped to model higher-order phase transformation from the β-phase (disordered) to γ-phase (ordered). Moreover, the calculated results were in terms of the literature data as shown in Fig. 14.
4 Different Processes Have Undergone and Its Effects
The major process used for investigating different properties of wrought Cu–Sn, Cu–Sn–Zn and Cu–Sn–Ti alloys are discussed below.
Sl. no. | Process undergone | Author and reference | Experiments and major conclusions |
|---|---|---|---|
1 | Solid–liquid interdiffusion (SLID) bonding | Munding et al. [62] | Explained the underlying principle of SLID bonding and its general characteristics |
Liu et al. [63] | Phase transition and metallographic behaviour at 400 °C were investigated. When the CuSn films were heated at higher-temperature ramp rate annealing, island-shaped IMCs were formed (diameter of 300–600 μm). Low ramp rate annealing is done before the bonding process will reduce the risks of fracture and voids. The activation energy (Q) and the intrinsic diffusivity (k0) of CuSn system were estimated to be 100 kJ/mol and 2.5 × 10−10m2/s, respectively | ||
Rautiainen et al. [64] | Vertical cracking and voiding phenomenon during the Cu–Sn SLID bonding process was explained using the thermal shock test | ||
Panchenko et al. [65] | Degradation of Cu6Sn5 increased with a rise in temperature and was found to degrade completely at 240 °C. This contributes to the spreading of pores from the edges exposed to the flux towards the centre, thereby suppressing the growth of Cu3Sn phase. Moreover, thermal storage in an N2 atmosphere promoted the transformation of Cu6Sn5 IMC layer to Cu3Sn IMC with a reduced amount of pores | ||
Liu et al. [66] | Wafer-level Cu–Sn SLID bonding process was an efficient method for 3D assembly and packaging. Interconnects possessed resistance of 100 mΩ and shear strength of 45 MPa at 260 °C. Uniformity of the electrochemical deposition was critical for obtaining the prerequisite bond strength and can be attained by combining pulse-reversed plating and by the optimization of an electroplated mask | ||
2 | Transient liquid phase bonding | Liu et al. [67] | Growth behaviour of Cu6Sn5 during the TLP bonding process was explained |
Bosco et al. [68] | Toughness and strength of Cu–Sn system fabricated by TLP bonding were explained. Uniform layer of δ-phase was formed on heating at 400 °C for 4 h, which exhibited a high strength (300 MPa) and low toughness (5 MPa√m). These properties can be improved to 30% by additions of Cu, i.e.: δ phase should be converted to δ + (Cu) microstructure thereby resulting in strength of 400 MPa and toughness of 7 MPa√m. Furthermore, TLP bonding done at 600 °C for 20 h will result in uniformly distributed solid Cu solution with enhanced toughness (13 MPa√m) and decreased strength (200 MPa). This experiment gave the functionality of Cu–Sn system for different temperature–time combinations that made them suitable for high-temperature bonding application between ceramic materials and wide bandgap devices | ||
Bosco et al. [69] | The critical interlayer thickness that would help to limit the formation of pores on the resulting bond was determined, which was found to be dependent on the height of the largest intermetallic grain. It was confirmed that there will be a rise in the time required to attain the intended microstructure whenever the magnitude of interlayer thickness exceeds critical interlayer thickness. Furthermore, an increase in heating rate along with a rise in bonding temperature will result in a significant reduction in critical interlayer thickness and time required for the bonding process | ||
Park et al. [70] | Phase-field simulations of IMC growth inside Cu/Sn/Cu sandwich structure under isothermal TLP bonding at 533 K was explained | ||
Park et al. [71] | Prediction of processing maps for TLP bonding in microelectronics packaging was proposed in this experiment | ||
3 | Annealing | Fan et al. [72] | Influence of annealing temperature on the microstructure and mechanical properties of the bronze alloy was investigated |
Takenaka et al. [73] | Evolution of IMCs in Sn/Cu/Sn diffusion couples during annealing at 433–473 K was investigated, and found that volume diffusion was the rate-controlling process for the reactive dispersion at higher annealing temperature and grain boundary diffusion at lower annealing temperatures | ||
4 | Hot pressing | Nassef et al. [48] | Experimented on the microstructural properties and mechanical behaviour of hot-pressed Cu–Sn powder alloys |
Han et al. [74] | Effects of hot pressing on the morphology of Cu–20 wt%Sn alloy was investigated. Variation in the magnitude of hardness and wear resistance was studied at 420, 520 and 600 °C | ||
5 | Ageing | Sobiech et al. [75] | Microstructural evolution, whiskering and interface thermodynamics during room temperature ageing process were explained in this study [100]. |
Kato et al. [76] | Influence of ageing on the pseudoelastic behaviour of Cu–15 wt%Sn single crystal alloys was explained. | ||
6 | Sintering | Dyachkova et al. [77] | Gave information regarding the microstructure, strength and wear behaviour of Fe-based P/M composites after sintering with Cu–Sn alloy |
Ruangdaj et al. [49] | Developed the characterization of liquid-phase sintered materials made from mechanically alloyed Cu + Sn powder compacts | ||
7 | Power spinning | Hui et al. [78] | Effects of annealing temperature and power spinning on the microstructural properties of Cu–Sn alloys was investigated and confirmed that power spinning increased the hardness and strength of bronze alloy without affecting ductility adversely |
Liu et al. [79] | Power-spun specimens of bronze alloys were treated at 200–400 °C for 0.5 h and the variation of microstructural properties was analyzed. There was a significant decrease in grain size when the temperature was set to 200–300 °C while setting up of temperature to 400 °C had resulted in increased grain size. Thus, high-temperature annealing improved the plasticity of bronze alloy by changing grain size and grain distribution through static recrystallization. Moreover, temperature above the upper critical recrystallization temperature (300 °C) had adversely affected YS and ultimate tensile strength (TS) of bronze alloys | ||
8 | Reduction diffusion method | Murase et al. [80] | Suggested the method for the preparation of bronze layers on polymer substrate using ionic liquid baths by the reduction–diffusion method |
Ito et al. [81] | Bronze alloy metallization of polymer substrate by a reduction–diffusion method in an ionic liquid bath at medium and low temperatures was explained by this study [106] | ||
9 | Induction heating | Yin et al. [82] | Investigated the accelerated formation of Cu3Sn in a Cu/Sn/Cu solder joint by the inductive heating process, under lower pressure (0.01 MPa) at ambient temperature. This resulted in enhancement of interfacial reaction at the Sn/Cu interface, i.e.: Cu6Sn5 grew from scalloped-like to dendritic-like microstructure, and the planar-like Cu3Sn grew to columnar-like microstructure in transition |
10 | Brazing | Buhl et al. [83] | Influence of brazing parameters (dwell duration and brazing temperature) on the properties of diamond-steel-joints was pointed out. It was found that the residual stress depends on the brazing temperature largely, and with longer holding, time and higher brazing temperature there was a drop in the shear strength of diamond-steel-joints |
11 | Hot extrusion | Hui et al. [84] | Grain structure and tensile properties were studied and found that a rise in temperature increased the size of sub-grains, and lead to nucleation and growth of recrystallized grains that further promote the growth of deformed grains. Hence, the competitive nature of growth was observed between deformed and recrystallized grains. Moreover, temperature beyond 700 °C would lead to overgrowth of deformed grains and will deteriorate the properties of the alloy |
12 | Hot compression | Hui et al. [85] | Gave the mechanical properties of bronze alloy that had undergone hot compression along with the influence of hot-compression on the distribution of Ʃ3n boundary |
The structure of β-phase and its decomposition products in Cu–14.8 wt%Sn (quenched) alloy using TEM was studied [86] and the observations revealed that the Cu–14.8% Sn matrix contained precipitates of α, δ or η phase with retained phase (β2), which were formed as a product of beam-heating in the electron microscope. Kuwano et al. [87] investigated the characteristic of martensite formation in Cu–15% Sn alloy. Furthermore, Cortie et al. [88] investigated on the decomposition behaviour of β phase in the Cu–Sn system.
5 Additive Manufacturing of Cu–Sn Alloys
The Cu–10Sn alloy specimens were fabricated by selective laser melting [139] (SLM) and phase formation process. The microstructure and mechanical properties of the SLM specimens were investigated and was compared to the corresponding material produced by casting process. Refined grains were observed as compared to the cast specimens in the SLM specimens, which was due to the high cooling rate characteristics of the process. The room-temperature mechanical properties were greatly influenced by the refined microstructure. The yield and ultimate tensile strength increased from 120 and 180 MPa (Cast specimen) to 220 and 420 MPa (SLM specimen). Moreover, improved ductility was observed in the SLM specimen. The observed enhancement in mechanical property proves the effectiveness of SLM and the capability of producing high-performance Cu based components by additive manufacturing. Another research was reported by Karthik et al. [140] on additive manufacturing of Cu–13Sn alloy by Selective Laser Melting [140] (SLM). Nano-precipitation and exceptional combination of high strength and ductility was noted in the alloy. The SLM processed Cu–13Sn was observed with improved microstructure compared to Cu–13Sn produced by casting. The observed microstructural characteristics were as follows: very fine cellular structure, spherical nano-precipitates, fine grain size and spatial heterogeneous microstructure. This lead to effective stress (nano-precipitates and fine grains) and back stress of fine second-phases and spatially heterogeneous microstructure which contributed to realizing high strength (~ 635 MPa) of the alloy. The SLM samples exhibited excellent tensile properties with minimum anisotropy properties and good strength-ductility trade-off. Thus the study shows the capability of SLM in controlling microstructure and developing novel high-performance material for critical application.
6 Applications of Bronze Alloys
High-temperature applications of the bronze alloy were reported [4, 8]. Some of the high-temperature applications involve automobile engine parts and turbine parts. Moreover, Hang et al. [89] came up with the grain orientation and phase transformation of Cu–Sn IMCs during the bonding process at low temperature, to affix its applications in low-medium temperature applications.
The microhardness, morphology, and tribological behaviour of Cu–Sn nanocomposites reinforced with carbon nanotubes (CNTs) were investigated [90]. Nanocomposites showed an increase in hardness with a rise in the mass fraction of CNTs but decreased electrical conductivity of the nanocomposite. At 2%CNT content, the nanocomposites showed a decrease in wear loss and friction coefficient by 68% and 72%, respectively, when compared to the commercial Cu–Sn alloy. Hence, this study can be incorporated for high-wear requirement applications like Cu–Sn alloys and its composites can be used in bearings and bushes in automobile applications. Yoshida et al. [91] investigated the corrosion resistance, anti-fretting characteristics, coefficient of friction and solder wettability of Cu–Sn alloy plating. This confirmed that the Cu–Sn plating possessed excellent anti-fretting characteristics, equivalent coefficient of friction, and good corrosion resistance that made them useful as Sn plating can be done on the surface of automobile terminals to prevent corrosion. Subrahmanian et al. [92] investigated the Cu–Sn alloy coating on mild steel substrate processed by a brush plating method. Results showed that the plated Cu–Sn alloy exhibited excellent corrosion resistance that made them applicable in marine industries.
Cu–Sn alloy sheets were fabricated by hot-rolling process [93] and an investigation was performed to determine the antibacterial properties of the alloy. A single-phase solid solution was maintained by the alloy sheets, which contained less than 15%Sn after hot-rolling. Alloy with 22%Sn possessed decomposition of solute atoms. With a rise in Sn content, there was a drop in corrosion potential of hot-rolled Cu–Sn sheets, thus they had more effective antibacterial properties than the as-cast Cu–Sn alloys because of supersaturation, and can be used for manufacturing medical apparatus because of its superior antibacterial properties. Kim et al. [94] investigated the influence of Sn doping on the nucleation of Cu films on TaN substrates. It was observed that pure Cu films agglomerated less than Cu–Sn alloys, and from Figs. 15 and 16, it can also be inferred that the agglomeration behaviour was more pronounced when done in H2 ambient environment rather than a vacuum environment. Figure 17 proved that with increasing Sn content, the melting point of the resultant alloy decreased. This decrease in the melting point of Cu–Sn alloy led to the coalescence of Cu nuclei and a decrease in nucleation density thus making it a possible applicant for manufacturing highly reliable interconnects. Research conducted had proved that the application of Cu–Sn alloy in MEMS and NEMS devices as it possessed good wettability and made strong interconnections between the substrates [95].
SEM images of a pure Cu, b Cu–5 at%Sn alloy, c Cu–10 at%Sn alloy on TaN substrate when annealed at 550 °C for 60 min in vacuum environment [94]
SEM images of a pure Cu, b Cu–5 at%Sn alloy, c Cu–10 at%Sn alloy on TaN substrate when annealed at 550 °C for 60 min in H2 ambient [94]
Variation of the melting point of Cu alloy with additions of Sn [94]
7 Effect on Cu–Sn Due to Additions of Other Elements
Effect of Sn content on bronze alloy was crucial for affixing its application in manufacturing material for diamond grinding wheels. Self-sharpening property of the alloy was obtained when Sn content was made to 25%. An investigation about the variation in microstructural properties of 75%Cu–25%Sn alloy during the additions of Ni, Fe and Co was conducted [96]. The hot-pressing temperature of 700 °C had resulted in a brittle type of fracture for all of the specimens like additions of Ni, Fe and Co on 75%Cu–25%Sn alloy. Weight loss of the specimen was greatly achieved on adding Co to 75%Cu–25%Sn alloy. Moreover, dissolution criteria for all the three specimens were also investigated. Furthermore, an increase in the wear resistance and transverse rupture strength was achieved by the addition of Ni element. Wang et al. [97] explored the abnormal growth of Cu3Sn through Cu6Sn5 grain boundaries in Ni/Sn/Cu (ternary) diffusion couple. It was observed that (Cu, Ni)3Sn phase grew with a layered morphology before the complete depletion of Sn. Along the grain boundary of (Cu, Ni)6Sn5 there was enriched Cu which made a favourable condition for the formation of (Cu, Ni)3Sn at the expense of (Cu, Ni)6Sn5, thereby affirming its use in microelectronic chip stacking technology. Schmetterer et al. [98] proposed four isothermal sections of Cu–Ni–Sn ternary system at various temperatures (220,400,500 and 700 °C) and the study also confirmed the presence of two ternary compounds at 25%Sn concentration, as proposed by Lee Park et al. [99].
Outstanding superconductive property of Nb3Sn compound made them usable in diverse applications. Muranishi et al. [100] had isothermally annealed Cu–8.3Sn/Nb/Cu–8.3Sn diffusion couples from 923 to 1053 K for 1038 h. Since the growth of Nb3Sn layer was towards Nb and scarcely towards Cu–Sn alloy, the growth rate of Nb3Sn layer was found to be dependent on the migration rate of Nb3Sn/Nb interface. The equation for finding the thickness of Nb3Sn layer was also coined by Muranishi et al. [99]. The experiment done on the same diffusion couples by Osamura et al. [101] revealed that the grain boundary diffusion facilitates interdiffusion at T = 923–1023 K, while inter-diffusion was found to be predominated by volume diffusion when the temperature was made to 1053 K. Yamashina et al. [101] theoretically examined the phase equilibrium of Cu–Nb–Sn ternary system to quantitatively account for the occurrence of uphill diffusion of Sn from Cu–Sn alloy to Nb3Sn at 1000 K (annealing temperature). Because of the reactive diffusion between Cu–Sn alloy/Nb, the Nb3Sn layer was formed, and the uphill dispersion took place from the Cu–Sn alloy to Nb3Sn. [102] also coined an equation to find the chemical driving force for the uphill diffusion and explained the growth behaviour of Nb3Sn.
Wang et al. [103] aimed to minimize the negative effect of Zn element in Sn–0.7Cu solder alloy by additions of Zn. Small additions of Zn had refined the microstructure and facilitated the growth of IMC layer at the solder/Cu interface during thermal ageing. Further additions of Zn (1.0 wt%of Zn) changed the composition of IMC (from Cu–Sn IMC to Cu–Zn IMC) at the interface. Moreover, Yin et al. [104] developed the effects of β-Sn3N4 whiskers on the mechanical properties of β-Sn3N4/Cu/Sn composite, which was processed by powder metallurgy. Addition of β-Sn3N4 whiskers increased the bending strength and hardness of the composite. The thermal conductivity was also high but less when compared to the Cu–Sn alloy. The whiskers (β-Sn3N4) also improved the work of fracture of the composite and brought grain refinement in the matrix, subsequently enhancing the mechanical properties of the composite.
Vuorinen et al. [105] explored the influence of Ti on the interfacial reactions between Sn–Ag solder and Cu substrates. After 3000 h of solid-state annealing, it was observed that the existence of Ti in solder doesn’t influence the growth of IMCs (Cu6Sn5 and Cu3Sn). However, the presence of Ti caused unevenness in the formed IMCs. Since Ti had very low solubility to Cu–Sn IMCs. Ti alloys possess exceptionally high corrosion resistance and high strength to weight ratio that made them useful in aerospace and automobile industries. Effect of Ti addition on bronze alloys and the characteristics of Cu–Sn–Ti alloy was of growing concern to widen its applications.
8 General Characteristics of Cu–Sn–Ti Alloy
Outstanding specific strength, non-toxic and non-allergenic nature of Ti alloys affix their application in biomaterials. The extent of corrosion resistance governs the life of alloy. Tsao et al. [18] solution treated (ST) Ti7CuxSn alloys (where x varies from 0 to 5 wt%) at 1000 °C for 2 h and quenched in water. Initially, Tu7Cu had martensite structure which was further refined with additions of Sn. After ST, there was the presence of pseudo-dendritic α-Ti phase in Ti7Cu5Sn alloy. Potentiodynamic polarization curves proved the improvement of corrosion resistance in the resultant alloy when Sn content was set to 5 wt%.
Nowadays, electrical industries are making use of connections with superconducting Nb3Sn composites wires. The experiment conducted on sandwich diffusion couples of CuSn/Nb/CuSn was aforementioned [100] and important conclusions were listed out. Since Ti is an element having excellent conductivity and toughness at high temperatures, the effect of Ti additions on the evolution of Nb3Sn layer was investigated by Hayase et al. [106]. The study experimentally proved that the addition of Ti increased the growth of Nb3Sn layer to a significant value. It was also noted that this growth of Nb3Sn layer took place only at high annealing temperatures, and confirmed that inter-diffusion took place in a direction perpendicular to the initial CuSn/Nb interface. Mikami et al. [107] had diffusion bonded Nb and Cu–9.3Sn–0.3Ti, and isothermally annealed the diffusion couple at 923 and 1053 K for 843 h. Rate controlling process was the migration of Nb3Sn/Nb interface due to the increased growth rate of Nb3Sn layer towards Nb. Moreover, an equation was derived [107] to determine the thickness of Nb3Sn layer that was formed at the interface. Evolution of Nb3Sn layer and reactive diffusion between the compounds were investigated, as they were the influencing factors for the conducting property being exhibited by Nb3Sn composite wire. In a study conducted Cu7.5SnTi/Nb/Cu7.5SnTi was considered as diffusion couples at T = 973–1073 K and investigated the improvement in superconducting property with additions of Ti into Cu–Sn alloy [108]. Tejima et al. [109] had reported that Tantalum (Ta) acts as an effective diffusion barrier against penetration of Sn into the Cu stabilizer from the bronze in superconducting Nb3Sn composite wire. Tejima et al. [109] made use of Cu9.3Sn0.3Ti/Ta/Cu9.3Sn0.3Ti as diffusion couples at T = 973–1053 K for 1462 h. Formation of Ta9Sn layer between Cu–Sn–Ti/Ta interfaces was also confirmed.
8.1 Wetting Criteria
Wetting and spreading of molten material are important factors that influence the brazing ability of a joint to be brazed. The wetting characteristics of Cu–Sn–Ti brazing alloy on graphite were studied [110] and experiments were carried out at 850–1000 °C to examine their phase developments. Increasing Ti content significantly increased wetting behaviour which means that increase in Ti content at 10 at%of Sn caused the formation of CuSn3Ti5 and SnTi3 IMCs, whereas a rise in Sn to 10 at% of Ti had resulted in Sn3Ti2 and Sn3Ti5 IMCs. Hence, an optimal concentration for the best wetting property of the alloy was found to be 10 at% Ti with more than 15 at% Sn content. A study [111] was conducted to achieve a contact angle of 10° with the addition of 3 at%Ti to Cu–15 at%Sn (best wetting property on vitreous carbon was obtained). A continuous layer of TiC (less than 0.5 μm thickness) was confirmed at the interface. The wettability behaviour [112] of Cu–Sn–Ti alloy on alumina was conducted and brazing was the preferred method to bond metal and ceramic joints. Minimum Ti content required to wet alumina was found to be 6 wt%. On increasing the content of Ti to 12wt%, there was a rise in the volume fraction of the intermetallic phase in the braze. Sessile tests revealed that 70Cu–21S–n9Ti exhibited the best wettability on polycrystalline alumina with less than 21 wt% of Sn. Lin et al. [112] also confirmed that Cu–Sn–Ti active braze alloy possessed a combination of both lower thermal expansion coefficient and lower wetting angle on alumina when compared to commercially used braze. The influencing factors on the reactive wetting of Cu–Sn–Ti on SiC were found to be temperature and surface roughness [113]. Wetting and spreading behaviour of the alloy was investigated by increasing the temperature and making all other parameters constant. The study had resulted in a reduction of wetting angle. The dominance of Cu–Sn–Ti over Ag–Cu–Ti alloys was confirmed as Cu–Sn–Ti possessed good wetting property even with an increased surface roughness of SiC.
8.2 Porous Cu–Sn–Ti Alloy
The pores in porous structures are divided as inherent pores and generated pores. During the sintering process, these inherent pores as the intrinsic characteristics are introduced. The influence of the porosity and pore morphology on the structure and mechanical properties of metallic porous composites are discussed. The porous Cu–Sn–Ti composites with carbide particles as space holders by sintering dissolution process (SDP) was fabricated [114] to examine the distribution of micropores and their effects on the mechanical properties. Increasing the concentration of graphite particles had resulted in a rise in the number of micropores along with a drop in compressive and flexural strength. Mechanical properties of the porous composites were found to improve by optimizing alloy composition and uniform distribution of carbamide particles. Flexural strength was the main property that governed wear resistance of tools. Hence, Back Propagation (BP) artificial network with Genetic Algorithm (GA) and particle swarm optimization algorithm (PSOA) was used [115] for the optimization of flexural strength of porous metallic materials. The dominance of PSOA-BP model over GA-BP model [116] was proved in predicting the flexural strength of metallic porous composites. Moreover, Cu–Sn–Ti alloy powder, alumina hollow particles and graphite particles were sintered to determine the influence of pore structure on the composite (porous Cu–Sn–Ti alumina composite) strength. With an increase in pore size there observed a decrease in strength, but this provided more space for the chip formation during the grinding process. This loss in TS can be compensated by effectively distributing the pore structures in the composite.
Porous Cu–Sn–Ti metal was fabricated with alumina bubble particles and Cu–Sn–Ti powder alloys by vacuum sintering process [117]. The study gave the influence of alumina bubble particles on the mechanical strength and microstructure of porous Cu–Sn–Ti metals. Highly porous bond with excellent mechanical strength (bond strength) was formed due to the formation of TiAl and TiO compounds. Moreover, a variation of bending strength with pore size and volume fraction was also determined as shown in Fig. 18.
Variation of bending strength with respect to pore size and pore volume fraction [117]
9 Isothermal Section and Phase Diagram of Cu–Sn–Ti Alloy
Application of Cu–Sn–Ti alloys as a binding material for manufacturing diamond tools by liquid phase sintering was established by Kizikov et al. [25] and Thibault et al. [26]. A detailed study on the ternary phase diagram was required for obtaining the composition of different phases. Thoiobault et al. [27] examined the ternary phases in the composition range between CuSnTi3 and CuSn5Ti6 at 900 °C. The experiment was conducted on alloys in powdered forms. These were annealed at 600–1000 °C for 10 h in a static argon (Ar) atmosphere. Presence of tightly packed homogeneous composition for Ti5Sn3Cu ternary phase was confirmed in two or three-phase domains. Moreover, the presence of Cu–Sn–Ti phase was confirmed along with the co-existence of Ti5Sn3Cu ternary phase with a hexagonal crystal structure similar to Ti5Ga4 type. Property of Cu–Sn–Ti alloy to produce high strength bonds made them useful in binding ceramic materials. The need for low-temperature bonding for ceramics (since the mode of failure of ceramics was creep) was satisfied by Cu–Sn–Ti alloy as it possessed low melting temperature. While the presence of CuSn3Ti5 ternary compound will weaken the strength of the bond. Hence, Naka et al. [28] came up with an investigation to determine the liquidus of the ternary Cu–Sn–Ti system and confirmed the presence of two ternary compounds (Cu–Sn–Ti and CuSn3Ti5). Figure 19 depicts a nutshell of all observed primary phase field of crystallization, and the existing phase reactions in the form of liquid surface projection. This data also confirms the presence of a large area of primary crystallization of Cu, Ti3Sn, Ti6Sn5 and CuSn3Ti5.
Liquidus projection of the Cu–Sn–Ti ternary system [28]
Zhang et al. [29] examined the Cu–Sn–Ti ternary system and developed its isothermal section at 473 K. The presence of 17 single-phase regions was confirmed through the investigation. As shown in Fig. 20 there observed 33 two-phase regions and 17 three-phase regions. The Observations on SEM, OM and XRD proved the presence of 12 binary compounds (CuTi, CuTi2, Cu3Sn, Cu3Ti2, Cu4Ti, Cu4Ti3, Cu6Sn5, Ti2Sn, Ti2Sn3, Ti3Sn, Ti5Sn3 and Ti6Sn5) and 2 ternary compounds (CuSnTi and CuSn3Ti5). Moreover, the experiment also ascertained 10 at% as the maximum solid solubility of Cu in Ti6Sn5.
Isothermal section of Cu–Sn–Ti ternary system at 473 K [29]
Solid–solid–liquid diffusion triple approach was investigated [30] to determine the isothermal section of Cu–Sn rich part in Cu–Sn–Ti ternary system at 823 K. Presence of one ternary compound (Cu–Sn–Ti), 10 three-phase fields (CuTi + Cu4Ti3 + Sn5Ti6, CuTi2 + CuTi + Sn5Ti6, Sn5Ti6 + Sn3Ti2 + CuSnTi, Liquid + CuSnTi + Cu3Sn, Liquid + Sn3Ti2 + CuSnTi, CuSnTi + Cu4Ti3 + Sn5Ti6, CuSnTi + Cu3Sn + Cu41Sn11, CuSnTi + Cu4Ti + Cu, CuSnTi + Cu41Sn11 + Bcc_a2 and CuSnTi + Bcc_a2 + Cu) were confirmed as shown in Fig. 21 along with the presence of the other two three-phase fields that were not confirmed in the scope of this experiment [30]. Further investigation should be done in the Ti–rich part of the ternary system.
Isothermal section of Cu–Sn–Ti ternary system at 823 K [30]
Since, a deep knowledge of the phase diagram is required to identify the formation of ternary compounds and to determine their properties, Wang et al. [31] constructed an isothermal section as shown in Figs. 22 and 23 at 300 and 800 °C and compared with the experimental work and the solubility ranges of Sn–Ti IMCs were established. The present study can be incorporated reliably in the Cu-rich side for Cu-based alloy brazing applications.
Isothermal section of the Cu–Sn–Ti at 397 °C [31]
Calculated isothermal section at 800 °C and compared with the experimental data as proposed by Wang et al. [31]
10 Applications of Cu–Sn–Ti Alloys
The use of Cu–Sn–Ti alloys in corrosive atmosphere made them suitable for marine industries. As mentioned above, additions of Sn up to 5 wt% had improved the corrosion characteristics of Cu–Sn–Ti alloy. The study conducted can be incorporated as a guide for manufacturing parts of automobile engines and boat hulls that require high corrosion resistive property. Furthermore, this ternary system served as efficient filler metal in brazing purpose [19, 20] because of its high-temperature workability.
11 Microstructural and Mechanical Properties of Cu–Sn–Zn Alloys
Copper–tin–zinc (Cu–Sn–Zn) ternary alloy has attracted tremendous research interests due to its unique properties, including, high corrosion resistance, remarkable solderability and good electrical conductivity The growth of Cu–Sn was not only investigated in pure Sn/polycrystalline Cu system but also Sn–0.5 wt% Zn/polycrystalline Cu system, and studied the microstructure of Cu3Sn grains during nucleation [118]. The Cu/Cu3Sn and Cu3Sn/Cu6Sn5 interfaces inherited the non-planar Cu/Cu6Sn5 interface since Cu6Sn5 was the first phase to form in the Cu–Sn system. In Sn–0.5 wt%Zn/Cu system, equiaxed Cu3Sn grains form during reflow and maintains the same shape even after prolonged ageing. The interfacial reaction between Zn and Cu was able to retard the columnar growth of Cu3Sn, which mainly contributes to the increase of Cu3Sn thickness, which was due to the grain coarsening of equiaxed grains. It was found that during prolonged ageing in pure Sn/Cu system many voids are present in the Cu3Sn phase. Figure 24 depicts the Cu3Sn grains separate into three parts after ageing at 160 °C for 400 h. Cu3Sn as layer 1 was the initial Cu3Sn with larger columnar shape. Cu3Sn as layer 2 was equiaxed originally, further became columnar. Cu3Sn as layer 3 was the newly formed Cu3Sn region with equiaxed grains.Cu3Sn as layer 1 (initial) became larger and more columnar. Whereas the original equiaxed Cu3Sn (layer 2) became columnar, and the newest Cu3Sn grains (layer 3) with equiaxed shape was formed in between Cu and Cu3Sn as layer 2. On increasing the ageing time, newly formed equiaxed Cu3Sn nucleates and further became columnar. Furthermore, old columnar Cu3Sn became larger and more columnar.
Separation of Cu3Sn into three parts after ageing at 160 °C for 400 h [118]
The effect of Fe addition to Cu–Sn–Zn alloy on the microstructure and mechanical properties was studied [119] and an improvement in hardness, strength and ductility was observed when the Cu–Sn–Zn–Fe was aged at 500 °C for 4 h after solution treating at 750 °C for 1 h. High-density precipitates and grain refinement (the two strengthening mechanism) was attributed to the improvement in yield strength. Figure 25 depicts the microstructure of peak aged state of alloy A (Cu–10 wt% Sn–1.76 wt% Zn) and alloy B (Cu–10.3 wt% Sn–1.81 wt% Zn–1.26 wt% Fe). It was inferred from the Fig. 25 that the grain size of alloy B is much finer than A. Also, a considerable amount of precipitation evolved in the alloy B matrix. This acted as an obstacle for the motion of dislocations in the grain boundaries that led to the strengthening of the alloy.
Optical micrographs of peak-aged specimens a alloy A b alloy B [119]
Figure 26 represents the isochronal ageing curve of alloy A and B (solution treated). It was observed from that the hardness of Alloy B is much higher than that of Alloy A, and concluded that the precipitation of secondary phase due to Fe addition had resulted in this typical ageing behaviour of Alloy B. Even though alloy A did not show any age-hardening response.
Isochronal ageing curves for solution treated alloys for 4 h [119]
Furthermore, Fig. 27 shows the engineering stress–strain curve of the two Cu alloys in the as-cast and peak-aged states. It was observed that the strength of alloy B was higher than alloy A. The heat treatment had led to a significant improvement in fracture elongation (FE) for both of the alloys. This increase in FE was attributed to the development of homogenized microstructure, resulting in enhanced ductility of the alloy.
Engineering stress–strain curve for the two Cu alloys in as-cast and peak-aged condition [119]
The microstructure of cold-worked FCC Cu–Sn–Zn alloys was characterized using the X-ray diffraction technique [120]. It was observed that the addition of Sn which act as a solute element in the near dilute range of 1 wt% to the alpha-brass system increased the net stacking fault concentration and had an arresting effect on the microstructural parameters like coherent domain size, the density of dislocation and deformation stacking fault. More than 20 wt% of Zn did not produce any significant change in the microstructural parameters, which was the main reason for observations of Halder et al. [121] as the experiment was conducted by ranging Zn concentrations from 24 to 34 wt% Zn. Hong [122] investigated the cause for the rough surface morphology and a method for improving the surface roughness of the metal precursor. RF-magnetron sputtering method was used to fabricate the metal precursor for solar application. Due to the co-sputtering process, the efficiency of the Cu (Zn, Sn) (CZT) based solar cell was enhanced. Sn bounded with Cu to produce phases such as CuSn, Cu6Sn5 and Cu81Sn22. Furthermore, the independent growth of Sn caused an increase in the surface roughness of the metal precursor.
As aforementioned, CZT and CZTS (Copper–Zinc–Tin–Sulphur) found use in photovoltaic cells and the demand for economical fabrication was of major concern. Cu/Sn/Zn clads or laminated metallic alloys find extensive use in industrial application where high specific strength, corrosion resistance and excellent wear and abrasion resistance are required. Dhakal et al. [123] came up with the characterization of a thin CZTS solar cell produced by the sputtering method. In addition to that, Scaragg et al. [124] and Vanalakar et al. [125] investigated on the electrodeposited and pulsed laser deposited CZTS films, respectively. Mathew et al. [126] fabricated trimetallic Cu/Sn/Zn multi-layered clad by roll bonding process as shown in Fig. 28a to examine the evolution of microstructure and to determine its tensile properties. Bonding process resulted in the strengthening of Cu/Sn interface thereby forming Cu6Sn5 IMC. During tensile testing at room temperature, the trimetallic Cu/Sn/Zn claddings showed typical composite strengthening and two-stage deformation behaviour. The overall deformation behaviour of the trimetallic clad was controlled by dynamic deformation activities such as recrystallization followed by significant grain growth in Sn.
Roll bonding process done on Cu/Sn/Zn [126]
The effects of isothermal annealing, mechanical deformation and Zn addition on the characteristics of Cu–5 wt% Sn was investigated [127] and the annealing temperature varied from 573 K to 773 K. Saturation of resistivity was found to occur through two annealing stages. The time exponent (n) of Cu–Sn alloy decreased with increasing deformation temperature except when worked for higher annealing times. While for the Cu–Sn–Zn alloy, time exponent for ε-precipitate dissolution decreased at both stages (at higher and lower annealing times). The softening behaviour was observed at higher stresses and temperature due to a drop in strength parameters. The thermodynamic properties of liquid Cu–Sn–Zn alloys with a constant Cu: Sn molar ratio of 2:1, 1:1, 1:2 and 1:3 was observed at four cross-sections [128]. The integral Gibbs free energy and the internal enthalpy for the ternary system at 1023 K were calculated by Gibbs–Duhem integration. The study yields a constant set of thermodynamic data for the liquid Cu–Sn–Zn alloys, which were used for the calculation of the ternary phase diagram. Figure 29 shows the enthalpy of mixing for the four cross-sections at 1023 K. This investigation helped to improve some industrial processes.
Integral enthalpy of mixing (ΔH) for all four cross-sections at 1023 K [128]
12 Isothermal Sections of Cu–Sn–Zn Alloy
An isothermal phase diagram shows the phases present in any alloy at a particular temperature and is useful in predicting the phases and their amounts and compositions at that temperature. Isothermal plots are useful because they allow compositional analysis, while liquidus and isopleth plots do not allow compositional analysis. The liquidus projection of the Sn–Zn–Cu system was determined [129] and the thermodynamic modelling was also conducted to calculate the isothermal sections. Since there were no ternary compounds in the Sn–Zn–Cu ternary system, all the primary solidification phases were solid solutions and binary IMCs. Thirty-two alloys were prepared and analyzed in this study. Their compositions were shown in the above Fig. 30a, b depicts the boundaries of different primary solidification phase regions and the liquidus troughs of the liquidus projection in the Sn–Zn–Cu ternary system based on the experimental results and phase diagrams of constituent binary systems [1, 130]. The ternary compound was absent, and the primary solidification phases were Sn, η-Cu6Sn5, ε-Cu3Sn, γ-(Cu–Sn),, Cu, γ-Cu5Zn8, δ-CuZn3, ε-CuZn5 and Zn.
Furthermore, Fig. 31 depicts the calculated isothermal sections at 523 and 873 K. Results and calculations in the present investigation was in accordance with the studies conducted by Yamaguchi et al. [131] and Chou et al. [132].
a Isothermal section at 523 K and b Isothermal section at 873 K [129]
The thermodynamic description of the ternary Cu–Pb–Zn and Cu–Sn–Zn system was studied [133]. For the Cu–Sn–Zn system, the ternary parameters were obtained using the PARROT module of Thermo-Calc. These parameters were used to fit experimental data for the bcc-A2 phase, which existed in Cu–Sn and Cu–Zn binary systems. Figure 32 shows the calculated isothermal section at 873 K. It was observed that the estimated solubilities of fcc-Al and bcc-A2 phases were in accordance with the experimental results of [131, 134]. The homogeneity range of Cu at other temperatures (723 K or 673–1073 K) is also presented satisfactorily by the calculations.
Calculated isothermal section at 873 K [133]
Figure 33 shows the calculated activities of Zn in the Cu-rich corner of the ternary system. This confirmed a negative deviation from Raoult’s Law. Activity of Zn increased with small additions of Sn. This behaviour was also presented by calculations.
Activity of Zn in liquid phase at 1423 K (as taken from [133])
The Sn rich corner of the Cu–Sn–Zn system was studied [135] and all the samples were solidified unidirectional at a very low rate. Reactions were detected by the change in phases, frozen out with the change in composition of the liquid due to segregation. Three quasi-peritectic reactions (U1–U3) and a ternary eutectic reaction were found (as shown in Fig. 34): U1, L + Cu6Sn5 = CuZn + Sn; U2, L + CuZn = Cu5Zn8 + Sn; U3, L + Cu5Zn8 = CuZn4 + Sn; and E, L = (Sn) + (Zn) + CuZn4.
Sn rich corner of Cu–Sn–Zn liquidus projection, demonstrating the presence of three quasi-peritectic reactions along with the region of primary solidification [135]
An optimized thermodynamic description for Cu–Al–Zn and Cu–Sn–Zn ternary systems was conducted [136] by applying phase equilibrium and thermodynamic data from the literature. The complete solubility of Cu–Al and Cu–Zn gamma phases were observed on substitutional solution treatment in the Cu–Al–Zn system. In the case of the Cu–Sn–Zn system, the solubility between the gamma phases was incomplete. Wada et al. [137] investigated the effect of the Zn addition to the Cu–Sn matrix of the Nb3Sn composite by measuring the work-hardening behaviour of the matrix, rate of Nb3Sn layer formation and superconducting properties. The addition of Sn increased Ic (critical current) through increasing the growth rate of the Nb3Sn layer at each Sn level. It was also observed that Zn got dissolved in Nb3Sn, and had no effect on the intrinsic superconducting properties such as Tc (critical temperature). However, Zn may introduce excess vacancies in the Cu–Sn matrix. This increases the distribution rate of Sn at the matrix–Nb3Sn diffusion interface, promoting the formation of an Nb3Sn layer. It was observed that the Zn addition caused an excellent enhancement in the growth rate of the Nb3Sn even at low Sn levels without compromising the ductility of the composite. Figure 35 depicts the Nb3Sn layer thickness as a function of heat treatment temperature. It is evident from the fig that the growth rate of Nb3Sn layer increased with increasing temperature. A rise in Sn content induced an apparent increase in the rate of growth for each temperature that was examined.
Variation of Nb3Sn layer thickness with heat treatment temperature [137]
Furthermore, Fig. 36 shows the variation of critical temperature (Tc) as a function of heat treatment temperature, heating period being 100 h. It was evident from the Fig. 36 that the critical temperature increased with increasing temperature and seemed to approach a saturation value at each Sn level. Raising the Sn level resulted in an increase in Tc at each heating temperature, but the Zn addition at any Sn level fails to make an essential increase in Tc.
Variation of critical temperature with respect to heat treatment temperature [137]
13 Application of Cu–Sn–Zn Alloys
Cu–Sn–Zn alloy possessed good cyclability and high theoretical capacity, hence they found use as an alternative for graphite electrodes in commercial lithium-ion batteries. Beattie et al. [138] investigated and successfully deposited a composition-spread library of Cu–Sn–Zn ternary alloys by electrodeposition and dripped immersion plating method. EDS and XRD were used to characterize the electrodeposition films. It was observed that for a successful immersion deposition, the third element should be of greater reduction potential than the other two elements. The production of combinatorial material was simple and economical. A simple phenomenological model was developed to describe the Cu, Sn and Zn composition variation in the experimental library. Furthermore, Cu–Sn–Zn alloys also found application as a solar cell [122].
14 Summary
A review was conducted on wrought Cu–Sn, Cu–Sn–Ti and Cu–Sn–Zn alloys in detail and the major findings from the present study are as follows
-
1.
General characteristics of wrought Cu–Sn alloy
-
Cu–Sn system was modelled thermodynamically and the phases were calculated using the CALPHAD method
-
The two important intermetallic compounds present in the Cu–Sn system were Cu3Sn and Cu6Sn5
-
The formation of voids and its effect on the intermetallic compound was studied and it was noted that the void density increased spontaneously
-
The grain distribution of intermetallic compound (Cu6Sn and Cu3Sn) and orientation of crystals were studied
-
The Kirkendall effect on the Cu–Sn system was explained
-
The orientation of grains and mechanical properties of Cu–Sn intermetallic compound joints by varying electric current was reported
-
-
2.
Different forms of bronze alloy and their properties
-
The phenomenon of inter-diffusion and intermetallic formation in Cu–Sn thin films were reported
-
The pressure sintering and plasma sintering technique to produce Cu–Sn powders were explained
-
A harmonic-structured Cu–Sn alloy was fabricated by mechanical milling (MM) and spark plasma sintering (SPS) process and it was observed that the hardness of the harmonic–structured Cu–Sn alloy was found to improve
-
The variation in mechanical properties during spray forming and cold rolling of Cu–Sn alloy were compared with cast ingots
-
The sprayed powders Cu–Sn had a regular surface with fine dendritic structure whereas, permanent mould cast samples possessed dendritic structure with Sn-rich δ phase in between the dendrites
-
The shape memory effect on Cu–Sn wrought alloys was studied
-
The anisotropy characteristics in mechanical properties would be formed in the building direction of the crystallographic structures
-
-
3.
Different processes undergone and its effects
-
SLID bonding and its general characteristics
-
Growth behaviour of Cu6Sn5 during the TLP bonding process
-
Influence of Annealing Temperature on wrought bronze alloys was investigated
-
Hot-pressed Cu–Sn powder alloys were studied
-
Microstructural evolution and whiskering of Cu–Sn alloys on ageing was explained
-
Morphological behaviour on sintering was reported
-
Power spinning technique was summarized
-
Reduction diffusion method was reported to coat Cu–Sn on polymer substrate
-
The inductive heating process, under lower pressure, was found to be 0.01 MPa
-
Grain structure and tensile properties of hot extrusion bronze was studied
-
-
4.
Applications of bronze alloys
-
High-temperature applications involve automobile engine parts and turbine parts.
-
Cu–Sn plating possessed excellent anti-fretting characteristics
-
Cu–Sn alloy are used in MEMS and NEMS devices as it possessed good wettability and made strong interconnections between the substrates
-
-
5.
Effect on Cu–Sn due to additions of other elements
-
Self-sharpening property of the alloy was obtained when Sn content was made to 25 wt%.
-
An increase in the wear resistance and transverse rupture strength was achieved by the addition of Ni element.
-
Ti had very low solubility to Cu–Sn intermetallic compounds
-
Presence of Ti in bronze alloys possesses exceptionally high corrosion resistance and high strength to weight ratio that made them useful in aerospace and automobile industries.
-
-
6.
General characteristics of Cu–Sn–Ti alloy
-
Cu–Sn–Ti alloys affix their application in biomaterials
-
Increasing Ti content significantly increased wetting behaviour
-
With an increase in pore size there observed a decrease in strength for Cu–Sn–Ti alloy
-
-
7.
Isothermal section and phase diagram of Cu–Sn–Ti alloy
-
The ternary phase diagram is required for obtaining the composition of different phases.
-
Presence of CuSn3Ti5 ternary compound will weaken the strength of the alloy
-
-
8.
Applications of Cu–Sn–Ti alloys
-
Cu–Sn–Ti alloys as a binding material for manufacturing diamond tools
-
Cu–Sn–Ti alloys suitable for marine industries
-
-
9.
Microstructural and mechanical properties of Cu–Sn–Zn alloys
-
Addition of Sn increased the net stacking fault concentration
-
Grain boundary strengthening led to the improvement of properties
-
Conducting ageing refines the microstructure
-
-
10.
Application of Cu–Sn–Zn alloys
-
Alternative for graphite electrodes in commercial lithium-ion batteries
-
Cu–Sn–Zn alloys find application in solar cells
-
15 Future Scope
Combining all the results of the investigation on wrought Cu–Sn, Cu–Sn–Ti and Cu–Sn–Zn alloys, it leads us to the conclusion that it can be used in electrical connections due to its conductive property, in marine industries due to its corrosive property, as the best filler material for brazing purpose and even in biomedical applications. Since Ti alloys were known for its high strength-to-weight ratio and toughness, applications of Cu–Sn–Ti alloy may be broadened in the field of aerospace and industrial purpose. But, due to the less number of researches conducted on wrought Cu–Sn–Ti alloys, it had made them less pronounced to the world. Thus future researches on wrought Cu–Sn, Cu–Sn–Ti and Cu–Sn–Zn may be focused on the following
-
Mechanical properties may be determined
-
Toughness
-
Creep
-
Fatigue like a high cycle and low cycle and electrical property
-
Anisotropic behaviour of Cu–Sn–Zn and Cu–Sn–Ti wrought alloys
-
Effect of quenching medium on the properties
-
High-temperature behaviour and wear behaviour
-
Wear behaviour at different load, sliding velocity and sliding distance
References
W. Gierlotka, S.W. Chen, S.K. Lin, J. Mater. Res. 22, 3158 (2007)
O. Minho, G. Vakanas, N. Moelans, M. Kajihara, W. Zhang, Microelectron. Eng. 120, 133 (2014)
A. Banerjee, R. Kumar, M. Dutta, S. Bysakh, A.K. Bhowmick, T. Laha, Surf. Coat. Technol. 262, 200 (2015)
J. Hui, Z. Feng, W. Fan, P. Wang, Mater. Res. Express 5, 046511 (2018)
H. Liu, K. Wang, K. Aasmundtveit, N. Hoivik, in 2010 Proceedings 60th Electronic Components and Technology Conference. ECTC 2010, Las Vegas, June 1–4 2010. (IEEE, Piscataway, 2010), pp. 853–857
I. Panchenko, K.J. Wolter, K. Croes, I. De Wolf, J. De Messemaeker, E. Beyne, M.J. Wolf, Microelectron. Reliab. 102, 113296 (2019)
Q. Lin, F. Li, J. Wang, J. Alloy. Compd. 767, 877 (2018)
V.A. Baheti, S. Kashyap, P. Kumar, K. Chattopadhyay, A. Paul, J. Alloy. Compd. 727, 832 (2017)
M. Onishi, H. Fujibuchi, T. Jpn. I. Met. 16, 539 (1975)
S. Kumar, C.A. Handwerker, M.A. Dayananda, J. Phase Equilib. Diff. 32, 309 (2011)
L. Révay, Surf. Technol. 5, 57 (1977)
A. Paul, C. Ghosh, W.J. Boettinger, Metall. Mater. Trans. A 42, 952 (2011)
S. Bader, W. Gust, H. Hieber, Acta Metall. Mater. 43, 329 (1995)
Y. Yuan, Y. Guan, D. Li, N. Moelans, J. Alloy. Compd. 661, 282 (2016)
M. Arita, H. Nakajima, M. Koiwa, S. Miura, Mater. T. JIM 32, 32 (1991)
J. Feng, C. Hang, Y. Tian, C. Wang, B. Liu, J. Alloy. Compd. 753, 203 (2018)
L. Wu, J.E. Graves, A.J. Cobley, Mater. Lett. 217, 120 (2018). https://doi.org/10.1016/j.matlet.2018.01.094
L.C. Tsao, M.-J. Heish, Y.-C. Yu, Corros. Eng. Sci. Techn. 53, 25 (2018)
J. He, W.F. Ding, Y. Wang, Z.W. Liu, Adv. Mater. Res. 753–755, 353 (2013)
J. Zhang, G.C. Wang, Y. Zheng, Mater. Sci. Forum 762, 602 (2013)
X.Y. Xue, X.F. Bian, H.X. Geng, M.H. Sun, X.B. Qin, Mater. Sci. Technol. 19, 557 (2003)
S. Fürtauer, D. Li, D. Cupid, H. Flandorfer, Intermetallics 34, 142 (2013)
X.J. Liu, C.P. Wang, I. Ohnuma, R. Kainuma, K. Ishida, Metall. Mater. Trans. A 35A, 1641 (2004)
D. Li, P. Franke, S. Fürtauer, D. Cupid, H. Flandorfer, Intermetallics 34, 148 (2013)
E.D. Kizikov, I.A. Lavrinenko, Mater. Sci. J. 17, 61 (1975)
S. Hamar-Thibault, C. Allibert, W. Tillman, in International Workshop on Diamond Tools Proceedings. EUROPM 99, Turin, November 8–10, 1999. (EPMA, Turnin, 1999), p. 57
S. Hamar-Thibault, C.H. Allibert, J. Alloy. Compd. 317, 363 (2001)
M. Naka, I. Nakade, J.C. Schuster, S. Urai, J. Phase Equilib. 22, 352 (2001)
X. Zhang, Y. Zhan, Q. Guo, G. Zhang, J. Hu, J. Alloy. Compd. 480, 382 (2009)
G.J. Zhou, Y. Zhou, Y. Luo, AIP Adv. 7, 025118 (2017)
J. Wang, C. Liu, C. Leinenbach, U.E. Klotz, P.J. Uggowitzer, J.F. Löffler, Calphad 35, 82 (2011)
B. Wierzba, W.J. Nowak, Physica A 523, 602 (2019)
M. De Bondt, A. Deruyttere, Acta Metall. 15, 993 (1967)
Z.J. Wang, K.J. Toyohiko, C.L. Ma, Rare Metals 32, 139 (2013)
G. Ross, V. Vuorinen, M. Paulasto-Kröckel, in 2016 IEEE 18th Electronics Packaging Technology Conference, EPTC 2016. Singapore, November 30–December 3, 2016 (IEEE, Piscataway, 2017), pp. 459–467
G. Ross, V. Vuorinen, M. Paulasto-Kröckel, J. Alloy. Compd. 677, 127 (2016)
K.N. Tu, Acta Metall. 21, 347 (1973)
K. Fujiwara, M. Ohtani, T. Isu, S. Nango, R. Kawanaka, K. Shimizu, Thin Solid Films 70, 153 (1980)
K.N. Tu, R.D. Thompson, Acta Metall. 30, 947 (1982)
K.N. Tu, Phys. Rev. B 49, 2030 (1994)
R. Chopra, M. Ohring, R.S. Oswald, Thin Solid Films 94, 279 (1982)
R. Chopra, M. Ohring, R.S. Oswald, Thin Solid Films 86, 43 (1981)
K.N. Tu, Mater. Chem. Phys. 46, 217 (1996)
F. Keraghel, K. Loucif, M.P. Delplancke, Physics Proc. 21, 152 (2011)
H. Fujiwara, T. Nishimoto, H. Miyamoto, K. Ameyama, in Proceedings of the 8th Pacific Rim International Congress on Advanced Materials and Processing, ed. by F. Marquis, Microstructure and Mechanical Properties of Cu-Sn Alloy with Harmonic Structure (Springer, Cham, 2013), pp. 2455–2460. https://doi.org/10.1007/978-3-319-48764-9_304
X. Wang, J. Zhao, J. He, J. Wang, J. Mater. Sci. Technol. 24, 803 (2008). https://doi.org/10.1155/2016/9796169
C. Lei, H. Huang, Y. Huang, Z. Cheng, S. Tang, Y. Du, Powder Technol. 301, 356 (2016)
A. Nassef, M. El-Hadek, Adv. Mater. Sci. Eng. 2016, 9796169 (2016)
R. Tongsri, N. Tosangtum, T. Yotkaew, P. Muthithamongkol, A. Sri-On, U. Patakham, Mater. Charact. 113, 52 (2016). https://doi.org/10.1016/j.matchar.2016.01.001
J.D. Stice, C.M. Wayman, Metall. Trans. A 13, 1687 (1982)
M. Kamal, Radiat. Eff. Defects Solids 161, 189 (2006)
M. Ceylan, R. Zengin, J. Mater. Process. Technol. 97, 148 (2000)
N. Kuwano, C.M. Wayman, T. Jpn. I. Met. 24, 504 (1983)
N. Kuwano, C. Wayman, T Jpn. I Met. 24, 561 (1983)
Z. Mao, D.Z. Zhang, J. Jiang, G. Fu, P. Zhang, Mater. Sci. Eng. A 721, 125 (2018)
S. Amore, E. Ricci, T. Lanata, R. Novakovic, J. Alloy. Compd. 452, 161 (2008)
W. Zhai, W.L. Wang, D.L. Geng, B. Wei, Acta Mater. 60, 6518 (2012)
W. Zhai, Z.Y. Hong, X.L. Wen, D.L. Geng, B. Wei, Mater. Design 72, 43 (2015)
Y. Su, D. Liu, X. Li, L. Luo, J. Guo, H. Fu, Metall. Mater. Trans. A 43, 4219 (2012)
H. Lu, S. Li, L. Liu, H. Fu, Special Casting & Nonferrous Alloys 26, 753 (2006)
J.W. Xian, S.A. Belyakov, M. Ollivier, K. Nogita, H. Yasuda, C.M. Gourlay, Acta Mater. 126, 540 (2017)
A. Munding, H. Hübner, A. Kaiser, S. Penka, P. Benkart, E. Kohn, in Wafer Level 3-D ICs Process Technology, ed. by C.S.Tan, R.J. Gutmann, L.R. Reif, Cu/Sn Solid–Liquid Interdiffusion Bonding (Springer, Boston, 2008). https://doi.org/10.1007/978-0-387-76534-1_7
H. Liu, K. Wang, K.E. Aasmundtveit, N. Hoivik, J. Electron. Mater. 41, 2453 (2012)
A. Rautiainen, V. Vuorinen, J. Li, M. Paulasto-Kröckel, Mater. Today Proc. 4, 7093 (2017)
I. Panchenko, K. Croes, I. De Wolf, J. De Messemaeker, E. Beyne, K.J. Wolter, Micro. Eng. 117, 26 (2014)
H. Liu, G. Salomonsen, K. Wang, K.E. Aasmundtveit, N. Hoivik, IEEE Trans. Compon. Packag. Manuf. Technol 1, 1350 (2011)
J. Liu, H. Zhao, Z. Li, X. Song, in 18th International Conference on Electronic Packaging Technology. ICEPT 2017, Harbin, August 16–19, 2017 (IEEE, Piscataway, 2017), pp. 10–13
N.S. Bosco, F.W. Zok, Acta Mater. 53, 2019 (2005)
N.S. Bosco, F.W. Zok, Acta Mater. 52, 2965 (2004)
M.S. Park, S.L. Gibbons, R. Arróyave, Acta Mater. 60, 6278 (2012)
M.S. Park, S.L. Gibbons, R. Arróyave, Microelectron. Reliab. 54, 1401 (2014)
W. Fan, J. Hui, Z. Xinfeng, W. Gao, C. Xue, Mater. Res. Express 5, 106520 (2018)
T. Takenaka, S. Kano, M. Kajihara, N. Kurokawa, K. Sakamoto, Mater. Sci. Eng. A 396, 115 (2005)
P. Han, F.R. Xiao, W.J. Zou, B. Liao, Mater. Des. 53, 38 (2014)
M. Sobiech, C. Krüger, U. Welzel, J.Y. Wang, E.J. Mittemeijer, W. Hügel, J. Mater. Res. 26, 1482 (2011)
H. Kato, N. Hirata, S. Miura, Acta Metall. Mater. 43, 361 (1995)
L.N. Dyachkova, E.E. Feldshtein, J. Mater. Sci. Technol. 31, 1226 (2015)
J. Hui, Z. Feng, W. Fan, X. Yuan, Mater. Charact. 144, 611 (2018)
J. Liu, W. Zheng, H. Yin, Microsc. Microanal. 26, 29 (2020)
K. Murase, A. Ito, T. Ichii, H. Sugimura, J. Electrochem. Soc. 158, D335 (2011)
A. Ito, K. Murase, T. Ichii, H. Sugimura, Electrochemistry 77, 677 (2009)
Z. Yin, F. Sun, M. Guo, Mater. Lett. 215, 207 (2017)
S. Buhl, C. Leinenbach, R. Spolenak, K. Wegener, Int. J. Refract. Met. Hard Mater. 3, 16 (2012)
J. Hui, Z.X. Feng, P. Wang, W. Fan, Z. Liu, Mater. High Temp. 36, 68 (2019)
J. Hui, Z. Feng, C. Xue, W. Gao, J. Mater. Sci. 53, 15308 (2018)
H. Morikawa, K. Shimizu, Z. Nishiyama, Trans. Jpn. Inst. Met. 8, 145 (1967)
N. Kuwano, C.M. Wayman, Trans. Jpn. Inst. Met. 24, 499 (1983)
M.B. Cortie, C.E. Mavrocordatos, Metall. Trans. A 22, 11 (1991)
C. Hang, Y. Tian, R. Zhang, D. Yang, J. Mater. Sci. Mater. Electron. 24, 3905 (2013)
H.M. Mallikarjuna, K.T. Kashyap, P.G. Koppad, C.S. Ramesh, R. Keshavamurthy, Trans. Nonferrous Met. Soc. 26, 1755 (2016)
K. Yoshida, S. Kitagawa, K. Mitose, K. Susai, Furukawa Rev. 40, 8 (2011)
B. Subramanian, S. Mohan, S. Jayakrishnan, Surf. Coat. Technol. 201, 1145 (2006)
J.H. Shin, J.S. Park, D.H. Bae, Met. Mater. Int. 17, 441 (2011)
H. Kim, T. Koseki, T. Ohba, T. Ohta, Y. Kojima, H. Sato, Y. Shimogaki, Thin Solid Films 491, 221 (2005)
N. Hoivik, K. Wang, K. Aasmundtveit, G. Salomonsen, A. Lapadatu, G. Kittilsland, B. Stark, in 3rd Electronics System Integration Technology Conference. ESTC 2010, Berlin, September 13–16, 2010. (IEEE, Piscataway, 2010), p. 526
P. Han, X. Lu, W. Li, W. Zou, Vacuum 154, 359 (2018)
Y.W. Wang, Z.X. Zhu, W.L. Shih, C.R. Kao, J. Alloy. Compd. 799, 108 (2019)
C. Schmetterer, H. Flandorfer, C. Luef, A. Kodentsov, H. Ipser, J. Electron. Mater. 38, 10 (2009)
J.S.L. Pak, K. Mukherjee, O.T. Inal, H.R. Pak, Mater. Sci. Eng. A 130, 83 (1989)
Y. Muranishi, M. Kajihara, Mater. Sci. Eng. A 404, 33 (2005)
K. Osamura, S. Ochiai, S. Kondo, M. Namatame, M. Nosaki, J. Mater. Sci. 21, 1509 (1986)
T. Yamashina, M. Kajihara, Mater. Trans. 47, 829 (2006)
F. Wang, X. Ma, Y. Qian, Scr. Mater. 53, 699 (2005)
J. Yin, Y. Zeng, D. Yao, Y. Xia, K. Zuo, J. Mater. Res. 29, 770 (2014)
V. Vuorinen, H.Q. Dong, T. Laurila, J. Mater. Sci. Mater. Electron. 23, 68 (2012)
T. Hayase, M. Kajihara, Mater. Sci. Eng. A 433, 83 (2006)
K.I. Mikami, M. Kajihara, J. Mater. Sci. 42, 8178 (2007)
Y. Muranishi, M. Kajihara, Mater. Sci. Eng. A 404, 33 (2005)
Y. Tejima, S. Nakamura, M. Kajihara, J. Mater. Sci. 45, 919 (2010)
Y.C. Hsieh, S.T. Lin, J. Alloy. Compd. 466, 126 (2008)
O. Dezellus, F. Hodaj, A. Mortensen, N. Eustathopoulos, Scr. Mater. 44, 2543 (2001)
C.C. Lin, R.B. Chen, R.K. Shiue, J. Mater. Sci. 36, 2145 (2001)
W. Tillmann, J. Pfeiffer, L. Wojarski, Adv. Sci. Technol. 88, 172 (2014)
B. Zhao, T. Yu, W. Ding, L. Zhang, H. Su, Z. Chen, Mater. Sci. Eng. A 730, 345 (2018)
B. Zhao, T. Yu, W. Ding, X. Li, H. Su, Prog. Nat. Sci. Mater. Int. 28, 315 (2018). https://doi.org/10.1016/j.pnsc.2018.04.002
B. Zhao, T. Yu, W. Ding, X. Li, Chin. J. Aeronaut. 30, 2004 (2017). https://doi.org/10.1016/j.cja.2017.08.008
C.Y. Ma, W.F. Ding, J.H. Xu, Y.C. Fu, Mater. Des. 65, 50 (2015)
M.Y. Tsai, S.C. Yang, Y.W. Wang, C.R. Kao, J. Alloy. Compd. 494, 123 (2010)
Y. Ye, X. Yang, C. Liu, Y. Shen, X. Zhang, T. Sakai, Mater. Sci. Eng. A 612, 246 (2014)
S.K. Ghosh, S.P. Sen Gupta, J. Appl. Phys. 54, 6652 (1983)
S.K. Halder, M. De, S.P. Sen Gupta, Ind. J. Pure Appl. Phys. 17, 492 (1979)
S. Hong, Mol. Cryst. Liq. Cryst. 565, 153 (2012)
T.P. Dhakal, C.Y. Peng, R. Reid Tobias, R. Dasharathy, C.R. Westgate, Sol. Energy 100, 23 (2014)
J.J. Scragg, D.M. Berg, P.J. Dale, J. Electroanal. Chem. 646, 52 (2010)
S.A. Vanalakar, G.L. Agawane, S.W. Shin, M.P. Suryawanshi, K.V. Gurav, K.S. Jeon, P.S. Patil, C.W. Jeong, J.Y. Kim, J.H. Kim, J. Alloy. Compd. 619, 109 (2015)
R.T. Mathew, D. Mule, K.R. Balasubramaniam, M.J.N.V. Prasad, J. Alloy. Compd. 725, 818 (2017)
F.A. El-salam, A. Fawzy, M.T. Mostafa, R.H. Nada, Egypt J. Solids 23, 341 (2000)
M. Peng, A. Mikula, J. Alloy. Compd. 247, 185 (1997)
Y. Huang, S. Chen, C. Chou, W. Gierlotka, J. Alloy. Compd. 477, 283 (2009)
W. Gierlotka, S.W. Chen, J. Mater. Res. 23, 258 (2008)
K. Yamaguchi, I. Nakamura, Rikagaku Kenkyusho-Iho 11, 1330 (1932)
C.Y. Chou, S.W. Chen, Acta Mater. 54, 2393 (2006)
T. Jantzen, P.J. Spencer, CALPHAD: Comput. Coupling Phase Diagr. Thermochem. 22, 417 (1998)
O. Bauer, M. Hansen, Z. MetuZZkd. 22, 405 (1930)
D.D. Perovic, L. Snugovsky, P. Snugovsky, J.W. Rutter, Mater. Sci. Technol. 28, 120 (2012)
J. Miettinen, CALPHAD: Comput. Coupling Phase Diagr. Thermochem. 26, 119 (2002)
H. Wada, M. Kimura, K. Tachikawa, J. Mater. Sci. 13, 1943 (1943)
S.D. Beattie, J.R. Dahn, J. Electrochem. Soc. 152, C542 (2005)
S. Scudino, C. Unterdorfer, K.G. Prashanth, H. Attar, N. Ellendt, V. Uhlenwinkel, J. Eckert, Mater. Lett. 156, 202 (2015)
G.M. Karthik, P. Sathiyamoorthi, A. Zargaran, J.M. Park, P. Asghari-Rad, S. Son, S.H. Park, H.S. Kim, Materialia 13, 100861 (2020)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karthik, M., Abhinav, J. & Shankar, K.V. Morphological and Mechanical Behaviour of Cu–Sn Alloys—A review. Met. Mater. Int. 27, 1915–1946 (2021). https://doi.org/10.1007/s12540-020-00899-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-020-00899-z