Abstract
A strongly divergent lineage, putatively a new cryptic species, of colonial ascidian was first detected as an anomalous sample in a population genomics study of the well-known worldwide invasive species Didemnum vexillum Kott, 2002. This putative new taxon, found in a marina in Roscoff, France, is indistinguishable from Didemnum vexillum in the external aspect and coexists with it in syntopy. However, morphological characters such as spicules and larvae allow a clear-cut distinction. In accordance with the preliminary results based on genome-wide analyses, morphological traits and mitochondrial sequences of the cytochrome oxidase I gene both support the establishment of a new species Didemnum pseudovexillum sp. nov. Previous unidentified sequences in public databases showed that the new species is also present in NW Mediterranean marinas. Didemnum pseudovexillum sp. nov. is assigned for the time being a cryptogenic species status, although its presently known disjoint distribution across two biogeographic regions and its presence in ports are suggestive of an introduced species. Further studies should be performed to ascertain its current distribution and putative natural range and settle its native vs. non-native status. This finding casts doubts on previous reports of Didemnum vexillum and also calls for caution when performing fast field surveys of non-indigenous species such as rapid assessment surveys (RAS) or BioBlitz surveys, based solely on external characters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taxonomy is at the heart of all biological studies (Bortolus 2008), and this holds particularly true in the study of introduced, non-indigenous species (NIS). Typically, an introduced species appears in a short time frame in a variety of geographic locations, often far away from its natural distribution range, as a result of human-mediated transport. In its introduction range, it is often identified by different specialists. If this happens in a group of difficult taxonomy and with few specialists, there are risks of misidentifications, repeated descriptions of new species, and overall failure of taxonomy to cope with a wide-scale perspective (Carlton 1999; Ojaveer et al. 2014).
Ascidians are a group of marine invertebrates which is paradigmatic in this respect. They are difficult to identify morphologically due to few diagnostic characters, which are often difficult to observe. In addition, morphological, chemical, and genetic variations within species suggest that many formally recognized species are in fact species-complexes (e.g. López-Legentil and Turon 2005; Bock et al. 2012; Teske et al. 2011). The problem is further complicated by declining taxonomic expertise (a global problem not limited to ascidians, Giangrande 2003). On the other hand, this group includes numerous and important introduced species (Lambert 2007; Shenkar and Swalla 2011; Zhan et al. 2015) with large-scale distributions, which has originated diverse taxonomic problems, as the long list of synonymies of some cosmopolitan species testifies (e.g. Botrylllus schlosseri (Pallas, 1766), Botrylloides leachii (Savigny, 1816), see Kott 1985).
When species had been well described, molecular barcoding can facilitate the correct identification of introduced species (Comtet et al. 2015), including cryptic introductions of widely distributed ascidians (e.g. Turon et al. 2003; Bishop et al. 2013; Ordóñez et al. 2016). Population-based genetic studies (e.g. population genetics, phylogeography) have also unveiled that even well-known introduced species had more variability than previously thought, revealing divergent lineages, and putative cryptic species (i.e. species not distinguishable with morphological traits) (Pante et al. 2015a). Indeed, cryptic speciation has proved to be widespread, and in some cases, the taxonomy has been resolved, such as in the case of the model “species” Ciona intestinalis (Linnaeus, 1767) (Brunetti et al. 2015; Malfant et al. 2018), while in other instances genetic clades remain to be formally named (e.g. Diplosoma listerianum (Milne Edwards, 1841) (Pérez-Portela et al. 2013), Botryllus schlosseri (López-Legentil et al. 2006; Bock et al. 2012; Griggio et al. 2014, but see Brunetti et al. 2020)).
Survey methods to detect introduced marine species (reviewed in Campbell et al. 2007, Kakkonen et al. 2019) include non-destructive visual surveys such as rapid assessment surveys (RAS, e.g. Cohen et al. 2005, Bishop et al. 2015, Nall et al. 2015), photographic methods (e.g. Grey 2009), or BioBlitz surveys (e.g. Cohen et al. 2011). Often, there is no time, money, or expertise for sampling followed by in-depth accurate morphological or molecular analyses of the specimens found. Thus, these surveys often rely on external characteristics such as general aspect and pigmentation, without morphological or molecular confirmation on voucher specimens. External characters are too variable in many ascidians, especially colonial species, to be deemed reliable, as demonstrated recently in surveys of Botrylloides spp. in Europe (Viard et al. 2019). Indeed, taxonomic issues such as misidentifications or lack of resolution at low taxonomic levels are common problems of all survey methods (Campbell et al. 2007).
Paramount among ascidian NIS is the case of Didemnum vexillum Kott, 2002, a global invader in temperate waters. This species has a highly convoluted identification story, including several misidentifications in different areas and two descriptions as new species (reviewed in Lambert 2009). Eventually, genetic analyses proved that all populations so far recorded were conspecific (Stefaniak et al. 2009) and the name Didemnum vexillum (wrongly described as a native species in New Zealand, Kott 2002, see Lambert 2009) was adopted.
Didemnum vexillum is a species in principle easily identified based on external morphological characters, particularly when abundant on artificial substrates where it often smothers other organisms. In a population genomics study of Didemnum vexillum (Casso et al. 2019), using genotyping-by-sequencing methods, we routinely obtained samples from diverse localities (marinas or aquaculture facilities) around the world. Unexpectedly, inclusion in the analyses of specimens sampled in one location of the NE Atlantic (Roscoff-Bloscon Marina, English Channel, France) resulted in a drop of more than 90% in the number of polymorphic loci shared among all samples, an outcome usually due to the mixing of several divergent species (Pante et al. 2015b). These preliminary results thus suggested that these specimens belong to a highly divergent lineage. This prompted a re-examination of these samples and further collections at the same marina, which uncovered the existence of a new species, “vexillum”-like in appearance and living in syntopy with “true” Didemnum vexillum, which is described in this paper.
Material and methods
Morphological observation
We examined 17 colonies of Didemnum spp. collected in Bloscon Marina, Roscoff, France (48° 41.95′ N, 3° 57.93′ W, Fig. 1), on 27 April 2015 and preserved in absolute ethanol. We also analysed five colonies from the same marina sampled on 29 June 2018, from each of which a fragment was preserved in formalin and a second fragment in absolute ethanol.
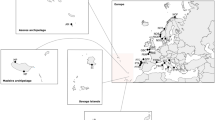
Southwestern Europe with indication of the type locality of Didemnum pseudovexillum sp. nov. (Atlantic) and the two localities where its presence has been inferred from previous data (Mediterranean). The map has been drawn with package rworldmap of R (https://cran.r-project.org/web/packages/rworldmap/index.html)
Morphological observation concentrated on the main features of colonies and zooids. Spicules were isolated from the tunic by dissolving tunic fragments in bleach (sodium hypochlorite, 35‰ concentration) in an oven at 80 °C. For scanning electron microscopy (SEM), the isolated spicules were then dehydrated in a graded alcohol series, sputter coated with gold, and observed in a Hitachi TM3000 microscope.
DNA extraction and amplification
We analysed six of the colonies collected in the sampling in April 2015 (hereafter colonies 1–6) and four of the colonies collected in June 2018 (colonies 7–10).
A fragment of about 590 bp of the cytochrome oxidase I (COI) mitochondrial gene was amplified and sequenced using primers designed by Stefaniak et al. (2009). For six colonies (1–6), the DNAs had been previously used to build the genomic libraries for GBS analyses and were obtained from a single thorax for each colony using a whole genome amplification (WGA) procedure as detailed in Casso et al. (2019). COI amplification was carried out in 20-μL final volume including 0.4 μL of each primer (10 mM), 1 μL MgCl2 (25 mM), 0.5 μL dNTPs (1 mM), 0.2 μL of Tq polymerase corresponding to 1 U (GoTaq, Promega), 4 μL 5× buffer (GoTaq, Promega), and 1 μL of DNA at a concentration of 50 ng/μL. PCR started with an initial denaturation at 94 °C for 5 min, followed by 35 cycles of a denaturation step at 94 °C for 1 min, an annealing step at 50 °C for 1 min, and an elongation step at 72 °C for 1 min, and a final elongation step at 72 °C for 7 min. The amplified DNA was purified with Exo-SAP (0.2 U/μL exonuclease and 0.2 U/μL shrimp phosphatase) at a proportion of 1:2 (ExoSap:PCR product). The sequences for both strands were obtained at the Scientific and Technical Services of the University of Barcelona. For the other four colonies (7–10), five thoraces were pooled per colony and extracted using the REDExtract-N-Amp tissue kit (Sigma-Aldrich), following manufacturer’s recommendations. PCR amplification was done in 20-μL total reaction volume with 10 μL of REDExtract-N-Amp PCR reaction mix (Sigma-Aldrich), 0.8 μL (10 mM) of each primer, 6.4 μL of ultra-pure water (Sigma-Aldrich), and 2 μL of DNA at a concentration of ca. 5 ng/μL. PCR conditions were set as before. Sequencing was carried out (both strands) at Macrogen facilities (Netherlands). The resulting sequences were assembled, edited, and aligned in BioEdit v.7.2.6 (Hall 1999).
Genetic analyses
To compare the obtained sequences with those already existing for the genus, we performed a search in the Barcode of Life Database (BOLD) at http://v3.boldsystems.org (accessed 20 Dec 2019). The query comprised all COI-5P sequences available in public databases with taxonomy = Didemnum. Sequences were recorded by species name and by Barcode Index Numbers (BINs, Ratnasingham and Hebert 2013). We aligned the sequences using the in-built BOLD aligner, eliminating sequences with contaminants and with stop codons.
The sequences were trimmed to a common length of 597 bp and collapsed into haplotypes using the online tool FaBox v.1.5 (Villesen 2007) at http://users-birc.au.dk/palle/php/fabox/index.php. The sequences obtained in the present study were added to the alignment, and a preliminary NJ tree was constructed using Mega7 software (Kumar et al. 2016). A perusal of this tree showed several inconsistencies among the downloaded sequences. For this reason and for ease of presentation of results, we selected a subset of sequences based on the following criteria: we deleted sequences without a species name when they did not fall close to our sequences in the tree, and for species or clades with many sequences, we randomly picked five haplotypes each. Finally, we deleted sequences that looked clearly divergent or misplaced in the trees and whose BLAST results suggested that they were erroneous sequences (possibly contaminations or errors in species identification).
The aligned sequences were then evaluated with the modelTest function of the R package phangorn (Schliep 2011) to select the best-fit evolutionary model of nucleotide substitution based on the Akaike information criterion (AIC). This model was then selected in a maximum likelihood tree search in Mega with default options and 1000 bootstrap replicates. A sequence of Diplosoma listerianum was used as an outgroup. A species delimitation analysis was performed in this tree using three approaches, different in nature and properties, to ensure confidence in the outcome of the species delineation. We used first multi-rate Poisson tree processes (mPTP, Kapli et al. 2016) as implemented in the web-service available at http://mptp.h-its.org using the default values. We also ran an Automatic Barcode Gap Discovery (ABGD, Puillandre et al. 2012) analysis using the web-service (https://bioinfo.mnhn.fr/abi/public/abgd/) with simple distance and a relative gap width of one. We explored a range of prior intraspecific divergences between 0.01 and 0.1. Finally, we used the single threshold general mixed Yule coalescent (GMYC) model (Pons et al. 2006); the analysis was performed with the R library splits (Ezard et al. 2009), using an ultrametric tree built with Mega using the RelTime method (Tamura et al. 2012).
Results
All of the colonies collected in 2015 and two of those collected in 2018 belonged to the new species, while another three colonies sampled in 2018 were morphologically assignable to Didemnum vexillum based on spicules and zooid characteristics (no larvae present) following Lambert (2009) and Ordóñez et al. (2015). This morphological distinctiveness was also confirmed with sequence data (see below).
Description
Didemnum pseudovexillum sp. nov. Turon & Viard
http://zoobank.org/F14EE06A-7A00-4FE2-8378-262FA464EF9C
Holotype: colony 8 Bloscon Marina, Roscoff, 29/06/2018. Paratypes: colonies 1 to 4, Bloscon Marina, Roscoff, 27/04/2015; colony 7, Bloscon Marina, Roscoff, 25/06/2018. Deposited at the Center of Resources for Animal Biodiversity (formerly Museum of Zoology) of the University of Barcelona, refs CRBA-90721 (holotype) and CRBA-90722 to CRBA-90726 (paratypes).
Etymology: the name pseudovexillum refers to the close external resemblance of this species to Didemnum vexillum, and thus calls for caution to avoid confusing the two species on the basis of the external aspect.
The colonies are large and encrusting and are highly abundant in the marina studied. When the available space is occupied, the colonies tend to generate uprising lobes giving them a tri-dimensional appearance. The colour is yellowish-orange, and the surface shows darker canals surrounding zones with zooidal apertures. Overall, the aspect is indistinguishable from Didemnum vexillum colonies found in close syntopy (exactly the same walls) in the marina studied (Fig. 2).
The colony surface has a whitish tinge due to the presence of spicules, with white rims corresponding to spicule accumulations in the oral siphons (Fig. 3a). The colony thickness reaches 2–3 mm. There is a thin distal tunic layer with more or less abundant spicules (never so abundant as to give this layer a coriaceous consistence) and a thick basal layer poor in spicules (Fig. 3b). In-between lie the thoraces of the zooids, whose abdomens are embedded in the upper part of the basal layer. The cavity of the colony runs between these two tunic layers, with the main canals penetrating the basal tunic between abdomens (Fig. 3b).
Didemnum pseudovexillum sp. nov. a Colony surface; b colony section, arrows point to canals; c ventral view of a thorax, showing a thoracic organ (arrow); d abdomen with testis; e abdomen with a large and a small oocyte; f–h Different larvae. Scale bars: a and b, 2 mm; c–h, 250 μm (note common scale bar)
The spicules are generally between 20 and 30 μm in diameter, reaching up to 40 μm (Fig. 4a–c). They have many somewhat bluntly tipped short rays, about 30 in the visible field and ca. 10 in the optical section. This is in stark contrast with the spicules of Didemnum vexillum from the same locality, with fewer (ca. 12 visible, 7 in optical section) and more pointed rays (Fig. 4d), in agreement with previous descriptions (Lambert 2009; Ordóñez et al. 2015).
The thoraces (Fig. 3c) are strongly contracted and measure ca. 0.5 mm. They have six small pointed lobes in the oral siphon, a wide atrial aperture exposing most of the branchial sac and no atrial languet. There are four stigmata rows, the exact number of stigmata could not be counted due to the strong contraction. The thoracic organs break away easily, but when present they lie in the lower part of the thorax and have an ear-like appearance (Fig. 3c). There is a muscular appendix of variable length, but generally shorter than the thorax itself, perhaps due to its contractibility. It originates in the anterior part of the oesophageal neck.
The abdomens reach ca. 0.6 mm; they contain a simple digestive system with an oval stomach. Many zooids have testis, consisting of a single follicle with a coiled sperm duct describing 6–7 turns (Fig. 3d). Some abdomens have also incubating oocytes, generally a single large one, sometimes a second smaller oocyte (Fig. 3e). In some cases, both testis and a small oocyte are present.
There are embryos and larvae in most of the colonies examined from both April 2015 and June 2018. They are free in the basal layer of the tunic. The larvae (Fig. 3f–h) measure ca. 0.5 mm. They have 3 adhesive papillae and a variable number of finger-like ectodermal ampullae. Four pairs are present in young larvae and, as they mature, more ectodermal ampullae are added. Careful examination is necessary to assess their number and disposition, but we never observed 6 pairs of ampullae. In contrast, there are always 6 pairs of them in mature Didemnum vexillum larvae (Lambert 2009; Ordóñez et al. 2015). Some arrangements found in our specimens are 4 pairs plus a dorsal unpaired ampulla, 4 pairs plus a single dorsal and a single ventral ampulla, 5 pairs, 5 pairs plus a single dorsal ampulla.
Genetic analyses
Of the sequenced specimens, colonies 1–6 (sampled in 2015) and colonies 7–8 (sampled in 2018), all morphologically assigned here to Didemnum pseudovexillum sp. nov., shared the same haplotype, while colonies 9 and 10 (2018), which were identified as Didemnum vexillum, had a different haplotype each. The three sequences have been uploaded to GenBank (accession numbers; colonies 1–8, MN952978; colony 9, MN952979; colony 10, MN952980).
The initial Didemnum dataset obtained from BOLD comprised 254 records, of which 214 had Barcode Index Numbers (BINs) assigned. They represented 36 nominal species and 51 BINs. This original alignment is available as Online Resource 1. Using the barcode gap analysis tool of BOLD, we found an intraspecific distance of 5.84 ± 0.32% (mean ± SE) and a distance to the nearest species of 13.29 ± 0.34%. The BIN discordance analysis tool of BOLD detected three discordant BINs with multiple species-level designations. Another 20 BINs were taxonomically concordant, while 28 BINs comprised only singletons.
After trimming to 597 bp and collapsing identical haplotypes, we obtained an alignment of 161 sequences, to which we added the sequences obtained in the present study. Congruent with the results of the BIN discordance analysis, a preliminary NJ tree (not shown) detected again some sequence misplacement (i.e. sequences assigned to the same species name but appearing in diverse clusters). We then prepared a refined dataset selecting a maximum of 5 sequences belonging to a given species or clade, deleting sequences without species names (except those topologically close to our sequences) and those that were highly divergent and/or had suspicious BLAST results. Note that, for Didemnum vexillum, we included sequences of the two main clades recognized in Stefaniak et al. (2012) that we named as in that work (clades A and B). This reduced dataset allowed us to refine the alignment, eliminating gaps introduced by the divergent sequences, to a final length of 582 bp. The final dataset, available as Online Resource 2, comprised 66 sequences, to which a sequence of Diplosoma listerianum (GenBank accession number KF791870) was added as outgroup.
The final dataset comprised 20 Didemnum species and 29 BINs. We re-ran the BOLD barcode gap analysis and obtained lower values of intraspecific distance (3.26 ± 0.24%, mean ± SE) and distance to the nearest species (12.93 ± 0.19%) than with the initial dataset. With the final dataset, there were no discordant BINs (assessed with the discordance analysis tool), with 11 concordant BINs and 18 singleton BINs.
The modelTest function of phangorn revealed that the best-fit model of nucleotide selection for our Didemnum dataset was the general time reversible model with a gamma distributed rate variation among sites and a proportion of invariable sites (GTR+G+I). This model was input in the ML tree construction algorithm of Mega and the corresponding phylogenetic tree obtained is depicted in Fig. 5 (G parameter = 0.795, I parameter = 17.61%). The sequences obtained from specimens sampled in Roscoff either grouped with Didemnum vexillum clade A (colonies 9 and 10), confirming morphological identification, or formed a clade (the single haplotype shared by colonies 1–8) with sequences of two unidentified Didemnum species from Catalan harbours, labelled as Didemnum sp1 and Didemnum sp2 in the work by López-Legentil et al. (2015). The distance between the Roscoff sequences and Didemnum sp2 was 2%, and with Didemnum sp1, it was 4.9%. This clade of three sequences had bootstrap support of 99%. The sister clade (albeit poorly supported, < 50%) in the tree comprised two sequences identified as Didemnum cineraceum (Sluiter, 1898) from Brazil (Oliveira et al. 2017) and one sequence from Australia identified as Didemnum cf. albopunctatum Sluiter, 1909 (Erwin et al. 2014). The Roscoff sequences had between 12.9 and 16.4% divergence with the sequences of this sister clade.
Maximum likelihood tree of the Didemnum dataset. For each branch, GenBank accession number and sequence id are provided. Numbers in main branches indicate bootstrap support values (when > 50%). Clades suggested to correspond to species are indicated by asterisks (mPTP method), by inverted triangles (ABGD method), and by triangles (GMYC method). The two clades of Didemnum vexillum (following the same names as in Stefaniak et al. 2012) are indicated
The species delineation analysis, made with mPTP, identified 19 putative species, mostly coherent with taxonomic identifications (20 nominal species in the tree), but with a few exceptions (Fig. 5). Interestingly, the clade comprising colonies 1–8, Didemnum sp2, and Didemnum sp1 was identified as one of these putative species. The ABGD method identified 29 distinct entities (i.e. putative species), with again some incongruences with taxonomic identification (Fig. 5). In agreement with the mPTP results, the colonies 1–8, Didemnum sp2, and Didemnum sp1 were identified as a single putative species. Finally, the GMYC method identified 30 groups, which were the same as in the ABGD analysis, with the only exception that Didemnum sp1 was placed as a separate entity from the one formed by colonies 1–8 and Didemnum sp2 (Fig. 5).
Discussion
The morphological analyses confirmed that in the Bloscon Marina in Roscoff (English Channel, France), Didemnum vexillum coexists with a new species, Didemnum pseudovexillum sp. nov. Both species are abundant and can be intermingled in the same micro-habitat (here, the same walls in the marina studied). There is virtually no external difference between them. On close examination, it seems that Didemnum pseudovexillum sp. nov. tends to have more oral siphon openings in the darker canal areas, and there is a more marked whitish tinge in the oral siphons due to spicule accumulation. However, in this species, as stated by Lambert (2009) for Didemnum vexillum as well, spicule density varies between colonies and even between various parts of the same colony. Clearly, these external characters are too unreliable to be used in the field. On the other hand, the spicules are clearly different and proved a useful diagnostic character. Larvae are also different, as Didemnum vexillum larvae have consistently 6 pairs of ectodermal finger-like antero-lateral ampullae, while Didemnum pseudovexillum sp. nov. has between 4 and 5 pairs. The number of coils in the sperm duct is also lower (6–7) than in Didemnum vexillum (8–11, Lambert 2009; Ordóñez et al. 2015). Finally, a recent study (Casso et al. 2020) showed that the microbiome communities of Didemnum vexillum and Didemnum pseudovexillum sp. nov. (referred to as Didemnum sp. in that work) were also markedly different. In Casso et al. (2020), the microbiome of Didemnum vexillum in its native and introduced range was examined, and samples of Didemnum pseudovexillum sp. nov. were used for comparison, showing that even congeneric species living in the same kind of environment had species-specific microbiomes.
The phylogenetic tree revealed a clade highly supported by bootstrap analysis (99%) comprising the Didemnum pseudovexillum sp. nov. sequences obtained in Roscoff and two sequences previously reported by López-Legentil et al. (2015) from Catalan harbours (NW Mediterranean, Fig. 1). In that work, they were named Didemnum sp1 (collected in L’Escala, 42° 07.00′ N; 3° 08.60′ E) and Didemnum sp2 (sampled in Port de la Selva, 42° 20.20′ N; 3° 11.90′ E). Unfortunately, the specimens from this study are no longer available, but one of us (XT) kept pictures of them and notes. The images revealed that colonies are small but with the same colouration as the ones from Roscoff. For Didemnum sp2, we kept morphological notes and, although the colony was not reproductive, spicules and zooid morphology were in complete agreement with the description of Didemnum pseudovexillum sp. nov. Unfortunately, there were no observations available on Didemnum sp1. The three methods of species delineation gave overall coherent results, but ABGD and GMYC tended to split the clades into species more than the mPTP method (29–30 vs 19 inferred species). It should be noted that the mPTP analysis yielded results that matched closely the nominal species assignment (20 species), albeit with some exceptions. Concerning our samples, the clade comprising Didemnum pseudovexillum sp. nov., Didemnum sp2, and Didemnum sp1 was recognized as a putative species by mPTP and ABGD, but Didemnum sp1 was placed as a distinct entity by GMYC. The Didemnum sp2 sequence was highly similar (98%) to the haplotype observed for the eight colonies sampled in Roscoff (98%), while Didemnum sp1 had 4.9% divergence. This slightly higher divergence is likely to explain the discrepancy between the results of the species delineation methods. However, the divergence between Didemnum pseudovexillum sp. nov. and Didemnum sp1 is well below the range of interspecies differences in the genus (Stefaniak et al. 2009, and present results). In addition, the tendency of GMYC to over-split has been pointed out in other studies (e.g. Pentinsaari et al. 2017). So, albeit further studies are necessary, we consider colonies 1–8, Didemnum sp1, and Didemnum sp2 to belong to the same species. Whatever the final placement of Didemnum sp1, Didemnum pseudovexillum sp. nov. is present both in Atlantic and Mediterranean harbours. This conclusion implies that, despite genetic COI uniformity in Roscoff, there may be a notable intraspecies genetic variability for that gene. Furthermore, during the genomic study of Didemnum vexillum performed by Casso et al. (2019), we sampled the population of Roscoff (not included in that work when it was realized that it was a different species), and we found 1716 polymorphic loci with a mean of 2.72 alleles/locus (authors’ unpublished results), a value in the range of the variability found in the Didemnum vexillum populations analysed (2.71–3.32 alleles/locus, Casso et al. 2019). Thus, the level of genetic variability of Didemnum pseudovexillum sp. nov. seems to be as high as that of similar introduced species. Further specific studies are necessary to assess the exact degree of genetic variation in populations of the new species.
The sister clade of Didemnum pseudovexillum sp. nov. comprised two sequences of Didemnum cineraceum from Brazil (Oliveira et al. 2017). This species has been reported from both sides of the Atlantic and the Pacific (Monniot 1983; Monniot and Monniot 1994; Monniot 1995; Rocha and Bonnet 2009; Lambert 2019). It has a very different type of larva (twice as large and gemmiparous, Monniot 1983; Neves 2015). The sister clade included also a sequence identified as Didemnum cf. albopunctatum by Erwin et al. (2014). This Australian specimen had a very different colony aspect and spicules. This sister clade is thus unlikely to be the same species, as also supported by the three methods used in the species delineation analysis.
The native versus non-native status of the new species is unclear, and it should be classed for the time being as cryptogenic (Carlton 1996). It is, however, noteworthy that Didemnum pseudovexillum sp. nov. has been found, so far, only on artificial structures, and it displays a disjoint distribution across the Mediterranean Sea and the English Channel, two distinct biogeographic provinces. It is thus tempting to classify the new species as non-native in these places, or at least in one of the two provinces. Numerous NIS, among them many ascidians, are shared by the Mediterranean and English Channel harbours, such as Botrylloides violaceus Oka, 1927 and Botrylloides diegensis Ritter & Forsyth, 1917 (Viard et al. 2019). This pattern might be due to bivalve aquaculture activities, known to host many native and non-native tunicates (Carman et al. 2010), which might act as a relay towards other artificial habitats such as marinas. Non-native colonial tunicates, including Didemnum and Botrylloides species, might have been “hitch-hiked” with imports of oysters and mussels between Mediterranean and Atlantic regions of France and Spain. A more complete knowledge of the current geographic distribution and habitat is necessary to assign a definite status to Didemnum pseudovexillum sp. nov.
In the presence of a species suspected of being introduced, extreme care should be taken before describing it as a new species to ensure that it has not been described elsewhere. Failure to recognize a species as introduced and the creation of a new name for it leads to the so-called pseudo-indigenous species (Carlton 2009), a problem that has already occurred in ascidians. For instance, Didemnum vexillum was “re-described” as Didemnum vestum Kott in Kott (2004a) in New England. Styela clava Herdman, 1881 was similarly “re-described” as Styela mammiculata Carlisle, 1954 in the English Channel (Millar 1960). Clavelina phlegraea Salfi, 1929 was the name given to Mediterranean specimens of Clavelina oblonga Herdman, 1880 (Ordóñez et al. 2016).
To avoid the pseudo-indigenous species problem, we revised all described species of Didemnum. There are 237 species recognized in the Ascidiacea World Database (http://www.marinespecies.org/ascidiacea/, Shenkar et al. 2019) as of December 2019. For each species, we consulted primary literature (original descriptions whenever possible) and assessed colony aspect and spicules in the first place. In species where these characters were coherent with Didemnum pseudovexillum sp. nov., we further checked the literature for zooid and larval descriptions. The results of this perusal showed that the species found in Roscoff had not been previously described. Some species showing similarities are listed below. Of note here is that, with a few exceptions, there are no COI data for these species, and obtaining genetic information would be invaluable to complement the morphological perusal done.
Didemnum perlucidum Monniot, 1983 is another introduced species that forms large investing colonies on artificial substrates and is widespread in tropical and subtropical waters worldwide (Smale and Childs 2012; Dias et al. 2016; Lambert 2019). However, this species is usually whitish, and the spicules are different, with fewer and more pointed rays, from those of Didemnum pseudovexillum sp. nov. (Monniot 1983; Neves 2015). Genetically, Didemnum perlucidum is also clearly different from the new species (Fig. 5).
Didemnum lahillei (Hartmeyer, 1909) has honey-coloured colonies with sparse spiculation. It can be abundant in shallow waters in Europe (Lafargue and Wahl 1987). However, the spicules are burr-like and the larvae have 5–6 pairs of ectodermal ampullae (Lafargue and Wahl 1987).
Didemnum psammatodes (Sluiter, 1895) is an invasive species, often reported from harbours, occurring in all warm waters (Kott 2001; Monniot 2016). It can form large colonies, sometimes with a tri-dimensional structure, and has brownish colour and sparse spiculation. It is characterized by the abundance of faecal pellets embedded in the colony, which is not observed in Didemnum pseudovexillum sp. nov. In addition, the spicules of Didemnum psammatodes include burr-like spicules (Monniot 1983; Kott 2001) not present in Didemnum pseudovexillum sp. nov. In our phylogenetic tree (Fig. 5), Didemnum psammatodes appears closely related to Didemnum vexillum, but markedly different from Didemnum pseudovexillum sp. nov.
Didemnum spumosum Kott in Kott 2004b, reported from Australia, has complex, three-dimensional colonies and similar zooid and spicule morphology. However, the sperm duct has more coils (10) and the larvae are larger than in Didemnum pseudovexillum sp. nov. (0.75 mm, Kott 2004b).
Didemnum mesenbrinum Monniot in Monniot et al. (2001) forms large crusts covering all substrata in South Africa. Its colour is whitish or cream, and the spicules are not very abundant (Monniot et al. 2001). The spicules are similar to the ones of Didemnum pseudovexillum sp. nov., but the atrial aperture of the zooids is different, being narrow or even slit-like (in contracted thoraces) instead of exposing most of the branchial sac as in the new species.
We summarize in Table 1 the main morphological differences between the new species and the three widespread invasive species in the genus (Didemnum vexillum, Didemnum perlucidum, Didemnum psammatodes) as well as with the closest species in our genetic tree (Didemnum cineraceum).
In conclusion, a new species of Didemnum is described which is present in some Atlantic and Mediterranean marinas. It can be dominant in fouling communities on artificial substrates, as it was the case in the marina of Roscoff (Brittany, France), where all the colonies sampled in 2015 and more than half of those collected in 2018 were Didemnum pseudovexillum sp. nov. Morphological and genetic data support the establishment of a new species. Its status should be considered cryptogenic until more information can be gathered, but it is likely an introduced species of unknown origin.
This case study adds to previous ones (e.g. Botrylloides spp., Viard et al. 2019) calling for caution when using field survey methods (such as RAS or BioBlitz surveys), based on easy-to-use external morphological characters, to monitor colonial tunicates. This is unfortunate as these taxa are among the most invasive species at a global level. It is important to note that fast field assessment surveys, such as RAS, are a powerful and needed tool, allowing a cost-effective surveillance of large territories with a high temporal frequency (Campbell et al. 2007; Kakkonen et al. 2019). They actually proved effective to monitor the spread of already-reported NIS (e.g. Cohen et al. 2005; Bishop et al. 2015) as well as to discover novel NIS (e.g. Asterocarpa humilis (Heller, 1878), Bishop et al. 2013). We thus certainly do not suggest that these field assessment methods should be abandoned. However, we do advocate for regular control of species lists obtained with these methods, for instance by means of genetic barcoding methods or by request to taxonomic specialists (if available). This would ensure the correctness of NIS lists, particularly in the context of surveillance programmes, such as those conducted under the Marine Strategy Framework Directive, as any mistake can be propagated in public databases. In the case of Didemnum vexillum, because of its external morphological similarity with Didemnum pseudovexillum sp. nov., observation of diagnostic molecular, such as COI sequencing, or morphological characters, such as spicules, should be compulsory, as well as keeping voucher specimens fixed in both formalin and ethanol. Our findings also imply the need for checking previous reports of Didemnum vexillum because of potential confusion with the new species.
References
Bishop JDD, Roby C, Yunnie ALE, Wood CA, Lévêque L, Turon X, Viard F (2013) The Southern Hemisphere ascidian Asterocarpa humilis is unrecognised but widely established in NW France and Great Britain. Biol Invasions 15:253–260
Bishop JD, Wood CA, Yunnie AL, Griffiths CA (2015) Unheralded arrivals: non-native sessile invertebrates in marinas on the English coast. Aquat Invasions 10:249–264
Bock DG, MacIsaac HJ, Cristescu ME (2012) Multilocus genetic analyses differentiate between widespread and spatially restricted cryptic species in a model ascidian. Proc R Soc B 279:2377–2385
Bortolus A (2008) Error cascades in the biological sciences: the unwanted consequences of using bad taxonomy in ecology. AMBIO 37:114–118
Brunetti R, Gissi C, Pennati R, Caicci F, Gasparini F, Manni L (2015) Morphological evidence indicates that Ciona intestinalis (Tunicata, Ascidiacea) type A and type B are different species: Ciona robusta and Ciona intestinalis. J Zool Syst Evol Res 53:186–193
Brunetti R, Griggio F, Mastrototaro F, Gasparini F, Gissi C (2020) Toward a resolution of the cosmopolitan Botryllus schlosseri species complex (Ascidiacea, Styelidae): mitogenomics and morphology of clade E (Botryllus gaiae). Zool J Linnean Soc zlaa023. https://doi.org/10.1093/zoolinnean/zlaa023
Campbell ML, Gould B, Hewitt CL (2007) Survey evaluations to assess marine bioinvasions. Mar Pollut Bull 55:360–378
Carlisle DB (1954) Styela mammiculata n.sp., a new species of ascidian from the Plymouth area. J. Mar. biol. Ass. UK 33:329–334
Carlton JT (1996) Biological invasions and cryptogenic species. Ecology 77:1653–1655
Carlton JT (1999) The scale and ecological consequences of biological invasions in the world’s oceans. In: Sandlund OT, Schei PJ, Viken Å (eds) Invasive species and biodiversity management. Kluwer Academic Publishers, Dordrecht, pp 195–212
Carlton JT (2009) Deep invasion ecology and the assembly of communities in historical time. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems. Springer-Verlag, Berlin Heidelberg, pp 13–56
Carman MR, Morris JA, Karney RC, Grunden DW (2010) An initial assessment of native and invasive tunicates in shellfish aquaculture of the North American east coast. J Appl Ichthyol 26:8–11
Casso M, Turon X, Pascual M (2019) Single zooids, multiple loci: independent colonisations revealed by population genomics of a global invader. Biol Invasions 21:3575–3592
Casso M, Turon M, Marco N, Pascual M, Turon X (2020) The microbiome of the worldwide invasive ascidian Didemnum vexillum. Front Mar Sci 7:201. https://doi.org/10.3389/fmars.2020.00201
Cohen AN, Harris LH, Bingham BL, Carlton JT, Chapman JW, Lambert CC, Lambert G, Ljubenkov JC, Murray SN, Rao LC, Reardon K, Schwindt E (2005) Rapid assessment survey for exotic organisms in southern California bays and harbors, and abundance in port and non-port areas. Biol Invasions 7:995–1002
Cohen AN, McCann L, Davis T, Shaw L, Ruiz G (2011) Discovery and significance of the colonial tunicate Didemnum vexillum in Alaska. Aquat Invasions 6:263–271
Comtet T, Sandionigi A, Viard F, Casiragi M (2015) DNA (meta)barcoding of biological invasions: a powerful tool to elucidate invasion processes and help managing aliens. Biol Invasions 17:905–922
Dias PJ, Rocha R, Godwin S, Tovar-Hernández MA, Delahoz MV, McKirdy S, de Lestang P, McDonaid JI, Snow M (2016) Investigating the cryptogenic status of the sea squirt Didemnum perlucidum (Tunicata, Ascidiacea) in Australia based on a molecular study of its global distribution. Aquat Invasions 11:239–245
Erwin PM, Pineda MC, Webster N, Turon X, López-Legentil S (2014) Down under the tunic: bacterial biodiversity hotspots and widespread ammonia-oxidizing archaea in coral reef ascidians. ISME J 8:575–588
Ezard T,Fujisawa T, Barraclough TG. 2009. SPLITS: SPecies’ LImits by threshold statistics. R package version 1.0-18/r45, 2009. http://R-Forge.R-project.org/projects/splits/. Accessed 14 Jan 2020
Giangrande A (2003) Biodiversity, conservation, and the ‘taxonomic impediment’. Aquat Conserv 13:451–459.
Grey EK (2009) Do we need to jump in? A comparison of two survey methods of exotic ascidians on docks. Aquat Invasions 4:81–86
Griggio F, Voskoboynik A, Iannelli F, Justy F, Tilak MK, Turon X, Pesole G, Douzery EJP, Mastrototaro F, Gissi C (2014) Ascidian mitogenomics: comparison of evolutionary rates in closely related taxa provides evidence of ongoing speciation events. Genome Biol Evol 6:591–605
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hartmeyer R (1909) Ascidien (continuation of work by Seeliger). In: Bronn HG (ed) Klassen und Ordnungen des Tier-Reichs. CF Winter’sche Verlagshandlung, Leipzig
Heller C (1878) Beiträge zur nähern Kenntnis der Tunicaten. Sitzber Acad Wiss Wien 77:2–28
Herdman WA (1880) Preliminary report on the Tunicata of the Challenger expedition. Part 2. Proc R Soc Edinburgh 10(2):714–726
Herdman WA (1881) Preliminary report on the Tunicata of the Challenger expedition. Cynthiidae Proc Roy Soc Edinburgh 11(3):52–88
Kakkonen JE, Worsfold TM, Ashelby CW, Taylor A, Beaton K (2019) The value of regular monitoring and diverse sampling techniques to assess aquatic non-native species: a case study from Orkney. Manag Biol Invasion 10:46–79
Kapli T, Lutteropp S, Zhang J, Kobert K, Pavlidis P, Stamatakis A, Flouri T (2016) Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 33:1630–1638
Kott P (1985) The Australian Ascidiacea. Part 1, Phlebobranchia and Stolidobranchia. Mem Qd Mus 23:1–440
Kott P (2001) The Australian Ascidiacea. Part 4, Aplousobranchia (3), Didemnidae. Mem Qd Mus 47:1–407
Kott P (2002) A complex didemnid ascidian from Whangamata, New Zealand. J Mar Biol Ass UK 82:625–628
Kott P (2004a) A new species of Didemnum (Ascidiacea, Tunicata) from the Atlantic coast of North America. Zootaxa 732:1–10
Kott P (2004b) New and little-known species of Didemnidae (Ascidiacea, Tunicata) from Australia (part 1). J Nat Hist 38:731–774
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Lafargue F, Wahl M (1987) The didemnid ascidian fauna of France. Ann Inst océanogr, Paris 63:1–46
Lambert G (2007) Invasive sea squirts: a growing global problem. J Exp Mar Biol Ecol 342:3–4
Lambert G (2009) Adventures of a sea squirt sleuth: unraveling the identity of Didemnum vexillum, a global ascidian invader. Aquat Invasions 4:5–28
Lambert G (2019) Fouling ascidians (Chordata: Ascidiacea) of the Galapagos: Santa Cruz and Baltra Islands. Aquat Invasions 14:132–149
Linnaeus C (1767) Systema naturae per regna tria naturae: secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Ed. 12. 1., Regnum Animale. 1 & 2. Holmiae, Laurentii Salvii. Holmiae Stockholm, Laurentii Salvii. pp 533–1327
López-Legentil S, Turon X (2005) How do morphotypes and chemotypes relate to genotypes? The colonial ascidian Cystodytes (Ascidiacea: Polycitoridae). Zool Scripta 34:3–14
López-Legentil S, Turon X, Planes S (2006) Genetic structure of the star sea squirt, Botryllus schlosseri, introduced in southern European harbours. Mol Ecol 15:3957–3967
López-Legentil S, Legentil ML, Erwin PM, Turon X (2015) Harbor networks as introduction gateways: contrasting patterns of native and introduced ascidians. Biol Invasions 17:1623–1638
Malfant M, Darras S, Viard F (2018) Coupling molecular data and experimental crosses sheds light about species delineation: a case study with the genus Ciona. Sci Rep 8:1480
Millar RH (1960) The identity of the ascidians Styela mammiculata Carlisle and S. clava Herdman. J. Mar. biol. Ass. UK 39:509–511
Milne Edwards H (1841) Observations sur les ascidies composées des côtes de la Manche. Mem Acad Sci Paris 18:217–326
Monniot F (1983) Ascidies littorals de Guadeloupe. I. Didemnidae. Bull Mus natn Hist nat, Paris, 4e Sér. 16, Section A, 1:5–49
Monniot F (1995) Ascidies de Nouvelle-Calédonie. XV. Le genre Didemnum. Bull Mus natn Hist nat, Paris, 4e Sér. 5, Section A, 2-4:299–344
Monniot F (2016) Ascidians (Tunicata) of the French Guiana expedition. Zootaxa 4114:201–245
Monniot C, Monniot F (1994) Additions to the inventory of Eastern tropical Atlantic ascidians: arrival of cosmopolitan species. Bull Mar Sci 54:71–93
Monniot C, Monniot F, Griffiths CL (2001) South African ascidians. Ann S African Mus 108:1–141
Nall CR, Guerin AJ, Cook EJ (2015) Rapid assessment of marine non-native species in northern Scotland and a synthesis of existing Scottish records. Aquat Invasions 10:107–121
Neves IM (2015) Didemnidae ascidians (Tunicata, Ascidiacea) from Bocas del Toro – Panamá. PhD Thesis Dissertation, Universidade Federal do Paraná
Ojaveer H, Galil BS, Gollasch S, Marchini A, Minchin D, Occhipinti-Ambrogi A, Olenin S (2014) Identifying the top issues of marine invasive alien species in Europe. Management of Biol Invasions 5:81–84
Oka A (1927) Zur Kenntnis der japanischen Botryllidae (Vorläufige Mitteilung). Proc Imp Acad 3:607–609
Oliveira FAS, Michonneau F, Lotufo TMC (2017) Molecular phylogeny of Didemnidae (Ascidiacea: Tunicata). Zool J Linnean Soc 180:603–612
Ordóñez V, Pascual M, Fernández-Tejedor M, Pineda MC, Tagliapietra D, Turon X (2015) Ongoing expansion of the worldwide invader Didemnum vexillum (Ascidiacea) in the Mediterranean Sea: high plasticity of its biological cycle promotes establishment in warm waters. Biol Invasions 17:2075–2085
Ordóñez V, Pascual M, Fernández-Tejedor M, Turon X (2016) When invasion biology meets taxonomy: Clavelina oblonga (Ascidiacea) is an old invader in the Mediterranean Sea. Biol Invasions 18:1203–1215
Pallas PS (1766) Elenchus zoophytorum sistens generum adumbrationes generaliores et specierum cognitarum succintas descriptiones, cum selectis auctorum synonymis. Fransiscum Varrentrapp, Hagae
Pante E, Puillandre N, Viricel A, Arnaud-Haond S, Aurelle D, Castelin M, Chenuil A, Destombe C, Forcioli D, Valero M, Viard F, Samadi S (2015a) Species are hypotheses: avoid connectivity assessments based on pillars of sand. Mol Ecol 24:525–544
Pante E, Abdelkrim J, Viricel A, Gey D, France SC, Boisselier MC, Samadi S (2015b) Use of RAD sequencing for delimiting species. Heredity 114:450–459
Pentinsaari M, Vos R, Mutanen M (2017) Algorithmic single-locus species delimitation: effects of sampling effort, variation and nonmonophyly in four methods and 1870 species of beetles. Mol Ecol Resour 17:393–404
Pérez-Portela R, Arranz V, Rius M, Turon X (2013) Cryptic speciation or global spread? The case of a cosmopolitan marine invertebrate with limited dispersal capabilities. Sci Rep 3:3197
Pons J, Barraclough TG, Gomez-Zurita J, Cardoso A, Duran DP, Hazell S, Kamoun S, Sumlin WD, Vogler AP (2006) Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst Biol 55:595–609
Puillandre N, Lambert A, Brouillet S, Achaz G (2012) ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol 21:1864–1877
Ratnasingham S, Hebert PDN (2013) A DNA-based registry for all animal species: the Barcode Index Number (BIN) system. PLoS One 8:e66213
Ritter WE, Forsyth RH (1917) Ascidians of the littoral zone of southern California. Univ California Publ Zool 16:439–512
Rocha RM, Bonnet NYK (2009) Ascidias (Tunicata, Ascidiacea) introduzidas no Arquipélago de Alcatrazes, Sao Paulo. Iheringia, Sér Zool, Porto Alegre 99:27–35
Salfi M (1929) Sulla blastogenesi in Clavelina e su una nuova specie del genere. Pub Staz Zool Napoli 9:195–201
Savigny JC (1816) Mémoires sur les animaux sans vertèbres, seconde partie. CLF Panckoucke, Paris
Schliep KP (2011) phangorn: phylogenetic analysis in R. Bioinformatics 27:592–593
Shenkar N, Swalla BJ (2011) Global diversity of Ascidiacea. PLoS One 6:e20657
Shenkar N, Gittenberger A, Lambert G, Rius M, Rocha R, Swalla BJ, Turon X (2019) Ascidiacea World Database. http://www.marinespecies.org/ascidiacea. Accessed 12 Dec 2019
Sluiter CP (1895) Tunicaten. In: Semon R (ed). Zoologische Forschungsreisen in Australien und den malagischen Archipel. Denkschr. Gesellsch, Jena 8:163–186
Sluiter CP (1898) Tuniciers recueillis en 1896 par la Chazalie dans la mer des Antilles. Mem Soc Zool France 11:5–34
Sluiter CP (1909) Die Tunicaten der Siboga-Expedition. Part 2. Die merosomen Ascidien. Siboga-Expedition 56:1–112
Smale DA, Childs S (2012) The occurrence of a widespread marine invader, Didemnum perlucidum (Tunicata, Ascidiacea) in Western Australia. Biol Invasions 14:1325–1330
Stefaniak L, Lambert G, Gittenberger A, Zhang H, Lin S (2009) Genetic conspecificity of the worldwide populations of Didemnum vexillum Kott, 2002. Aquat Invasions 4:29–44
Stefaniak L, Zhang H, Gittenberger A, Smith K, Holsinger K, Lin S, Whitlatch RB (2012) Determining the native region of the putatively invasive ascidian Didemnum vexillum Kott, 2002. J Exp Mar Biol Ecol 422–423:64–71
Tamura K, Battistuzzi FU, Billing-Ross P, Murillo O, Filipski A, Kumar S (2012) Estimating divergence times in large molecular phylogenies. PNAS 109:19333–19338
Teske PR, Rius M, McQuaid CD, Styan CA, Piggott MP, Benhissoune S, Fuentes-Grünewald C, Walls K, Page M, Attard CRM, Cooke GM, McClusky CF, Banks SC, Barker NP, Beheregaray LB (2011) “Nested” cryptic diversity in a widespread marine ecosystem engineer: a challenge for detecting biological invasions. BMC Evol Biol 11:1–13
Turon X, Tarjuelo I, Duran S, Pascual M (2003) Characterising invasion processes with genetic data: an Atlantic clade of Clavelina lepadiformis (Ascidiacea) introduced into Mediterranean harbours. Hydrobiologia 503:29–35
Viard F, Roby C, Turon X, Bouchemousse S, Bishop J (2019) Cryptic diversity and database errors challenge non-indigenous species surveys: an illustration with Botrylloides spp. in the English Channel and the Mediterranean Sea. Front Mar Sci 6:615. https://doi.org/10.3389/fmars.2019.00615
Villesen P (2007) FaBox: an online toolbox for fasta sequences. Mol Ecol Res 7:965–968
Zhan A, Briski E, Bock DG, Ghabooli S, MacIsaac HJ (2015) Ascidians as models for studying invasion success. Mar Biol 162:2449–2470
Acknowledgments
We are grateful to Laurent Lévêque and the diving team (Mathieu Camusat, Yann Fontana, Wilfried Thomas) of the Marine & Diving Facilities of the FR2424 - Station Biologique de Roscoff, for the field sampling. We thank Andrea Fernández and Gustavo Carreras for help with the sequencing work. The comments of three anonymous reviewers helped to improve the article. All necessary authorizations for field sampling by diving in Roscoff were given by decisions of the Prefect of the Brittany Region (Decision 85/2015 of 18/02/2015 and Decision 154/2018 of 02/02/2018).
Funding
This research was funded by the project PopCOmics (CTM2017-88080, MCIU/AEI/FEDER/UE) from the Spanish Government. Additional support for sampling and surveys in Brittany came from the AquaNIS2.0 project, supported by the Foundation TOTAL. This is a contribution from the Consolidated Research Group “Benthic Biology and Ecology” SGR2017-1120 (Catalan Government).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for animal testing, animal care, and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling have been obtained by the authors from the competent authorities and are mentioned in the “Acknowledgments”. This study is compliant with CBD and Nagoya protocols.
Data availability
The sequences obtained in this study have been deposited in GenBank with accession numbers MN952978–80. All datasets analysed during this study are included as supplementary information files.
Author contributions
XT and FV conceived the research. FV contributed samples. MC and MP generated and analysed genetic data, with contribution from FV and XT. XT analysed morphological details and wrote the first draft of the manuscript. All authors contributed to the manuscript and approved its contents.
Additional information
Communicated by K. Kocot
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is registered in ZooBank under http://zoobank.org/A10F8027-8DB8-46EB-8F2F-BB1E8CD4468D
Rights and permissions
About this article
Cite this article
Turon, X., Casso, M., Pascual, M. et al. Looks can be deceiving: Didemnum pseudovexillum sp. nov. (Ascidiacea) in European harbours. Mar. Biodivers. 50, 48 (2020). https://doi.org/10.1007/s12526-020-01083-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-020-01083-7