Abstract
Okenia polycerelloides (Ortea & Bouchet, 1983) is a small goniodoridid nudibranch originally described from the Canary Islands. Its taxonomic history has been problematic since its original description, a situation that worsened after this taxon was synonymized with Okenia zoobotryon (Smallwood, 1910), one of the most problematic Okenia Menke, 1830 species. Because of their external similarity, it has been difficult to determine the differences between the two taxa without a meticulous anatomical study. Thus, we present herein the first complete anatomical study of O. polycerelloides, based on specimens from the type locality and from Brazil (the latter previously identified as O. zoobotryon). A topotype of O. zoobotryon was also analyzed. Additionally, we also performed a preliminary molecular analysis on these species. Okenia polycerelloides can be distinguished externally from O. zoobotryon by the absence of integumentary spicules and the presence of a posterodorsal papilla, and internally by characteristics of the digestive (shape of the salivary glands, length of the typhlosole) and reproductive systems (diameter and length of the penial sac; cilia on the penis; and shapes of the seminal receptacle, bursa copulatrix, and ampulla). Molecular analysis revealed a high genetic divergence in both COI and H3 genes between O. polycerelloides and O. zoobotryon, which support both species as distinct. Therefore, we present a redescription and propose to restore the species status of O. polycerelloides, and consider the records of O. zoobotryon in the South Atlantic Ocean as attributable to O. polycerelloides, until new evidence may suggest otherwise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Okenia polycerelloides (Ortea & Bouchet, 1983) was first described in 1983 as Bermudella polycerelloides from Tenerife, Canary Islands (Ortea and Bouchet 1983). Until then, Bermudella Odhner, 1941 was monospecific, with Bermudella zoobotryon (Smallwood, 1910) as the type species (originally Polycerella zoobotryon Smallwood, 1910) (Odhner 1941), with which B. polycerelloides would be later synonymized (Rudman 2004). The histories of these two species are mixed and confused, making it impossible to refer to one without referring to the other.
Okenia polycerelloides was originally attributed to the genus Bermudella by mistake, since the species has a radular formula of 1.1.0.1.1 (see Ortea and Bouchet 1983), characteristic of the genus Okenia Menke, 1830 (see Leuckart 1828; Menke 1830; Vayssière 1901); whereas in Bermudella, the formula is 3.1.0.1.3 (see Smallwood 1912; Odhner 1941). Similarly, Clark (1984) noted that the type species of Bermudella (P. zoobotryon) also has a radular formula of 1.1.0.1.1, instead of 3.1.0.1.3 as described by Smallwood (1910), and consequently, Clark proposed a new combination for the type species and synonymized the genera Bermudella and Okenia. Even after this, Ortea et al. (2009), referring to nudibranchs from the Canary Islands, considered the type species of Bermudella as Okenia zoobotryon (Smallwood, 1910), but still maintaining B. polycerelloides. Rudman (2004) recorded O. zoobotryon from Australia, considering B. polycerelloides as a synonym, without further remarks. Rudman (2006) briefly discussed this decision, suggesting that the synonym was established based only on analysis of the original descriptions of both species. Since then, the synonymy has been accepted by several authors, and many photographs of specimens identified as O. zoobotryon from different locations across the world have been published (e.g., Poddubetskaia 2004; Redfern 2004; Grune 2005; Valdés et al. 2006; García et al. 2008). However, not a single specimen of O. polycerelloides was analyzed by Rudman (2004), and until now, except by the radula described by Ortea and Bouchet (1983), no information was available about its internal anatomy.
The original description of O. polycerelloides provides only drawings and was based on external morphology and radular features (Ortea and Bouchet 1983). Similarly, despite the anatomical description by Smallwood (1912), the anatomy of the reproductive system of O. zoobotryon remained unknown until its recent redescription (see Pola 2015).
We present the first complete anatomical study of O. polycerelloides, based on specimens from the type locality (Canary Islands) and from Brazil (previously reported as O. zoobotryon). Comparing external and internal anatomical features, and molecular data, we discuss the systematic and nomenclatural status of O. polycerelloides and O. zoobotryon, concluding that both are valid species and that their world distributions need to be reviewed in light of the new data herein provided.
Material and methods
Morphological study
Most specimens were collected by the authors and collaborators at several locations along the Brazilian coast, mainly in the state of São Paulo, but also from Bahia and Santa Catarina states. Material referred to O. polycerelloides and previously identified as O. zoobotryon from the Malacological Collection of the Museu de Zoologia da Universidade de São Paulo (MZSP), the type material of O. polycerelloides from the Muséum national d’Histoire naturelle (MNHN), a specimen from the type locality (Tenerife, Canary Islands), and a topotype of O. zoobotryon lent by the Department of Invertebrate Zoology and Geology at the California Academy of Sciences (CASIZ 18106, Bermuda Island, Hamilton Parish, VI/2009) were also examined. Several individuals of O. polycerelloides were examined and photographed alive, as were some of the spawn and one juvenile. The animals were anesthetized with a mixture of isotonic magnesium chloride solution and menthol dissolved in seawater. Specimens were fixed in 70% ethanol or 10% formalin in seawater; all of them were preserved in 70% ethanol and deposited in the MZSP.
The description of external morphology is based on living and preserved specimens. Internal morphology was studied by means of standard dissection techniques (see Cunha and Simone 2019), and the drawings were done under a stereomicroscope fitted with a camera lucida. The radula, jaw, and penis were analyzed by scanning electron microscopy (SEM), following the methods of Alvim and Pimenta (2016).
Molecular study
Taxon sampling
Molecular sampling included three specimens of O. polycerelloides from Brazil (São Paulo state) and one specimen from Canary Island. Sequences of others eight Okenia species were retrieved from GenBank, and the genera Triopha Bergh, 1880, Tyrannodoris Willan & Chang, 2017, and Ancula Lovén, 1846 composed the outgroups for the phylogenetic analysis. Voucher information and accession numbers of all sequences are in Table 1.
DNA extraction, amplification, and sequencing
Total genomic DNA was extracted using Instagene Matrix Kit (Biorad). One mitochondrial (cytochrome oxidase I, COI) and one nuclear gene (histone 3 gene, H3) were sequenced using the following primers, respectively: dgLCO 5′-GGTCAACAAATCATAAAGAYATYGG-3′ and dgHCO: (5′-TAAACTTCAGGGTGACCAAARAAYCA-3′) (Meyer et al. 2005); H3AD F (5′-ATGGCTCGTACCAAGCAGCVGC-3′) and H3BD R (5′-ATATCCTTRGGCATRATRGTGAC-3′) (Colgan et al. 1998).
Polymerase chain reaction (PCR) amplification was carried out using a Mastercycler Nexus machine (Eppendorf) in a 25-μl total volume. Reactions were as follows, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM of MgCl2, 200 μM of each primer, and 1.0 unit of Taq DNA Polymerase (Thermo Fisher Scientific). All amplifications of COI and H3 genes wereperformed using a PCR program a dNTPs, 0.4 μM of each primer, and 1.0 unit of Taq DNA Polymerase (Thermo Fisher Scientific). All amplifications of COI and H3 genes wereperformed using a PCR program a of each primer, and 1.0 unit of Taq DNA Polymerase (Thermo Fisher Scientific). All amplifications of COI and H3 genes were performed using a PCR program according to the following protocol, 3-min denaturation at 95 °C, 35 subsequent cycles of 94 °C for 30 s, 50–52 °C for 30 s, 72 °C for 1 min, and a final extension step at 72 °C for 7 min. PCR products were purified using the Agencourt Ampure XP PCR Purification Kit (Beckman Coulter) and sequenced using the BigDye terminator v.3.1 sequencing kit and analyzed on an ABI 3730 capillary sequencer (Applied Biosystems).
Sequence alignment and analysis
Chromatographs were checked and consensus sequences were constructed from the forward and reverse sequences using CodonCode Aligner (v. 7.0.1, CodonCode Corporation). Final consensus sequences, together with the additional sequences of the Okenia spp. and the outgroups, were aligned using MUSCLE (Edgar 2004).
To measure the genetic distance between the species, a distance matrix based on the uncorrected p-distance was calculated in PAUP (as implemented on CIPRES Science Gateway, Miller et al. 2010). Phylogenetic relationships were recovered using a Bayesian Inference (BI), based on concatenated COI and H3 datasets, as implemented in MrBayes 3.2.6 (Ronquist and Huelsenbeck 2003), also available at CIPRES Science Gateway (Miller et al. 2010). The best-fitting models of sequence evolution were determined based on the Akaike Information Criterion (AIC) as implemented in jModelTest2 2.1.6 (Darriba et al. 2012). The Bayesian analysis performed two independent runs and four Markov Chain Monte Carlo (MCMC), starting with a random seed. Each run consisted of 10,000,000 generations, sampled at every 1000th generation. Convergence between chains and ESS (effective sample size) values were checked in Tracer 1.6. (Rambaut et al. 2018). After discarding the first 25% trees as burn-in, a 50% majority-rule consensus tree and posterior probabilities (PP) for node support were calculated using the remaining trees from both chains and values > 0.95 were considered strongly supported. Final trees were visualized and edited with FigTree v.1.4.2 (Rambaut 2007).
Results
Systematics
Order Nudibranchia Cuvier, 1817
Family Goniodorididae H. Adams & A. Adams, 1854
Genus Okenia Menke, 1830
Okenia polycerelloides (Ortea & Bouchet, 1983)
Bermudella polycerelloides Ortea & Bouchet, 1983: 51; Ortea et al. 1996: 128; Ortea et al. 2009: 79 (pl. 2, fig. B); Caballer-Gutiérrez et al. 2015: 242 (pl. 4, fig. C).
Okenia zoobotryon: García et al. 2008: 102 (non Smallwood 1910); Rios 2009: 422 [in part] (non Smallwood 1910).
Material examined
Type material
Canary Island, Tenerife, Los Cristianos Beach, no collection data, MNHN (holotype, MNHN-IM-2000-28179; paratype, MNHN-IM-2000-28180) (Holotype, Fig. 1a–c).
Additional material
Canary Islands, Tenerife, Los Cristianos, 3 m depth, MZSP 139548, 1 specimen (dissected, 6 mm long when preserved, 23/I/2003). Brazil, São Paulo state: Ubatuba, Marina Kawai, 1–2 m depth, MZSP 139553, 38 specimens (6 dissected, 3–6 mm long preserved, 21/I/2013); São Sebastião, Araçá Bay, MZSP 139549, 4 specimens (4 dissected, 3–5 mm long preserved, 25/X/2011), MZSP 139550, 6 specimens (2 dissected, 1–2 mm long preserved, 20/VII/2012), MZSP 143644, 1 specimen (dissected and sequenced, 5 mm long preserved, L. Sales coll., 22/VIII/2015), MZSP 143645, 1 specimen (dissected and sequenced, 6 mm long preserved, V. Queiroz coll., 10/VIII/2017), MZSP 143646, 1 specimen (dissected and sequenced, 4 mm long preserved, V. Queiroz coll., 10/VIII/2017), Segredo Beach, MZSP 103213, 7 specimens (3–5 mm long preserved, 17/I/2012), Itaçucê, MZSP 103278, 30 specimens (5 dissected, 1–4 mm long preserved, 20/I/2012); Ilhabela, Yacht Club of Ilhabela (YCI), 1–2 m depth, MZSP 139551, 60 specimens (10 dissected, 1–7 mm long preserved, L. Sales coll., 28/II/2012), MZSP 139552, 5 specimens (4 dissected, 3–5 mm long preserved, L. Sales coll., 16/I/2013), MZSP 139554, 6 specimens (2 dissected, 2–4 mm long preserved, 11/IX/2013), MZSP 139555, 4 specimens (6–8 mm long preserved, 18/IX/2013); Santos, Alemoa Port, 10–12 m depth, MZSP 32460, 1 specimen (4 mm long preserved, 09/V/2000); São Vicente, Porchat Island, 0.5 m depth, MZSP 109952, 28 specimens (2 dissected, 3–8 mm long preserved, C. M. Cunha coll., 11/XI/2012), MZSP 109953, 1 specimen (7 mm long preserved, 11/XI/2012). Santa Catarina state, Porto Belo, Araçá Beach, MZSP 47262, 11 specimens (1 dissected, 1–4 mm long preserved, 31/I/2005). Bahia state, Salvador, Ribeira Beach, 1 m depth, MZSP 139556, 20 specimens (1 dissected, 1–3 mm long preserved, 06/XI/2013).
Comparative material examined
Okenia zoobotryon: Bermuda Island, Hamilton Parish, Tom Moore Pond, 4 m depth, CASIZ 18106, 1 specimen (dissected, 5 mm long preserved, 12/VI/2009).
Redescription
Diagnosis
Integumentary spicules absent. Single papilla (here termed “posterodorsal papilla”) in dorsal midline between gills and posterior tip of foot. Gill composed of 7–9 bipinnate branchial leaves, two distal leaves smaller than others. Typhlosole extending from cecum chamber to about four fifths of intestine total length. Penis ciliated, about 4 times vagina length, fully covered by penial sac; extending from its base located lateroventrally on right side posterior to buccal bulb, to dorsal region, looping around with several irregular turns and running to right lateral region. Distal end of penial sac approximately twice width of remainder of sac. Seminal receptacle and bursa copulatrix oval.
External morphology
Body (Figs. 1a–c, 2a, d, and 3a–c), elongated, up to 8 mm long (juvenile 1 mm); middle third of body 1.5 times higher than remainder, forming slight hump (Figs. 1c, 2a, d, and 3c). Gill (Figs. 2a, d, 3a, c, and 4c: gi) on posterior third of body, around anus, with 7–9 bipinnate branchial leaves, two distalmost leaves smaller than others; juvenile with 5 bipinnate branchial leaves. Foot (Figs. 1a and 3b–c: ft) wider than body; metapodium triangular (Fig. 3a, c: mt), occupying posterior end of body and extending posteriorly about one fourth of body length, forming tail. Genital pore (Figs. 3c and 4a: gp) on right side of body, usually below second lateral papilla. Notum (Fig. 3a–c: no) variable, generally continuous with width of body, limited by presence of lateral papillae or with distinct edge surrounded by papillae. Papillae (Figs. 2a, d and 3a–c) up to 22 in number, cylindrical in shape, base slightly wider than rounded apex, about one fourth–one third length of rhinophores; anterior papillae (Fig. 3a–c: ap), 2–3 just anterior to rhinophores, central papilla usually smaller than others; lateral papillae (Fig. 3a–c: lp), 9–12 in number, distributed symmetrically or asymmetrically around notum; one posterodorsal papilla in dorsal midline between gills and posterior tip of foot (Fig. 3a, c: pdp); dorsal papillae, up to 7 (usually 6–7 in number), located between rhinophores and gill, most same size as lateral papillae, in a constant position; number of dorsal papillae varying by the absence of up to 5 in the general arrangement; when 7 papillae are present, they are arranged in two triangles with 3 papillae each and 1 anterior to them, generally smaller than the others, parallel to central papillae; juvenile (Fig. 2d) with 4 dorsal papillae arranged in lozenge pattern. Mouth vertical, slit-like (Fig. 3b: mo). Oral tentacles (Fig. 3a–c) digitiform, smooth, short, approximately one third of rhinophore length, dorsolateral to mouth. Rhinophores (Fig. 3a–c: ri) non-retractile, about 1–1.5 times body width, each rhinophore with 5–9 cup-like lamellae; rhinophores smooth in juvenile (Fig. 2d).
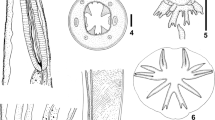
Okenia polycerelloides (Ortea & Bouchet, 1983), external morphology; a dorsal view; b ventral view of the anterior region; and c right lateral view. Scale bars: 1 mm. ap anterior papillae, dp dorsal papillae, ey eye, ft foot, gi gill, gp genital pore, lp lateral papillae, mo mouth, mt metapodium, no notum, pdp posterodorsal papilla, ot oral tentacle, ri rhinophore
Okenia polycerelloides (Ortea & Bouchet, 1983), internal anatomy; a–b visceral mass; a right lateral view; b ventral view; and c circulatory system with gill, dorsal view. Scale bars, 1 mm. aa anterior aorta, ag albumen gland, am ampulla, an anus, apt posterior aorta, au auricle, bc bursa copulatrix, blg blood gland, bu, buccal pump, cc cecum chamber, dg digestive gland, ebr efferent branchial vessel, es esophagus, gi gill, go gonad, gp genital pore, in intestine, kd kidney, mo mouth, ne nephrostome, nr nerve ring, og oral gland, p penis, pc pericardium, pr prostate, ps penial sac, rp renopericardial duct, sr seminal receptacle, va vagina, vd vas deferens, ve ventricle, vs venous sinus
Color
Body background color (Fig. 2a, b) whitish, translucent; mottled with light- and dark-brown spots and clusters of opaque white threadlike lines, both irregularly distributed; bluish pigmentation present or not; foot surface translucent white. Body of juvenile (Fig. 2d) predominantly translucent white, with few brown spots and opaque white clusters. Papillae, gills, and rhinophores (Fig. 2a) with coloration similar to body, but with brown spots concentrated on bases and extending to proximal half of rhinophores and stems of branchial filaments; clusters of opaque white rays more concentrated at distal end of rhinophores.
Visceral mass
Visceral mass (Fig. 4a–b) with anterior buccal bulb (bb); circumesophageal nerve ring (nr), posterodorsal to buccal bulb. Reproductive system anterior to and occupying about one third length of visceral mass, posterior to nervous system; gonad (go) posterior to reproductive system, fully covering digestive gland, occupying about two thirds length of visceral mass; cecum chamber (cc) dorsal to reproductive system and anterior to pericardium; esophagus (es) partially ventral to cecum chamber and dorsal to ampulla (am); intestine (in) located to right of kidney, dorsal to reproductive system and gonad; kidney (kd) ventral to pericardium; venous sinus (vs) to left of anus (an) and posterior to kidney.
Circulatory and excretory systems
Pericardium (Fig. 4a, c: pc) elongate, covering about one fourth length and one half width of visceral mass, located between cecum chamber and gill, dorsally to kidney; branchial circle composed of afferent and efferent rings at gill base (Fig. 4c: ebr). Venous sinus (Fig. 4a: vs) ventrally connected to afferent ring. Auricle (Fig. 4c: au) located posteriorly in pericardial cavity; about two times volume of ventricle; subtriangular, posterior end about three times larger than anterior, wall thin and tuberculated. Ventricle (Fig. 4c: ve) subtriangular, posteriorly connected to auricle and anteriorly to ventral pericardium wall, giving rise ventrally to aortic trunk, which bifurcates into anterior and posterior aortas. Anterior aorta (Fig. 4c: aa) running anteriorly in visceral mass, to right and dorsolaterally to reproductive system, connecting to blood gland (blg); blood gland located dorsal to mucus gland. Posterior aorta (Fig. 4c: apt) running posteriorly in visceral mass and ventrally to kidney, entering at median point of digestive gland. Kidney (Fig. 4a: kd) thin-walled, granular, partly attached to ventroposterior pericardium wall, connected to it by renopericardial duct (rp) that opens internally in posterior right ventrolateral region of pericardium wall. Nephrostome (Fig. 4a: ne) small, located close to right side and on anterolateral edge of anus.
Digestive system
Buccal bulb (Figs. 4a–b and 5a: bb) short, occupying about 1/11 length of visceral mass. Buccal pump (Figs. 4a, 5a, c, and 6a: bu) muscular, dorsal, about one half of buccal bulb. Oral glands (Figs. 4a and 5c: og) conical, composed of several elongated lobes with free distal end, surrounding about one half of anterior end of buccal bulb. Buccal bulb muscles (Fig. 5d): odontophore protractor muscle (mp) arising on buccal bulb ventral surface, inserting around odontophore; circular muscle (mc) consisting of peri-buccal sphincter surrounding approximately anterior one third of buccal bulb; protractor muscles (m1) consisting of several long narrow muscles, arising on lateral surface of buccal mass, inserting around mouth; retractor muscles (m2) consisting of pair of wide muscles, about one half diameter of mouth, same length as buccal bulb, arising on ventrolateral surface of mouth and inserting on internal ventrolateral surface of body wall; superficial muscles of buccal bulb (m3) consisting of several long narrow interlaced muscles, covering about two thirds of dorso-posterior surface of radular sac; ventral protractor muscles of buccal bulb (m10) consisting of pair of muscles, 2 times wider than m2, arising on ventral surface of mouth, covering ventral surface of buccal mass, inserting anteroventral to radular sac; retractor muscles of buccal pump (ms) consisting of pair of short muscles, about 4 times longer than wide, arising on posteroventral surface of buccal pump, running ventrally over m3, inserting on lateral surface of buccal bulb, over mp; intrinsic buccal pump muscles (mb) consisting of several large fibers covering sides of bulbs and connecting its dorsal and ventral limits. Jaws (Figs. 6a and 7e: jw), a reduced pair, smooth, located on anteroventral surface of mouth; size about one third of mouth diameter. Pair of ventral folds of buccal cavity (Fig. 6a: vf), gradually appearing in midregion of ventral inner surface of buccal cavity, running longitudinally toward anterior end, connected to jaws at its lateral edge. Odontophore (Fig. 6a: od) slightly ellipsoid, occupying about one third of volume of buccal cavity. Radular sac (Figs. 5a, c and 6a: rn) elongated, about one half odontophore length. Radula (Figs. 6a and 7a–c: ra) formula 29–31 × 1.1.0.1.1.; lacking rachidian teeth; lateral teeth hook-shaped, base elongated, about 2 times wider than width of cuspid; cuspid long, about 4 times narrower than base, inner surface concave with shallow groove, inner edge covered with pectinate denticulation, about 15–18 long thin denticles, smaller at ends of row and longer in middle; marginal teeth about one third length of lateral teeth, base wide, rectangular; distal end bicuspid, cusps parallel or intersecting at tips, about one half size of teeth. Esophagus (Figs. 4b, 5a–d, and 6a: es) cylindrical, about three fourths of intestine length; two pairs of esophageal folds (Fig. 6a: ef) arising at upper base of internal opening of buccal pump on right and left sides. Pair of salivary glands (Fig. 5c, d: sg), rounded, located on posterior lateral portion of buccal bulb near point of connection with esophagus, about two times wider than buccal ganglia. Pair of esophageal glands (Fig. 5c, d: eg) similar to salivary glands in shape, each esophageal gland located among salivary gland, esophagus and buccal ganglion, on both sides of buccal bulb. Stomach (Fig. 5a: st) connected anteriorly to esophagus and posteriorly to cecum chamber, ventral to digestive gland and connected to it by two ducts (dd), one on right side and one ventral to stomach wall. Cecum chamber (Figs. 4a, b and 5a, b: cc) with internal folds extending from connection with stomach to beginning of intestine; typhlosole (Fig. 5a, b: tf) originating from upper part of inner wall and extending through intestine to about four fifths of its length. Intestine (Figs. 4a and 5a, b: in) starting from end of cecum chamber, similar in diameter to esophagus, ending at anus; anus opening in center of gill circle.
Okenia polycerelloides (Ortea & Bouchet, 1983), digestive system; a digestive system with esophagus and buccal bulb deflected, ventral view; b cecum chamber opened, dorsal view; c buccal bulb with nerve ring, left lateral view; and d buccal bulb muscles, left lateral view. Scale bars; a 1 mm; b–d 500 μm. an anus, bb buccal bulb, bg buccal ganglion, bu buccal pump, cc cecum chamber, ccb cerebrobuccal connective, ce cerebral ganglion, cpr pleurovisceral connective, dd digestive gland duct, eg esophageal glands, es esophagus, ey eye, gf gastric folds, in intestine, mb intrinsic buccal pump muscles, mc circular muscle of the buccal bulb, mo mouth, mp protractor muscle of the odontophore, ms retractor muscles of the buccal pump, m1 protractor muscles of the buccal bulb, m2 retractor muscles of the buccal bulb, m3 superficial muscles of the buccal bulb, m10 ventral protractor muscles of the buccal bulb, nr nerve ring, og oral gland, pd pedal ganglion, pl pleural ganglion, rin rhinophoral nerve, rn radular nucleus, sc statocysts, scc connection between stomach and cecum chamber, sg salivary gland, st stomach, tf typhlosole, vg visceral ganglion
Okenia polycerelloides (Ortea & Bouchet, 1983); a buccal bulb opened, half of the buccal pump removed, left lateral view; b reproductive system; c–d central nervous system (nerve ring); c anterior view; and d posterior view. Scale bars, 500 μm. ad allosperm duct, ag albumen gland, am ampulla: bc bursa copulatrix, bg buccal ganglion, bu buccal pump, cbg buccal commissure, ccb cerebrobuccal connective, ccp cerebropedal connective, cce cerebral commissure, ce cerebral ganglion, cp pedal commissure, cpr pleurovisceral connective, ef esophageal folds, es esophagus, ey eye, fc fertilization chamber, hd hermaphrodite duct, jw jaw, mg mucus gland, mo mouth, na nidamental opening, od odontophore, p penis, pd pedal ganglion, pl pleural ganglion, pp parapedal commissure, pr prostate, ps penial sac, ra radula, rd seminal receptacle duct, rg rhinophoral ganglion, rin rhinophoral nerve, rn radular nucleus, sr seminal receptacle, ud uterine duct, va vagina, vd vas deferens, vf ventral fold, vg visceral ganglion
Okenia polycerelloides (Ortea & Bouchet, 1983), scanning electron micrographs; a–c radula; a overview; b detail of the lateral tooth; c detail of the marginal tooth, specimen from Canary Islands (MZSP 139548); d penis (penial sac removed), inset—detail of the penis showing the cilia, a specimen from Brazil; e jaw (MZSP 139548). Scale bars: a 80 μm; b, e 20 μm; c 9 μm; d 500 μm, inset 5 μm. jw jaw
Reproductive system
Posterior to nerve ring and anterior to digestive gland, occupying about one third length of visceral mass, except for hermaphrodite gonad (Fig. 4a, b). Hermaphrodite gonad (Fig. 4a, b: go) thin-layered, fully covering digestive gland like a membrane, occupying about two thirds of visceral mass. Hermaphrodite duct (Fig. 6b: hd) long and narrow, connected on right side to dorsolateral anterior edge of gonad, ventrally to intestine, connecting lateral and subterminal regions of ampulla. Ampulla (Figs. 4b and 6b: am) elongate, tubular, over and between edges of mucus (mg) and albumen (ag) glands; emitting short duct, about one fifth length of ampulla; duct bifurcates connecting to anterior end of fertilization chamber and prostate. Prostate (Figs. 4b and 6b: pr) tubular, ventral to mucus gland, about two times length of ampulla, median region turning, running and narrowing at distal end, forming vas deferens. Vas deferens (Figs. 4a, b and 6b: vd) narrow and long, about three times longer than prostate length, distal end connecting to base of penial sac. Penis (Figs. 4a and 6b: p) cylindrical, elongate, ciliated, about four times length of vagina; fully covered by penial sac (ps); extending from its base lateroventrally on right side, posterior to buccal bulb, to dorsal region, looping around with irregular turns and running to right lateral region; opening externally in genital pore, atrium common to vagina and nidamental opening (Fig. 4a). Fertilization chamber (Fig. 6b: fc) slightly immersed between glands and ducts of hermaphrodite system. Uterine duct (Fig. 6b: ud) arising from fertilization chamber, anterolateral to ampulla duct insertion, bifurcating into short seminal receptacle duct and allosperm duct. Seminal receptacle duct (Fig. 6b: rd) about two times wider and shorter than uterine duct. Allosperm duct (Fig. 6b: ad) about same width as seminal receptacle duct and about three times length of vagina. Seminal receptacle (Figs. 4a and 6b: sr) and bursa copulatrix (Figs. 4a, b and 6b: bc) oval, variable in size, depending on amount of sperm inside. Vagina (Figs. 4 and 6b: va) similar in shape and width to penial sac, about one fourth its length, located dorsolateral to mucus gland and on side of penial sac, near genital pore.
Nervous system
Nerve ring (Figs. 4a, b and 5a, c: nr) located posteriorly to buccal mass, surrounding esophagus and salivary glands anteriorly. Cerebral (ce) and pleural ganglia (pl) (Figs. 5c and 6c, d) fused with each other, forming cerebropleural ganglia, located dorsolateral to esophagus. Cerebral commissure (Fig. 6c, d: cce) short, about one third width of cerebropleural ganglia. Pleurovisceral connectives (Figs. 5c and 6c, d: cpr) short, left connective as long as cerebropleural ganglion length, right connective about one third of cerebropleural ganglion length; both arising on inner side of and posterior to cerebropleural ganglia. Visceral ganglion (Figs. 5c and 6d: vg) elongate, ventral to esophagus, longer than right pleurovisceral connective. Pedal ganglia (Figs. 5c and 6c, d: pd) located ventrolaterally to esophagus and slightly posterior to cerebropleural ganglia; cerebropedal connectives inconspicuous (Fig. 6c: ccp); antero-dorsal side of pedal ganglia leaning to anteroventral side of cerebropleural ganglia (Figs. 5c and 6c). Pedal commissure (Fig. 6c, d: cp) located ventrally, parallel to esophagus, about three times wider than and about same length as parapedal commissure (Fig. 6c, d: pp); pedal and parapedal commissures variable in length, one fourth to one half width of pedal ganglion. Buccal ganglia (Figs. 5c and 6c–d: bg) located dorsally to buccal bulb, ventrally to esophagus and posterolaterally to salivary and esophageal glands; buccal commissure short (Fig. 6c, d: cbg). Cerebrobuccal connectives (Figs. 5c and 6c, d: ccb) arising on anteroventral side of cerebropleural ganglion, about two times length of left pleurovisceral connective, running laterally to esophagus and salivary glands, crossing posterior superficial muscles of buccal bulb ventrally (Fig. 5d: m3) and connecting to latero-ventral side of buccal ganglia. Rhinophoral ganglia (Fig. 6c, d: rg) bulb-shaped, located on anterolateral edge of cerebral ganglia, giving rise to rhinophoral nerve. Eyes (Figs. 5c and 6c, d: ey) on sides of cerebropleural ganglia, closer to cerebropedal connectives. Statocysts (Fig. 5c: sc) dorsal to pedal ganglia, closer to cerebropedal connectives, covered by cerebropleural ganglia.
Distribution
Although many previous records of O. zoobotryon seem to be attributable to O. polycerelloides (see the “Discussion” section), we were unable to obtain specimens from these localities. Therefore, we were able to confirm only that the geographical range of O. polycerelloides includes Brazil (Bahia, São Paulo, and Santa Catarina states) and the Canary Islands (type locality). Moreover, the species has been reported for Venezuela (Caballer-Gutiérrez et al. 2015, fig. 4C).
Biological notes
The type material of O. polycerelloides was reported as found among algae, hydroids and the bryozoan Amathia vidovici (Heller, 1867), while the topotype was collected associated to the bryozoan Amathia verticillata (Delle Chiaje, 1822) (= Zoobotryon verticillatum), on which there were also attached egg masses. In Brazil, O. polycerelloides are found living, feeding, and spawning on colonies of both bryozoan species. Actually, these bryozoans are sympatric in the area where most of the collections were carried out (São Sebastião, São Paulo), their colonies commonly occurring intermingled. However, since the colonies of A. verticillata are commonly bigger and more abundant, we generally focused our efforts on them. The egg masses from the Brazilian specimens and those that came attached to the bryozoans where the topotype was collected look exactly the same: cylindrical, variable in size (4–10 mm), gelatinous, with translucent walls, the eggs and embryos white and arranged in several rows inside the spawning (Fig. 2c). The nudibranch feeds by suctioning the bryozoan zooids, probably by means of the buccal pump present in the buccal bulb. During copulation, they exhibit promiscuous behavior, mating several times with different partners. Sperm exchange appears to be reciprocal during copulation. When placed in an aquarium, they spawn continuously on the glass walls.
Molecular results
The three O. polycerelloides samples from Brazil were effectively amplified and sequenced, but our attempts to amplify the topotype did not work. We sequenced 636 bp of COI and 328 bp of H3 genes, resulting in a concatenated dataset with 964 bp. Partitioned gene analysis revealed a higher diversity in COI, which recovered two haplotypes with three segregating sites (0.47% of intraspecific divergence). For H3, only one allele was found.
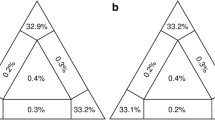
Among all taxa, pairwise sequence divergence in COI varies from 17.13 to 19.34% and in H3 from 7.92 to 12.19% (see Table 2). Comparison between O. polycerelloides and O. zoobotryon revels a genetic distance about 17% for COI and 10% for H3.
In the phylogenetic analysis, log likelihood ratio tests indicate that GTR + I + G and TPM2uf + G were the best models for COI and H3 genes, respectively. The Bayesian inference tree shown in Fig. 8 revealed a highly supported clade that corresponds to O. polycerelloides species with a PP equal 1.00. This O. polycerelloides clade is included in a major clade that comprises Okenia pellucida Burn, 1967, Okenia vena Rudman, 2004 and Okenia brunneomaculata Gosliner, 2004, also well supported (PP = 1.00). This clade is nested within a polytomy which includes O. zoobotryon, Okenia harastii Pola, Roldán & Padilla, 2014b, Okenia amoenula (Bergh, 1907), and Okenia rosacea (MacFarland, 1905) (O. rosacea is also considered as part of the polytomy as its weak support at a sister position tend to collapse it). This topology clearly demonstrates O. polycerelloides as distinct species.
Phylogenetic relationship of Okenia spp. based on Bayesian analysis of mitochondrial (COI) and nuclear (H3) genes, on a concatenated dataset. Numbers associated with nodes represent posterior probabilities from Bayesian MCMC searches conducted in MrBayes. Scale bar represents percent sequence divergence
Discussion
Okenia polycerelloides was considered a synonym of O. zoobotryon, after Rudman (2004). Since then, only Ortea et al. (2009) and Caballer-Gutiérrez et al. (2015) have treated O. polycerelloides as a distinct species, although they assigned it to the genus Bermudella. However, the internal structure of both species remained undescribed until recently, when Pola (2015) published a redescription of O. zoobotryon, including data on the internal anatomy and radula. We analyzed a topotype of O. zoobotryon and found that the reproductive system is completely in accordance with Pola (2015); the other systems (circulatory, nervous, and details of the digestive system), which she did not describe, are similar in both species.
Although similar in general appearance, there are clear distinctions between the two species in many external (see Ortea et al. 2009; present study, Table 3) and internal characters (Table 3). Externally, two conspicuous differences are apparent: (1) the presence of spicules on the mantle of O. zoobotryon, while in O. polycerelloides they are completely absent and (2) the existence of a posterodorsal papilla on the dorsal midline between the gills and the posterior tip of the foot in the latter species, which is absent in the former. The usual number of dorsal papillae is also different, four in O. zoobotryon and seven in O. polycerelloides. However, our analysis of a large number of O. polycerelloides specimens revealed that the number and distribution of these papillae vary among individuals, although there is a regular pattern common to most adult specimens. Therefore, the number and arrangement of the dorsal papillae are not constant as described for O. zoobotryon (see Smallwood 1910; Pola 2015), and are not a reliable diagnostic character for O. polycerelloides. In contrast, the posterodorsal papilla was present and in the same position in all specimens of O. polycerelloides analyzed, including the topotype, holotype, and juvenile (Figs. 1c, 2a, b, d, and 3a, c). Internally, the differences between O. polycerelloides and O. zoobotryon occur in the anatomy of the foregut and reproductive system. The salivary glands are rounded in O. polycerelloides (Fig 5c, d) and drop-shaped in O. zoobotryon (see Pola 2015, fig. 2A); the plica on the inner side of the radular innermost tooth base of O. zoobotryon (see Pola 2015, fig. 3D) is absent in the other species. The reproductive system of O. polycerelloides differs from O. zoobotryon in the shape of the bursa copulatrix, seminal receptacle, and ampulla; diameter and length of the penial sac; and diameter of the vagina (Table 3). Thus, based on all these differences, we re-establish O. polycerelloides as a valid species, distinct from O. zoobotryon.
The penis of O. polycerelloides is ciliated throughout its length (Fig. 7d). However, we do not know for sure whether this feature is present or absent in O. zoobotryon, since it was not possible to verify the presence of this character in the topotype studied here. However, it seems that the penis of O. zoobotryon illustrated by Pola (Pola 2015, fig. 3F, G) does not have cilia, as she made no mention of this character, which would be another difference between the two species. The ciliation on the penis could be a useful distinguishing character for other Okenia species, but most of them have not been studied at this level of detail (e.g., Gosliner and Bertsch 2004; Rudman 2004, 2007). The penis of the type species of the genus (Okenia elegans (Leuckart, 1828)) is not ciliated, but its surface is completely armed with spines (Vayssière 1901). More-detailed descriptions of Okenia species generally report the presence or absence of penial spines (e.g., Gosliner 2004, 2010; Paz-Sedano et al. 2017). The cilia are not easily detected, since the species of Okenia are usually small and their penises are commonly involved by a thin-walled sac (penial sac). In this study, under the stereomicroscope, the penial cilia of O. polycerelloides were initially mistaken for possible penial spines. The presence of cilia was confirmed only after examination of living specimens with an everted penis under the stereomicroscope and fixed specimens under SEM.
Externally, O. polycerelloides is also similar to O. harastii and Okenia mija Burn, 1967. Their background colors range from translucent brownish to whitish, with scattered dark-brown and white spots (Burn 1967; Pola et al. 2014b), but in O. mija the dark-brown spots are surrounded by a yellow band (Burn 1967; see Rudman 2004, fig. 29C), which easily distinguishes it from the others. Color, however, is not a reliable character to distinguish the other species, since both have the same general coloration pattern. Nevertheless, since O. harastii does not have the posterodorsal papilla that is present in O. polycerelloides, the number and arrangement of the papillae, and the number of lamellae in the rhinophores (see Rudman 2004; Pola et al. 2014b) are good distinguishing characters for these species. In O. mija, there is a notch in the posterior midline of each lamella of the rhinophores (see Rudman 2004, fig. 12B), and this notch is absent in all the other species mentioned. Although O. polycerelloides and O. mija share the presence of an unpaired posterior papilla (Fig. 3a, c; Rudman 2004, fig. 12B), in the latter, two smaller papillae are present on each side of this papilla. Therefore, O. polycerelloides can be distinguished from its congeners by a combination of external characters: color pattern, number of lamellae in the rhinophores, and number and arrangement of papillae. Moreover, O. polycerelloides can also be distinguished from O. harastii and O. mija by characteristics of the reproductive system. For example, the latter two species present a broad vagina (see Rudman 2004, fig. 4C for O. mija and Pola et al. 2014b, fig. 3D for O. harastii) while O. polycerelloides present a thin vagina; the seminal receptacle is pyriform in O. harastii and oval in O. mija and O. polycerelloides; the bursa copulatrix is oval in O. harastii and O. polycerelloides, and spherical in O. mija; the penis of O. harastii is coiled in a large thin-walled sac while in O. polycerelloides this penial sac cover the penis over its entire length, and in O. mija, according Rudman (2004), the penis is not contained in a thin-walled sac (Rudman 2004; Pola et al. 2014b; present study).
Although the original description of O. polycerelloides did not provide an anatomical study of the reproductive system, the description and illustration of the external morphology (see Ortea and Bouchet 1983, fig. 1A–D) are well documented and unambiguous with respect to the presence of the posterodorsal papilla. Furthermore, the study of several specimens revealed that the numbers of dorsal papillae and branchial leaves as originally described are within the range of variation that we observed for the species. The radular morphology of the topotype and the Brazilian specimens also agrees with the original description (see Ortea and Bouchet 1983, fig. 2), as do the general shape and coloration of the body. Moreover, the morphological analysis of the type material allowed us to confirm that the penis, ampulla, and bursa copulatrix of the Brazilian specimens and the topotype present the same characteristics of the type material. Although we were not allowed to dissect the type material, the paratype had a cut on the head that was probably made by the authors of the species in order to remove the buccal mass and study the radula, what allowed us to see the general aspect of the penis, ampulla, and bursa copulatrix. Thus, the Brazilian specimens and the topotype analyzed herein fit the original description and the observed characteristics of the type material of O. polycerelloides.
Besides O. polycerelloides, two other species of Okenia have been reported in Brazil: Okenia evelinae Marcus, 1957 and Okenia impexa Marcus, 1957. Both were originally described from the coast of São Paulo state (Marcus 1957) and are easily distinguishable from O. polycerelloides: O. evelinae has violet rhinophores and yellow spots spread over the body, and O. impexa has claviform and pointed papillae with a continuous axial bundle of spicules, only one dorsal papilla between the rhinophores and the gill, and the last pair of lateral papillae arises from a common base. Both species have spicules in the tegument, and neither of them has the posterodorsal papilla (see Marcus 1957; Sales et al. 2016) that is present in O. polycerelloides.
Okenia zoobotryon has been recorded in many locations around the world since its original description, e.g., in Bermuda (Smallwood 1910; Clark 1984), Barbados, (Edmunds and Just 1985), Cuba (Valdés and Ortea 1995), the Bahamas (Redfern 2004), Senegal (Poddubetskaia 2004), Australia (Rudman 2004), and Venezuela (Grune 2005; Valdés et al. 2006; Caballer-Gutiérrez et al. 2015). However, comparison of the illustrations provided by these reports with the redescription of O. zoobotryon (Pola 2015) and O. polycerelloides (present study) suggests the possibility that many of these records are attributable to the latter species, and even to other, similar ones. As Pola et al. (2014b) stated, in spite of some differences, the specimens reported as O. zoobotryon for Australia (Rudman 2004) are more similar to O. harastii than to the former species. Similarly, the reports of O. zoobotryon for Senegal (Poddubetskaia 2004), Venezuela (Grune 2005; Valdés et al. 2006), and the USA (Ianniello 2007) seem to belong to O. polycerelloides, as do the previous reports for Brazil (García et al. 2008), since the specimens illustrated have the posterodorsal papilla that is absent in O. zoobotryon. Based on this, it is possible that the geographical distribution of O. zoobotryon is more restricted than that of O. polycerelloides, but we think that any conclusion on this matter should await the examination of specimens from these localities.
The abundance of O. polycerelloides in Brazil, together with the current difficulty in finding the other species of the genus previously reported for this country (O. evelinae and O. impexa), raises an interesting question: How did Ernst and Eveline Marcus, the pair of researchers who worked intensively with sea slugs in Brazil for three decades (1950–1980), not find this now-abundant species? A possible answer would be that O. polycerelloides did not occur in Brazil at that time. Amathia verticillata (= Zoobotryum verticillatum), the bryozoan with which this nudibranch is commonly associated, occurs worldwide in temperate and warm waters (e.g., Delle Chiaje 1822; Bullivant and Bills 1968; Fox 2001; Hill 2001; Elkhorn Slough Research 2002), and is considered a cryptogenic species (McCann et al. 2007). Although it has a short-lived, non-feeding larva (Santagata 2008) that probably lacks good dispersal capabilities, its colonies could possibly travel long distances by means of fouling on ship hulls and rafting on seaweed or other substrata (Miranda et al. 2018). It is therefore possible that their associated biota, including the nudibranchs, could be transported along with them. Amathia verticillata has been reported in Brazil since at least 1860 and was recently considered an exotic species there (Farrapeira 2011; Micael et al. 2018; Miranda et al. 2018), whereas O. polycerelloides was first recorded in the western South Atlantic (as O. zoobotryon) only in 2008 (García et al. 2008). Thus, if the occurrence of O. polycerelloides in Brazil is related to the introduction of A. verticillata, probably there were multiple arrivals of the bryozoan in the region, at least some coming from places where the nudibranch was present. This hypothesis should be tested in future studies involving population genetics.
Although the Brazilian specimens of Okenia studied here closely fit the holotype and the topotype of O. polycerelloides analyzed, it is important to consider the possibility that they are part of a species complex. Recent molecular studies have shown that sea slug species that are morphologically similar and were previously considered as only a single taxon are in fact a complex of different species with more restricted distributions than first thought (e.g., Carmona et al. 2011, 2014a, 2014b, 2014c, 2014d). In order to test that, we tried to obtain DNA sequences of O. polycerelloides from Brazil and the Canary Islands, but we failed in amplifying the topotype. However, considering the strong morphological similarity among the Brazilian specimens, the topotype, and the type material of O. polycerelloides, as well as the differences between them and O. zoobotryon, the more parsimonious decision, for now, is to attribute the Brazilian species to O. polycerelloides until molecular studies can be performed with specimens from the Canary Islands. Thus, considering the Brazilian specimens belong to O. polycerelloides, until new evidence may suggest otherwise, we compared the obtained sequences to those of the neotype of O. zoobotryon and other Okenia species.
The molecular data clearly corroborate the morphological results concerning the validity of O. polycerelloides as a distinct species from O. zoobotryon. Okenia polycerelloides has an expressive genetic distance when compared with other Okenia species available on Genbank (see Table 2), compatible with a status of a taxonomic unit. The genetic divergence between O. polycerelloides and O. zoobotryon, previously considered the same species, is about 17% to the COI gene and 10% to the H3 gene, which strongly suggests that they belong to different species. COI distances between pairs of O. polycerelloides and all Okenia species studied here were similar to the reported by Pola et al. (2014b) regarding O. harastii with other Okenia species. The divergence between O. polycerelloides and O. zoobotryon has also been reinforced by Bayesian tree topology (Fig. 8), which recovered each species in distinctive clades with high PP support as well.
The distinguishing morphological (external and internal) features found in our study are more than sufficient to recognize O. polycerelloides as a separate species. Although our molecular study presented here also suggests the same, it is still important to obtain DNA sequences of O. polycerelloides from the type locality to compare them with the Brazilian ones. Therefore, we propose to restore the species status and consider the records of O. zoobotryon in the South Atlantic Ocean as attributable to O. polycerelloides, until new evidence may suggest otherwise.
References
Adams H, Adams A (1854) The genera of recent Mollusca; arranged according to their organization. van Voorst, London
Alvim J, Pimenta AD (2016) Comparative morphology and redescription of Pleurobranchus species (Gastropoda, Pleurobranchoidea) from Brazil. Zool Stud 55(15)
Bergh LSR (1880) On the nudibranchiate gasteropod Mollusca of the North Pacific Ocean, with special reference to those of Alaska. Part II. Proc Acad Nat Sci Philadelphia 32:40–127
Bergh LSR (1907) The Opisthobranchia of South Africa. Trans South Afr Philos Soc 27(1):1–144
Bullivant JS, Bils RF (1968) The pharyngeal cells of Zoobotryon verticillatum (Delle Chiaje) a gymnolaemate bryozoan. N Z J Mar Freshw Res 2:438–446
Burn R (1967) Descriptions of two new species of Okenia (Nudibranchia, Doridacea) from south-eastern Australia. Proc R Zool Soc NSW 1965(/1966):52–57
Caballer-Gutiérrez M, Ortea J, Rivero N, Tucker GC, Malaquias MA, Narciso S (2015) The opisthobranch gastropods (Mollusca: Heterobranchia) from Venezuela: an annotated and illustrated inventory of species. Zootaxa 4034(2):201–256
Carmona L, Malaquias MAE, Gosliner TM, Pola M, Cervera JL (2011) Amphi-Atlantic distributions and cryptic species in sacoglossan sea slugs. J Molluscan Stud 77:401–412
Carmona L, Bhave V, Salunkhe R, Pola M, Gosliner TM, Cervera JL (2014a) Systematic review of Anteaeolidiella (Mollusca, Nudibranchia, Aeolidiidae) based on morphological and molecular data, with a description of three new species. Zool J Linnean Soc 171:108–132
Carmona L, Lei RB, Pola M, Gosliner TM, Valdés A, Cervera JL (2014b) Untangling the Spurilla neapolitana (Delle Chiaje, 1841) species complex: a review of the genus Spurilla Bergh, 1864 (Mollusca: Nudibranchia: Aeolidiidae). Zool J Linnean Soc 170:132–154
Carmona L, Pola M, Gosliner TM, Cervera JL (2014c) Review of Baeolidia, the largest genus of Aeolidiidae (Mollusca: Nudibranchia), with the description of five new species. Zootaxa 3802:477–514
Carmona L, Pola M, Gosliner TM, Cervera JL (2014d) The Atlantic-Mediterranean genus Berghia Trinchese, 1877 (Nudibranchia: Aeolidiidae): taxonomic review and phylogenetic analysis. J Molluscan Stud 80:482–498
Clark KB (1984) New records and synonymies of Bermuda opisthobranchs (Gastropoda). The Nautilus 98:85–97
Colgan DJ, McLauchlan A, Wilson GDF, Livingston SP, Edgecombe GD, Macaranas J, Cassis G, Gray MR (1998) Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust J Zool 46:419–437
Cooper JG (1863) On new or rare Mollusca inhabiting the coast of California. Proc Calif Acad Sci 1(3):56–60
Cunha CM, Simone LRL (2019) Morphological re-description of Aplysia depilans (Gastropoda: Anaspidea): new insights into the anatomy of the anaspideans. J Mar Biol Assoc U K 99(3):595–610
Cuvier G (1817) Le règne animal distribué d’après son organisation, pour servir de base a l’histoire naturelle des animaux et d’introduction a l’anatomie compare. Deterville, Paris
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9(8):772
Delle Chiaje S (1822) Memorie sulla storia e notomia degli animali senza vertebre del Regno di Napoli. Fratelli Fernandes, Napoli
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Edmunds M, Just H (1985) Dorid, dendronotoid and arminid nudibranchiate Mollusca from Barbados. J Molluscan Stud 51:52–63
Elkhorn Slough Research (2002) Least wanted aquatic invaders, spaghetti bryozoan (Zoobotryon verticillatum). http://www.elkhornslough.org/research/aquaticinvaders/aquatic21.htm. Accessed 05 September 2016
Farrapeira CMR (2011) The introduction of the bryozoan Zoobotryon verticillatum (Delle Chiaje, 1822) in northeast of Brazil: a cause for concern. Biol Invasions 13:13–16
Fox R (2001) Zoobotryon verticillatum, Ctenostome, Bryozoan. Invertebrate anatomy online. http://webs.lander.edu/rsfox/invertebrates/zoobotryon.html. Accessed 05 September 2016
García FJ, Domínguez M, Troncoso JS (2008) Opistobranquios de Brasil: descripción y distribución de opistobranquios del litoral de Brasil y Del Archipiélago Fernando de Noronha. Feito S.L., Vigo
Gosliner TM (2004) Phylogenetic systematics of Okenia, Sakishimaia, Hopkinsiella and Hopkinsia (Nudibranchia: Goniodorididae) with descriptions of new species from the tropical Indo-Pacific. Proc Calif Acad Sci 55(5):125–161
Gosliner TM (2010) Two new species of nudibranch mollusks from the coast of California. Proc Calif Acad Sci 61(16):623–631
Gosliner TM, Bertsch HW (2004) Systematics of Okenia from the Pacific coast of North America (Nudibranchia: Goniodorididae) with descriptions of three new species. Proc Calif Acad Sci 55(22):414–430
Grune S (2005) Okenia zoobotryon from Venezuela. Sea Slug Forum. http://www.seaslugforum.net/find/13487. Accessed 04 May 2018
Heller C (1967) Die Bryozoen des adriatischen Meeres. Verh Zool-Bot Ges Wien 17:77–136
Hill K (2001) Zoobotryon verticillatum. Smithsonian Marine Station at Fort Pierce. http://www.sms.si.edu/irlspec/zoobot_vertic.htm. Accessed 11 September 2016
Ianniello LM (2007) Okenia zoobotryon from southeast Florida, USA. Sea Slug Forum. http://www.seaslugforum.net/find/21015. Accessed 03 May 2018
Leuckart FS (1828) Breves animalium quorundam maxima ex parte marinorum descriptiones. Augusti Osswaldi, Heidelbergae
Lovén SL (1846) Index Molluscorum litora Scandinaviae occidentalia habitantium. Öfversigt af Kongliga Vetenskaps Akademiens Förhandlingar 3:134–160
MacFarland FM (1905) A preliminary account of the Dorididae of Monterey Bay, California. Proc Biol Soc Wash 18:35–54
Marcus E (1957) On Opisthobranchia from Brazil. Zool J Linnean Soc 43:390–486
McCann LD, Hitchcock NG, Winston JE, Ruiz GM (2007) Non-native bryozoans in coastal embayments of the southern United States: new records for the Western Atlantic. Bull Mar Sci 80(2):319–342
Menke KT (1830) Synopsis methodica molluscorum generum omnium et specierum earum, quae in Museo Menkeano adservantur, cum synonymia critica et novarum specierum diagnosibus. Editio altera, auctior et emdatior. Georg Uslar, Pyrmonti
Meyer C, Geller J, Paulay G (2005) Fine scale endemism on coral reefs: archipelagic differentiation in turbinid gastropods. Evolution 59:113–125
Micael J, Gillon A, Jardim N, Rodrigues P, Costa AC (2018) Sexual reproduction in the invasive bryozoan Amathia verticillata (Ctenostomatida: Vesiculariidae). J Coast Conserv 22:305
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Gateway cComputing Environments Workshop (GCE). New Orleans, LA
Miranda AA, Almeida ACS, Vieira LM (2018) Non-native marine bryozoans (Bryozoa: Gymnolaemata) in Brazilian waters: assessment, dispersal and impacts. Mar Pollut Bull 130:184–191
Odhner NJ (1941) New polycerid nudibranchiate mollusca and remarks on this family. Göteborgs Kungl Vetensk Samhälles handl, Ser B, Mat Naturvetensk Skr 1:1–20
Ortea J, Bouchet P (1983) Un nuevo Goniodorididae (Mollusca: Nudibranchiata) de las islãs Canarias. Vieraea 12:49–54
Ortea J, Moro L, Bacallado JJ, Pérez Sánchez JM, Vallès Y (1996) Nuevos datos sobre la fauna de dóridos fanerobranquios (Gastropoda, Nudibranchia) delas islas Canarias. Rev Acad Canaria Cienc 8:125–138
Ortea J, Moro L, Espinosa J (2009) El género Okenia Menke, 1830 (Mollusca: Nudibranchia) en las islas Canarias con notas sobre Okenia zoobotryon (Smallwood, 1910) una especie en controversia permanente. Vieraea 37:75–83
Paz-Sedano S, Ortigosa D, Pola M (2017) A new Okenia Menke, 1830 from the Azores Islands, Portugal (Mollusca, Nudibranchia, Goniodorididae). Spixiana 40:13–22
Poddubetskaia M (2004) Okenia zoobotryon? from Senegal. Sea Slug Forum. http://www.seaslugforum.net/find/10265. Accessed 04 May 2018
Pola M (2015) The identity of Okenia zoobotryon (Smallwood, 1910) (Nudibranchia: Goniodorididae): redescription and proposed designation of a neotype. Am Malacol Bull 33:72–77
Pola M, Cervera LJ, Gosliner TM (2008) Description of the first Roboastra species (Nudibranchia, Polyceridae, Nembrothinae) from the Western Atlantic. Bull Mar Sci 83(2):391–399
Pola M, Padula V, Gosliner TM, Cervera JL (2014a) Going further on an intricate and challenging group of nudibranchs: description of five novel species and a more complete molecular phylogeny of the subfamily Nembrothinae (Polyceridae). Cladistics 30(6):607–634
Pola M, Roldán P, Padilla S (2014b) Molecular data on the genus Okenia (Nudibranchia: Goniodorididae) reveal a new cryptic species from New South Wales (Australia). J Mar Biol Assoc UK 94:587–598
Rambaut A. (2007) FigTree Tree Figure Drawing Tool, Version 1.4. http://tree.bio.ed.ac.uk/software/figtree/ Accessed 26 January 2019
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst Biol 67:901–904
Redfern C (2004) Okenia zoobotryon from the Bahamas. Sea Slug Forum. http://www.seaslugforum.net/find/12421. Accessed 04 May 2018
Rios EC (2009) Compendium of Brazilian sea shells. Evangraf, Rio Grande
Risso A (1818) Mémoire sur quelques Gastropodes nouveaux, Nudibranches et Tectibranches observés dans la mer de Nice. J Phys Chim Hist Nat Arts 87:368–377
Ronquist F, Huelsenbeck JP (2003) MrBayes: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Rudman WB (2004) Further species of the opisthobranch genus Okenia (Nudibranchia: Goniodorididae) from the Indo-West Pacific. Zootaxa 695:1–70
Rudman WB (2006) Comment on re: radula of Senegalese Okenia zoobotryon by angel Valdes. Sea Slug Forum. http://www.seaslugforum.net/find/16883. Accessed 11 April 2018
Rudman WB (2007) Two new species of Okenia (Gastropoda: Nudibranchia: Goniodorididae) from eastern Australia and Tanzania. Zootaxa 1657:57–67
Sales L, Queiroz V, Migotto AE (2016) Rediscovery of Okenia impexa Marcus 1957 on the Brazilian coast: the first image of a living specimen from Brazil. Spixiana 39:203–204
Santagata S (2008) The morphology and evolutionary significance of the ciliary fields and musculature among marine bryozoan larvae. J Morphol 269:349–364
Smallwood WM (1910) Notes on the hydroids and nudibranchs of Bermuda. Proc Zool Soc London 80:137–147
Smallwood WM (1912) Polycerella zoobotryon. Proceedings of the American Academy of Arts and Sciences 47:609–630
Valdés A, Ortea J (1995) Revised taxonomy of some species of the genus Okenia Menke, 1830 (Mollusca: Nudibranchia) from the Atlantic Ocean, with the description of a new species. The Veliger 38:223–234
Valdés A, Hamann J, Behrens DW, Dupont A (2006) Caribbean Sea Slugs: a field guide to the opisthobranch mollusks from the tropical northwestern Atlantic. Sea Challengers Natural History Books, Etc., Gig Harbor
Vayssière AJ (1901) Recherches zoologiques et anatomiques sur les mollusques Opisthobranches du Golfe de Marseille (suite et fin). Annales du Musée d’histoire naturelle de Marseille 6:1–130
Willan RC, Chang Y-W (2017) Description of three new species of Tambja (Gastropoda, Nudibranchia, Polyceridae) from the western Pacific Ocean reveals morphological characters with taxonomic and phylogenetic significance for traditional Polyceridae and related ‘phaneorobranch’ nudibranchs. Basteria 81(1–3):1–23
Acknowledgments
We are grateful to Elizabeth Kools and Terry Gosliner for the loan of specimens held by the California Academy of Sciences (CASIZ), and to Leopoldo Moro Abad and Manuel Caballer Gutiérrez (Manuel Caballer MNHN, project E-RECOLNAT: ANR-11-INBS-0004) for the donation of the topotype and images of the holotype. We thank Philippe Bouchet and Virginie Héros for the opportunity to analyze the type material of O. polycerelloides deposited in the MNHN and again to Manuel Caballer Gutiérrez for the support during the visit to the collection. We also thank the Universidade de São Paulo (USP), particularly the staffs of the laboratories of electron microscopy of the Museu de Zoologia (MZSP) and the Instituto de Biociências (IB-USP), for their help in the SEM preparations, and of the Centro de Biologia Marinha for their assistance in the laboratory and field work during this study (CEBIMar-USP; project no. 810).
Funding
This study was supported by fellowships from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – Finance Code 001) and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) to LS (grant 2013/08425-0). Partial funding was also provided by FAPESP to CMC (grant 2010/11253-9) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to AEM (grant 308056/2015-9). This paper is a contribution of NP-BioMar, USP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable.
Data availability statement
The datasets generated and/or analyzed during the current study are available in the GenBank (National Institutes of Health genetic sequence database) repository, https://www.ncbi.nlm.nih.gov/genbank/.
Additional information
Communicated by V. Ugorri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sales, L., Migotto, A.E., Baroni, S. et al. Taxonomic reassessment and redescription of Okenia polycerelloides (Ortea & Bouchet, 1983) (Nudibranchia: Goniodorididae) based on morphological and molecular data. Mar Biodiv 49, 2351–2368 (2019). https://doi.org/10.1007/s12526-019-00972-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-019-00972-w