Abstract
Hydrochemistry, sources of ions, identification of pollution hotspot, and potability of groundwater in shallow and deep aquifers of Palakkad and Chittur taluks (area = 1875 km2) of Bharathapuzha river basin, the largest river basin in Kerala, India, are discussed in this paper. The study area belongs to one of the rice bowls of the State. Geologically, the study area consists of Archaean crystallines, overlaid by laterite. Due to the unique geography, subhumid to semiarid climate, and intense agricultural activities, the quality of groundwater is a serious concern and warrants attention. For delineating the major ions and nutrients, water samples were analyzed for shallow (dug wells) and deep (bore wells) aquifers during monsoon (MON) and postmonsoon (POM) seasons. Physicochemical parameters showed distinct spatiotemporal variation and quality degradation, which was more prominent in deep groundwater. The relative abundance of cations and anions is in the order Na > Ca > Mg > K and HCO3 > Cl > CO3 for both the aquifers. The spatial distribution trend of parameters in deep and shallow aquifers showed a similar pattern indicating a common influence on water quality. Multivariate factor analysis (R-mode) employed in the study derived three main factors controlling the groundwater chemistry, viz. geogenic, anthropogenic, and pH. Among these, water chemistry is chiefly controlled by the geogenic factor and partly by the other two. It is also reflected well in the correlation analysis of various parameters. Since the eastern part of Chittur taluk in the study area is more impacted by anthropogenic and climatic influences, a pollution hotspot has been identified in this region through the application of Geographic Information System (GIS) overlay analysis. Thus, the study reveals agrochemical, climatic, and geologic influences on the chemistry and quality of groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is considered to be a pure form than surface water, as it undergoes purification in the soil column by way of anaerobic decomposition, percolation, and ion exchange. This is the main reason for the over-exploitation of groundwater in both rural and semi-urban regions around the world (Fasunwon et al., 2008; Tyagi et al., 2002). When water slowly moves into the subsurface porous region, it may remain there for long periods in contact with the minerals of the soil or bedrock and gets saturated with the dissolved solids until attaining equilibrium. Optimal and sustainable use of groundwater is possible only through the proper assessment of quality and quantity (Kumar et al., 2016). Periodic assessment of groundwater is highly essential for its use in drinking and irrigational purposes (Berhe et al., 2017). Rapid population growth has demanded large quantities of food grain that, in turn, resulted in the utilization of water resources to a great extent, and it is estimated that freshwater withdrawals increased from 580 km3 per year in 1990 to around 3700 km3 per year in 2000 (Gleick, 2000). Presently, the annual global withdrawal of water for all purposes accounts for about 4600 km3. It will increase by 20–30% by 2050, up to 5500 to 6000 km3 per year (Boretti & Rosa, 2019). The negative impacts of industrialization, increase in population, and agriculture have put the surface water sources under the pressure of pollution, and therefore, the demand on the use of groundwater has increased (Dubey et al., 2002). Groundwater provides nearly 50% of all drinking water globally, with 2.5 billion people relying chiefly on it to satisfy their day-to-day activities, and India records 85% of the total population depending on groundwater for drinking (Gronwall & Danert, 2020).
Geochemistry and water quality are closely related, which determines whether groundwater is suitable or not for drinking and irrigation (Kumar et al., 2016). The three main processes that determine the chemistry of groundwater are atmospheric inputs, water-soil/rock interaction, and human activities. The atmospheric inputs comprise precipitation, change in climate pattern, and natural disasters, while the interaction of rocks and minerals with water facilitates dissolution and weathering of minerals (Jarvie et al., 1998). The chemical composition of groundwater collected from a well depends on the composition of the recharge water, evapotranspiration, interaction of gases between the land surface and the groundwater table within the unsaturated zone, chemical reactions between water and solid materials within the aquifer or unsaturated zone, and mixing with other sources such as groundwater, surface water or water from anthropogenic activity (www.deq.state.or.us/wq/groundwater/docs/lubgwma/hydrogeologyrpt/appx4a.pdf).
Groundwater degradation results from the introduction or removal of certain substances beyond their natural variations, ultimately affecting its quality (Ramesh, 2001; Todd, 2001). Human activities such as industrialization, agricultural pollution, soaring consumption, and rapid urbanization deteriorate the quality of groundwater and damage their use in drinking, agricultural, industrial, and domestic purposes (Pawar et al., 1998; Jeong, 2003; Shivran et al., 2006; Stites & Kraft, 2001; Ledoux et al., 2007; Garcia-Garizabal, 2012). Several studies are available that expose nitrate contamination of groundwater due to anthropogenic activities (Takem et al., 2010), groundwater deterioration due to rapid urbanization (Purushotham et al., 2011), and spatiotemporal variation and identification of groundwater potential zones using Geographic Information System (Bagyaraj et al., 2012). Groundwater quality in Palakkad district has also been studied by various researchers (Shaji et al., 2007, 2018; Parimala Gandhi, K., 2008; Raj & Azeez, 2009; Kumar et al., 2016; Manjula & Warrier, 2019).
The quality assessment and management are the key steps and shall be implemented conjointly for an unpolluted environment for the generations to come. For understanding the quality of groundwater for its use by human beings and agriculture, the estimation of various relevant chemical constituents in water is indispensable (Dass et al., 2001). Further, statistical analyses such as correlation and factor analysis help us to identify the association of various chemical components, which in turn reveals the source of ions. Principal component factor analysis is an effective multivariate statistical method to identify the likely factors that cause variation in hydrochemical composition (Jayaprakash et al., 2007; Olmez et al., 1994). The factor analytical method has extensively been used by many researchers to delineate several geochemical and anthropogenic influences (Bakac, 2000; Subbarao et al., 1996). Factor score studies shed light on the variation of geochemical factors that control the composition of groundwater, such as precipitation, percolation, groundwater flow patterns, and soil and aquifer characteristics (Jayaprakash et al., 2007).
With the unique geography, climate, and rampant agricultural activity, groundwater is a major concern in the study area and needs utmost consideration. The present study intends to appraise the hydrochemical characteristics, sourcing of ions, and identification of pollution hotspot using multivariate statistical analysis and GIS application. Besides, the study also aims to assess the potability of groundwater in the shallow and deep aquifers of Palakkad and Chittur taluks of Kerala, India.
Study Area
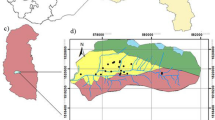
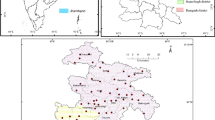
Palakkad (area = 723.4 km2) and Chittur (= 1152.0 km2) taluks of Palakkad district in the Bharathapuzha River Basin—BRB, of northern Kerala, India, were selected for the present study. The study area lies between N. Lat. 10° 15′ 59′′ to 10° 55′ 44′′ and E. Long. 76° 26′ 49′′ to 76° 54′ 29′′. The Bharathapuzha river (Order = 8th; L = 209 km; Area = 6186 km2), one of the two largest rivers in Kerala, originates from the Western Ghats at Anaimalai Hills (elevation = 1964.0 m. above MSL) and flows through the highland, midland, and lowland parts of the study area and joins the Arabian Sea at Ponnani (Anirudhan & Thrivikramji, 1996; Anirudhan et al., 1994). The ‘Palakkad Gap’ (L = 32 km.) is a distinct structural discontinuity in the 960-km-long Western Ghats on the border of Kerala with Tamil Nadu (CGWB, 2013). Palakkad and Chittur taluks lie within the gap, in juxtaposition, with Palakkad towards the north and Chittur towards the south. This area belongs chiefly to the gentle slope category (Fig. 2). Palakkad Gap is the most influential factor for the unique climate and other characteristics such as commercial and cultural exchanges between the state and the rest of the country (http://spb.kerala.gov.in/index.php/district-profile-palakkad.html). Most parts of the BRB in the midland and parts of lowland are under intense cultivation of wet crops such as paddy and sugar cane, and dry crops such as cotton, red pepper, and legumes. Palakkad district accounts for nearly 34% of the entire rice production in the State and is known as one of the ‘rice bowls’ of Kerala (Athira & Kumar, 2016). Palakkad district registers an annual average rainfall of 2106.6 mm. The major rainfall is received during June to September in the southwest monsoon (71%). But the northeast monsoon contributes only about 18%. The annual average maximum and minimum temperatures are 32.3 and 23.4 °C, respectively (Source = IMD, Pune). The location map showing sampling points, slope map, geology map, and land use/land cover map with major rivers and tributaries is shown in Figs. 1, 2, 3, and 4, respectively.
The Archaean crystallines are the chief rock types found in the study area. These comprise charnockites, khondalites, calc-granulites, hornblende gneiss, migmatites, and gneisses (Kumar et al., 2016). Khondalites and calc-granulites are seen north of Palakkad from Dhoni to Valayar and are traversed by a number of pegmatites veins. The Palakkad gap area, the eastern part of Palakkad district, is covered mainly by hornblende–biotite gneiss, which is migmatized (Shaji et al., 2007). Pegmatites and quartz veins are observed all over the district, especially in the eastern and north eastern parts (Soman, 1997). Granite is exposed on the southeastern part of Attapady and isolated patches in western and southern parts. Mica schists are noticed as relicts within the hornblende–biotite gneiss in Chittur taluk (Ravindrakumar & Chacko, 1994). A small occurrence of crystalline limestone is also reported in parts of the district. The crystalline limestones show intense fracturing and large scale development of solution cavities.
Materials and Methods
The study area was divided into rectangular grids of ~ 16.0 km2 (4.0 X 4.0 km). Representative shallow aquifer dug wells were selected from each grid (n = 40; depth < 15 m below ground level). The groundwater samples (total n = 80) were collected for monsoon—MON (August 2005), and postmonsoon—POM (April 2006), seasons. In addition, 20 bore well sites were selected near to the dug well sampling stations for gathering the water quality of deep aquifers, and samples (total n = 40) were collected for the above two seasons.
The physicochemical attributes were analyzed following the standard procedure of APHA (1995). The measurement of pH was carried out with handheld pH meter, and Electrical Conductivity (EC) with conductivity meter. Total dissolved solids (TDS) were determined by the evaporation method. Total hardness (TH), Ca, and Mg were estimated by titration using standard EDTA solution. Total alkalinity (TA), carbonates (CO3), and bicarbonates (HCO3) were measured by titration against standard sulfuric acid solution. Flame photometry was employed for measuring Na and K. Chloride (Cl) was determined by argentometric titration, and nitrate (NO3), phosphate (PO4), and silicate (SiO2) by colorimetry with UV–visible spectrophotometer. The analytical results were compared with standard values set by BIS (2012) and WHO (1996) for drinking purposes.
The statistical analysis of water quality parameters was carried out using SPSS (1997). The widely used R-mode factor analysis was employed for the present study. It investigates the relationship between the variables within the samples. Other methods, for example, the Q-mode, examine the relationship of samples in the environment. Since we need to investigate variable interactions within the samples, the R-mode factor analysis was considered (Aryafar & Ardejani, 2013). Following varimax rotation, eigenvalues and eigenvectors were extracted from the correlation matrix, and the values with the least importance were rejected (Davis, 2002).
Spatial analysis and identification of hotspot of pollution were carried out by GIS application using ArcGIS 9.1 software (Kannan & Sabu, 2010). The sampling locations were mapped with the help of SOI toposheet (1:50,000) and GPS. The thematic layers such as land use/land cover, geology, slope, and drainage were prepared from the hard copies issued from Kerala State Land Use Board (2005). The data were utilized for this study after thorough field verification. The hard copies were geo-referenced and digitized using GPS points and Survey of India toposheets.
Results and Discussion
Physicochemical Attributes
Shallow Aquifer
Statistical summary of the physicochemical attributes of shallow groundwater samples for MON and POM is given in Table 1. The pH, in general, falls slightly in acidic range. The amount of chemical constituents contributed to ground water by precipitation is probably minor except for the presence and action of dissolved carbon dioxide (www.deq.state.or.us/wq/groundwater/docs/lubgwma/hydrogeologyrpt/appx4a.pdf). Precipitation in chemical equilibrium with atmospheric carbon dioxide will be slightly acidic. This pH makes the water more aggressive and capable of dissolving constituents encountered in the subsurface. Low pH is also attributed to the acidic lateritic soil (Raghunath et al., 2001), and to some extent, the effect of chemical fertilizers like superphosphate and ammonium sulfate that have been widely used in agriculture (Appelo & Postma, 2005). Since EC is directly related to TDS (Eq. y = 0.677x-13.092; R2 = 0.9977), the locations with higher values of EC follow correspondingly higher TDS values too. MON samples exhibited relatively higher values for both these parameters, which relates to the leaching of ions from soils, rocks, and agricultural areas (Garg, 2003). The TDS content of about 25% of the total samples in each season showed values beyond the desirable limit.
The total hardness values of almost 60% of the samples during each season fall between hard and very hard types (Sawyer & McCarty, 1967). These samples mostly belong to the eastern side (i.e., Chittur taluk). The total alkalinity value of samples above the permissible limit accounts for nearly 50% each season. The spatial variation of alkalinity clearly shows elevated values in the central and eastern side of the study area.
Most of the cations exhibit concentrations below the permissible limits. The abundance of cations is in the order Na > Ca > Mg > K, and their corresponding seasonal average in percentage is 41%, 35%, 19%, 5%. The anions such as HCO3 and Cl show dominance with seasonal averages 57.6% and 40%, respectively. Carbonate is secondary in importance, representing an average of 2.3%. The order of anionic abundance is HCO3 > Cl > CO3.
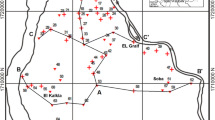
Water masses have various geochemical constituents that help to analyze the source and transport of groundwater (Lloyd & Heathcote, 1985). Saha and others in 2008 classified water masses according to a distinct abundance (> 50%) of any cation among total cations or anion among total anions, and if there is no clear dominance, 1/3 or 33% shall be considered as the cut-off value. Among the water masses identified in the study area, four were dominant in most of the locations, with three Na-dominated class and one Ca-dominated. The content of nitrate, phosphate, and silicate showed higher values in MON, owing to the leaching of nutrients out of agricultural fields in heavy rains. After evaluating the attributes in terms of potability, it is found that 23% of samples in MON and 35% in POM are not suitable for drinking.
Deep Aquifer
Statistical summary of the physicochemical attributes of groundwater samples of deep aquifer for MON and POM is given in Table 1. The results revealed relatively higher values for almost all the parameters. Spatial distribution of parameters such as TDS, EC, hardness, and alkalinity showed higher contents towards the eastern part. Nearly 30% of samples in each season exhibit TDS content outside the desirable limit. The hardness content of approximately 60% of the total samples in both the seasons falls between hard and very hard types. Total alkalinity shows wide variation in all the seasons. Of the total samples, 95% in MON and 90% in POM fall beyond the permissible limit. However, major cations show values within permissible limits. Among them, Na is the dominant one and the order of abundance is Na > Ca > Mg > K. Bicarbonate is the dominant anion, followed by chloride and carbonate. Ca-HCO3 is the dominant water type identified in the deep aquifer. It is represented by nearly 50% of samples during MON and 35% during POM. The second dominant group identified during MON is Na-HCO3, and that of POM is Ca-Na-HCO3. All the nutrients (nitrate, phosphate, and silicate) in deep groundwater exhibit relatively higher concentrations in POM in contrast to their abundance in MON in shallow wells. The analysis of bore well samples shows that all the samples during MON and 60% samples in POM are not suitable for drinking.
GIS Application
The parameters such as pH, EC, TDS, TH, and TA were considered for GIS modeling (Kannan & Sabu, 2010). The seasonal hydrochemical data were integrated with its spatial dimension, and inverse distance weighted method (IDW) had been used for spatial interpolation. Overlay analysis using GIS application was employed and proper weightages were given to the thematic layers generated for these parameters and are given in Table 2. EC was given a weightage = 30, TH = 20, TA = 20, TDS = 15, and pH = 15. The weightage of each layer and corresponding feature scores were assigned and added to the thematic layers, based on their importance to influence the potability of groundwater (Multi-criteria evaluation). Among the various methods available for finding interclass or inter-map relationship, a probability-weighted method has been followed. It involves a linear combination of probability weights for each thematic layer (Wt) and assigned scores (Wi) given to the particular categories of derived maps. The final integrated maps are generated after deriving the total weights as the sum or product of the weights assigned to the different layers based on their suitability (Bagyaraj et al., 2012).
GIS analysis was carried out to identify the pollution hotspot or the stress zone in the study area. The final output maps of overlay analysis of the selected thematic layers for MON and POM are shown in Fig. 9. The output maps were generated following multi-criteria evaluation based on suitability for drinking. In the figure, the green regions represent good quality, i.e., the water samples collected from these regions show parameters with values within the limits of drinking quality standards. The quality degradation starts from the regions of yellow to red. The dark red region represents the samples with all the selected parameters falling outside the limits of drinking quality standards. This is the ‘pollution hotspot’ in the study area, which is the eastern part falling in Chittur taluk. A pollution hotspot is a region at which the parameters defining the water quality such as pH, electrical conductance, Total Dissolved Solids (TDS), chloride, fluoride, iron, nitrate, sulfate, total hardness, calcium, and magnesium are beyond the permissible limit in the absence of alternate sources (CWC, 2011). This region is a rain shadow place in the State, characterized by low rainfall, over-exploitation of groundwater and industrial and agricultural activities. Comparatively higher sand content in the soils of Palakkad district and higher content of silt and clay in Chittur taluk were already established by Kannan et al., 2009. The higher concentration of organic carbon was also noted in silt and clay-rich soil.
Correlation Analysis
Shallow Aquifer
The correlation between various elements for shallow groundwater is presented in Table 3. The parameters EC, TH, HCO3, and Cl showed significant positive correlation with Ca, Mg, and Na. It reveals that the simultaneous increase or decrease of the ions in the groundwater is mainly attributed to the dissolution/precipitation and concentration effects (Kannan & Sabu, 2010; Kumar et al., 2016). Further, Gibb’s diagrams (Gibbs, 1970), representing the ratios of Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−) as a function of TDS has been employed to assess the functional sources of dissolved chemical constituents. The Gibb’s diagrams for the preset study show points falling chiefly in the rock dominance field, revealing rock–water interaction as the major mechanism controlling the surface water chemistry (Fig. 5). Nutrients such as NO3 and PO4 were not significantly correlated with any of the other ions, and it indicates that the sources of these ions are not lithological but anthropogenic. The correlation study shows that the chemistry of ions in the groundwater depends mostly on rock–water interactions and partly on human activities.
Deep Aquifer
The results of the correlation analysis of deep aquifer (Table 4) for MON and POM show similar relationships between ions. Therefore, the chemistry of the deep groundwater is chiefly influenced in a similar way for both the seasons (Fig. 6). A very noticeable change has been observed between total hardness and its correlated ions during POM separately for shallow and deep groundwaters. Hardness showed a significant positive correlation with nitrate in shallow groundwater irrespective of other ions. Such a relationship between hardness and nitrate has already been established by Jeevanandam and others in 2006. The concentration of several inorganic chemicals such as NO3, Cl, SO4, PO4, K, Mg, and Ca is influenced directly or indirectly by agricultural activities (Bohlke, 2002).
Assessment of the Relation Between Deep Aquifer and Geogenic Factor
Major Geogenic Constituents in the Study Area
The geogenic constituents originate from the rocks, and thus, the geology of an area has a significant influence in determining the quality of groundwater. The major rock types seen in the study area were investigated by various researchers (Kumar et al., 2016; Ravindrakumar & Chacko, 1994; Shaji et al., 2007; Soman, 1997) and given in detail in the study area part of this paper. Among them, khondalite group comprises calgranulite with limestone and garnet–sillimanite gneiss found in the Palakkad gap area. Hornblende–biotite gneiss covers a wider area, especially along the Palakkad gap. Granite gneiss is exposed along the intra-state boundary of the district. Garnet–biotite gneiss occurs in the northern parts of the district in close association with khondalite, charnockite and hornblende gneiss (Table 5).
Characteristics of Garnet–Biotite Gneiss
Satheesh Kumar (2001) studied the geology and structure of a part of Achankovil shear zone. The characteristics of garnet–biotite gneiss are given in Table 5.
Correlation of Water Quality of Deep Aquifer with Garnet–Biotite Gneiss
Correlation analysis was conducted between the water quality parameters of deep aquifer with the constituents of garnet–biotite gneiss (Table 6). The constituents, namely SiO2, CaO, MgO, Na2O, and K2O in garnet–biotite gneiss, were correlated with the mean, minimum, and maximum concentration of Si, Ca, Mg, Na, and K in the waters of deep aquifer. The study revealed that garnet–biotite gneiss is positively correlated with maximum concentration of water quality. This reveals the influence of garnet–biotite gneiss with the water of deep aquifer.
Factor Analysis
Factor analysis has been carried out to identify the significant factors affecting both shallow and deep ground water quality (Table 7).
Shallow Aquifer
Factor I of MON which explains 44.1% of the total variance with high loadings of EC, TDS, Ca, Mg, Na, and Cl indicates that these ions have been derived from a common source. This is termed as the geogenic factor. For halite dissolution to occur, the ratio between Na and Cl should almost equal to 1, and if the ratio is more than 1, silicate weathering could be the reason for the release of Na (Meyback, 1987). In the present study, the Na/Cl ratio of groundwater samples varies between 0.09 and 1.96. When Na is plotted against Cl (Fig. 7), most of the points lie under the 1:1 line, showing relatively excess Cl. Since the anthropogenic sources in the study area, chiefly chemical fertilizers, do not add much of the Na into the groundwater, Na might be released from weathered soil, which was also reported by Shaji and others in 2007. Further, chloride contamination in groundwater results from an enormous quantity of manure and fertilizer application (Komor & Anderson, 1993; Wassenaar, 1995).
The strong correlation between both magnesium–calcium and magnesium–bicarbonate (0.87 and 0.57, respectively) and the abundance of these ions in groundwater indicate that the dissolution reaction indeed is happening (Jalali & Khanlari., 2007). Thus, factor I shows mineral dissolution as the main process determining the water chemistry i.e., geogenic. Almost the same condition can be observed during POM. Additionally, NO3 found in the water, which has no relevant lithologic origin in the study area, must relate to human interventions (Subramani et al., 2005).
Factor II of MON explains 20.1% of the total variance with high loadings of variables TA, CO3, and HCO3. The pH of groundwater relies on the rock chemistry and the sediments surrounding the travel course of the recharge water percolating to the groundwater (www.agwt.org/content/acid-rain-and-ground-water-ph) and the duration of contact between groundwater and a particular rock. Groundwater moving through carbonate-rich rocks usually shows a pH value of more than 7 since the acidic water gets neutralized. If the aquifer holding the groundwater has few carbonate rocks, the groundwater will remain acidic. In the study area, bicarbonate is the dominant anion. The major contribution is attributed to the dissolution of crystalline limestone in the Palakkad Gap region (Soman, 1997). Earlier studies also reported high concentrations of these ions in the study area (Bajpayee & Verma, 2001). Moreover, the above-mentioned variables correlate positively with pH, reflecting the relationship between alkalinity and the dissolution process. It also comes under the geogenic factor.
During POM, Factor II accounts for 25.6% of the total variance with high loadings in the variables Na, TA, CO3, and HCO3. Mineral weathering could be the reason for its high loading (Shaji et al., 2007). The ion exchange between Ca or Mg and Na also results in relatively high Na content in groundwater (Stimson et al., 2001).
The sources of Ca and Mg in groundwater can be explained using Ca + Mg/HCO3 ratio. If the dissolution of carbonate rocks in the aquifer and associated mineral weathering causes the release of Ca and Mg into the groundwater, the ratio would nearly be around 0.5 (Sami, 1992). In the present study, the majority of the samples (90%) fall under this value with an average of 0.4 (Fig. 8). The low Ca + Mg/HCO3 ratio (< 0.5) may be either due to the depletion of (Ca + Mg) over subsequent Na exchange or by HCO3 enrichment. The excess HCO3 ions then release the alkali ions, chiefly Na, into the solution through ion exchange (Jalali & Khanlari, 2007). The presence of migmatized hornblende–biotite gneiss in the study area establishes the geological influence on the concentration of the cations like Na (Soman, 1997).
Factor III of MON is responsible for 12.8% of the total variance. This factor is having high loadings of NO3, PO4, and K. The presence of these ions clearly indicates human influence on the quality of groundwater in the study area. This is termed as anthropogenic factor. The use of chemical fertilizers is a common practice in Kerala, and in the study area, it is prevalent as the BRB is chiefly an agricultural one (Chattopadhyay et al., 2005). The MON enrichment of this factor substantiates the relation between nutrient leaching from soil and their corresponding concentrations in groundwater (Babu et al., 2007). Since nitrate is loosely bound to the soil, it tends to be high in the runoff, causing its elevated concentration during MON (Rao et al., 2004). During POM, the factor has high loadings of PO4 and K indicating the application of chemical fertilizers. Nitrate showed a low loading during POM since the runoff is relatively low compared to that in MON.
Deep Aquifer
The factor analysis of various parameters of deep groundwater extracted four major groups of loading that represent the chief influencing factors determining the water chemistry. The results are summarized in Table 7.
Factor I of both MON and POM samples in the deep groundwater shows high loadings of similar parameters such as TDS, TH, Ca, Mg, Na, and Cl due to the contribution by the rocks, chiefly hornblende–biotite gneiss. Hence, the factor I is chiefly geogenic. However, both in shallow and deep groundwater, chloride was found to be present in high concentrations. Human interventions account for the contribution of chloride, as the chloride-rich minerals are not prevalent in the study area. Since paddy cultivation is the chief land use in the study area, fertilizers such as potash may be applied to a great extent. The major constituent of potash is carnallite (KMgCl3—6H2O), which is rich in chloride.
Factor II of MON in deep groundwater is abundant with the loadings of pH, TA, and HCO3, and it accounts for 18% of the total variance. Since these three parameters are very much related, this factor can be termed geogenic factor, indicating that the lower values of principal component scores in some stations are due to low residence time of groundwater. Similar loadings were observed in POM. High concentrations of bicarbonate ions have already been reported (Bajpayee & Verma, 2001). The dissolution of crystalline limestone in the Palakkad Gap region was also established (Soman, 1997).
Factor III of MON is loaded with high contents of potassium and phosphate. It accounts for nearly 13% of the total variance. This factor is also present in the shallow aquifers, explained earlier, indicating human influence through fertilizer application.
Principal Component Factor Analysis assessment
The spatial variation of principal component scores indicating anthropogenic factor, geogenic factor, and precipitation factor was studied. The study reveals the dominance of anthropogenic factor towards the central and eastern side (Chittur taluk) of the study area. Phosphate, nitrate, and potassium contributed from intense agricultural activities are mainly responsible for the anthropogenic factor. Further, dominance of the geogenic factor also exists in the eastern part of the area. The eastern part is a semi-arid region. However, the sub-humid western region shows dominance of precipitation factor.
GIS Weighted Map
GIS application has been used to identify the pollution hotspot or the stress zone in the study area. The final output maps of overlay analysis of the selected thematic layers for MON and POM are shown in Fig. 9. The final overlay maps were generated using the sum or product of the weights given to each layer depending on their suitability for drinking. In the figure, the green regions represent good quality. i.e., the water samples collected from these regions show parameters with values within the limits of drinking quality standards. The dark red region represents the samples with all the selected parameters falling outside the limits of drinking quality standards. This is the ‘pollution hotspot’ in the study area, which is the eastern part falling in Chittur taluk.
Conclusion
The present study reveals that in the shallow aquifer, 23% of samples in MON and 30% in POM are not suitable for drinking since majority of the parameters show contents above the permissible limits. The quality is further degraded in deep groundwater, where more than 60% of samples are not potable. Seasonal variation is distinct with shallow aquifer exhibiting higher concentrations during MON for most of the parameters, while deeper counterpart registers higher values during POM. The shallowness of dug wells (ave. = 7.33 ft.) and the greater dissolution of salts and minerals during MON account for their high content of ions. But, the bore wells are very deep (> 100 ft.) and the movement of ions to the deep aquifer is relatively low. Here, rock–water interaction is the chief mechanism controlling the water chemistry. The correlation analysis confirms the relation of garnet–biotite gneiss with phreatic aquifer. The multivariate factor analysis identified chiefly two factors, viz., geogenic factor due to dissolution and ion exchange process, and anthropogenic factor due to nutrients from irrigation return flow which in turn control the chemistry of both shallow and deep groundwater in the study area. The principal component analysis map shows the spatial distribution of affected areas. This is in conformity with the identification of the pollution hotspot towards the eastern part, which falls mainly in Chittur taluk by the GIS model analysis.
References
Anirudhan, S., & Thrivikramji, K. P. (1996). Sediments of the Bharathapuzha, Kerala: Implications of downstream trends of texture and grain shape. Journal of the Geological Society of India, 47, 459–465.
Anirudhan, S., Thrivikramji, K. P., & Roy Chacko, P. T. (1994). Roles of relief and climate on composition of detrital sediments of Bharathapuzha Basin Kerala. Journal of the Geological Society of India, 43, 425–433.
APHA. (1995). Standard Methods for the Examination of Water and Wastewater. (19th ed.). Washington D.C.
Appelo, C. A. J., & Postma, D. (2005). Geochemistry, groundwater and pollution. (2nd ed., p. 404p). Balkema publishers.
Aryafar, A., & Ardejani, F. D. (2013). R-mod factor analysis, a popular multivariate statistical technique to evaluate water quality in Khaf-Sangan basin, Mashhad, Northeast of Iran. Arabian Journal of Geosciences , 6, 893–900. https://doi.org/10.1007/s12517-011-0367-7.
Athira, H., & Kumar, K. N. (2016). Scenario analysis of rice cultivation in Kerala. J. Ext. Educ. https://doi.org/10.26725/JEE.2016.4.28.5760-5763.
Babu, K. N., Padmalal, D., Maya, K., Sreeja, R., & Arun, P. R. (2007). Quality of surface and groundwater around tile and brick clay mines in the Chalakudy river basin southwestern India. Jour. Geol. Soc. India, 69, 279–284.
Bagyaraj, M., Ramkumar, T., Venkatramanan, S., & Gurugnanam, B. (2012). Application of remote sensing and GIS analysis for identifying groundwater potential zone in parts of Kodaikanal Taluk. . Front. Earth Sci. https://doi.org/10.1007/s11707-012-0347-6.
Bajpayee, S. K. & Verma, A., 2001. Water quality of rivers of Kerala, South Western, India. In: V. Subramanian & A. L. Ramanathan, (Eds.), Proceedings of the International Workshop on Ecohydrology. Capital publishing company, pp. 307–308.
Bakac, M. (2000). Factor analysis applied to a geochemical study of suspended sediments from the Gediz River, western Turkey. Environmental Geochemistry and Health, 22(2), 93–111.
Berhe, B. A., Dokuz, U. E., & Celik, M. (2017). Assessment of hydrogeochemistry and environmental isotopes of surface and groundwaters in the Kutahya Plain Turkey. Journal of African Earth Sciences, 134, 230–240.
BIS. (2012). Specifications for drinking water, IS:10500:1991. . Bureau of Indian Standards.
Bohlke, J. K. (2002). Groundwater recharge and agricultural contamination. Hydrogeology Journal, 10, 153–179.
Boretti, A., & Rosa, L. (2019). Reassessing the projections of the World Water Development Report. npj Clean Water. https://doi.org/10.1038/s41545-019-0039-9.
CGWB (2013) Central Ground Water Board, Kerala region. Technical reports: Series ‘D’. Ground water information Booklet of Palakkad District.
Chattopadhyay, S., Asa Rani, L., & Sangeetha, P. V. (2005). Water quality variations as linked to landuse pattern: A case study in Chalakudy river basin Kerala. Current Science, 89(12), 2167p.
CWC (2011) Water quality hot-spots in rivers of India. Central Water Commission, India water portal, https://www.indiawaterportal.org/articles/water-quality-hot-spots-rivers-india-report-central-water-commission
Dass, S., Agarwal, M., Chaudhary, Y.S., Shrivastav, R., (2001) A study on fluoride and other water quality parameters of groundwater of district Agra (U.P.). In: V. Subramanian, A. L. Ramanathan (Eds.), Proceedings of the international workshop on ecohydrology. Capital Publishing Company, 213p.
Davis, J. C. (2002). Statistics and data analysis in geology. (pp. 526–540). Wiley.
Dubey, C. S., Pattanayak, S. K., Sharma, B. K., & Sirohi, A. (2002). Groundwater pollution in the satellite city Faridabad, India. In C. S. Dubey & P. S. Saklani (Eds.), Geoindicators and related environmental studies: focus on India. (pp. 47–56). Pilgrims Publishing.
Fasunwon, O., Olowofola, J., Akinyemi, O., Fasunwon, B. and Akintokun, O. (2008) Contaminants evaluation as water quality indicator in ago-iwoye, South-western, Nigeria, African Physical Review, 2, p. 12.
Garcia-Garizábal, I. (2012). Dynamics of an aquifer and water quality in a traditional irrigated land in the Ebro river basin. ITEA, 108, 115–130.
Garg, S. S. (2003). Water quality of well and bore well of ten selected locations of Chitrakoot region. In A. Kumar (Ed.), Aquatic environment and toxicology. (p. 114p). Daya Publishing House.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 170, 1088–1090.
Gleick, P. H. (2000). The changing water paradigm- a look at twenty-first century water resources development. International Water Resources Association Water International, 25(1), 127–138.
Gronwall, J., & Danert, K. (2020). Regarding groundwater and drinking water access through a human rights lens: Self-supply as a norm. Water. https://doi.org/10.3390/w12020419.
Jalali, M., & Khanlari, Z. V. (2007). Major ion chemistry of groundwaters in the Damagh area, Hamadan, western Iran. Environmental Geology. https://doi.org/10.1007/s00254-007-0795-6.
Jarvie, H. P., Whitton, B. A., & Neal, C. (1998). Nitrogen and phosphorus in east coast British rivers: Speciation, sources, and biological significance. Science of the Total Environment, 210, 79–109.
Jayaprakash, M., Giridharan, L., Venugopal, T., Krishna Kumar, S. P., & Periakali, P. (2007). Characterization and evaluation of the factors affecting the geochemistry of groundwater in Neyveli, Tamil Nadu, India. Environmental Geology. https://doi.org/10.1007/s00254-007-0868-6.
Jeevanandam, M., Kannan, R., Srinivasalu, S., & Rammohan, V. (2006). Hydrogeochemistry and groundwater quality assessment of lower part of the Ponnaiyar River Basin, Cuddalore district South India. Environmental Monitoring and Assessment, 132, 263–274. https://doi.org/10.1007/s10661-006-9532-y.
Jeong, C. H. (2003). Effect of landuse and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. Journal of Hydrology, 235, 194–210.
Kannan, N., & Sabu, J. (2010). Quality of ground water in the shallow aquifers of a paddy dominated agricultural river basin, Kerala, India. International Journal of Environmental Science and Engineering, 2, 3.
Kannan, N., Sabu, J., & Jobin, T. (2009). Textural characteristics, organic carbon, and nitrogen content in the paddy soils of Palakkad district, Kerala India. Pollution Research, 28(2), 161–163.
Komor, S. C., & Anderson, H. W. (1993). Nitrogen isotopes as indicators of nitrate sources in Minnesota sand-plain aquifers. Ground Water, 31, 260–270.
Kumar, V. S., Amarender, B., Dhakate, R., Sankaran, S., & Kumar, K. R. (2016). Assessment of groundwater quality for drinking and irrigation use in shallow hard rock aquifer of Pudunagaram, Palakkad District Kerala. Applied Water Science, 6, 149–167. https://doi.org/10.1007/s13201-014-0214-6.
Ledoux, E., Gomez, E., Monget, J. M., Viavattene, C., Viennot, P., Ducharne, A., & Mary, B. (2007). Agriculture and groundwater nitrate contamination in the Seine basin. The STICS–MODCOU modelling chain. Science of the Total Environment, 375(1), 33–47.
Lloyd, J. W., & Heathcote, J. A. (1985). Natural inorganic hydrochemistry in relation to groundwater. (p. 294p). Claredon Press.
Manjula, C., & Warrier, U. (2019). Evaluation of water quality of Thuthapuzha Sub-basin of Bharathapuzha, Kerala India. Applied Water Science, 9, 70. https://doi.org/10.1007/s13201-019-0937-5.
Meyback, M. (1987). Global chemical weathering of surficial rocks estimated from river dissolved loads. American Journal of Science, 287, 401–428.
Olmez, L., Jack, W. B., & Villaume, J. F. (1994). A new approach to understanding multiple-source groundwater contamination: Factor analysis and chemical mass balance. Water Research, 28, 1095–1101.
Parimala Gandhi, K. (2008). Analysis of water quality parameters in selected areas of Palakkad district. Current World Environment, 3(2), 283–287.
Pawar, N. J., Pondhe, G. M., & Patil, S. F. (1998). Groundwater pollution due to sugar mill effluent at Sonai, Maharashtra, India. Environmental Geology, 34(2–3), 151–158.
Purushotham, D., Prakash, M. R., & Rao, A. N. (2011). Groundwater depletion and quality deterioration due to environmental impacts in Maheshwaram watershed of R.R. District, AP (India). Environmental Earth Science, 62(8), 1707–1721.
Raghunath, R., Sreedhara Murthy, T. R., & Raghavan, B. R. (2001). Spatial distribution of pH, EC and total dissolved solids of Nethravathi river basin, Karnataka state, India. Poll. Res., 20(3), 413–418.
Raj, N. and Azeez, P.A., (2009) Spatial and temporal variation in surface water chemistry of a tropical river, the river Bharathapuzha, India. Current Science, 96(2).
Ramesh, R., (2001) Point and non-point sources of groundwater pollution: case studies along the East Coast of India. In: V Subramanian and A. L. Ramanathan (Eds), Proceedings of the international workshop on ecohydrology. Capital Publishing Company, 107p.
Rao, P. M., Sekhar, P., & Yadav, Y. S. (2004). Water quality studies on Kolleru lake and its infalling drains of A.P., India. In A. Kumar (Ed.), Water pollution. (p. 171p). APH publishing corporation.
Ravindrakumar, G. R., & Chacko, T. (1994). Geothermobarometry of mafic granulites and metapelite from the Palghat Gap, South India: petrological evidence for isothermal uplift and rapid cooling. Journal of Metamorphic Geology, 1, 479–492.
Saha, D., Dhar, Y. R., & Sikdar, P. K. (2008). Geochemical Evolution of Groundwater in the Pleistocene Aquifers of South Ganga Plain Bihar. Journal of the Geological Society India, 71, 473–482.
Sami, K. (1992). Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern cape South Africa. Journal of Hydrology, 139, 27–48.
Satheesh Kumar, R., (2001) Geology and Structure of a part of Achankovil Shear Zone (Unpublished doctoral thesis, University of Kerala)
Sawyer, C. N., & McCarty, P. L. (1967). Chemistry for sanitary engineers. (2nd ed., p. 518p). McGraw-hill.
Shaji, E., Bindu, J. V., & Thambi, D. S. (2007). High fluoride in groundwater of Palghat district, Kerala. Current Science, 240(92), 2–25.
Shaji, E., Gomez-Alday, J. J., Hussein, S., Deepu, T. R., & Anilkumar, Y. (2018). Salinization and deterioration of groundwater quality by Nitrate and Fluoride in the Chittur Block, Palakkad Kerala. Journal of the Geological Society of India, 92, 337–345.
Shivran, H. S., Dinesh Kumar, D., & Singh, R. V. (2006). Improvement of water quality though biological denitrification. Journal of Environmental Science & Engineering, 48(1), 57–60.
Soman, K. (1997). Geology of Kerala, published by Geological Society of India, Bangalore, India-560019, 280p.
SPSS, (1997) SPSS base statistics, version 13.0. Chicago, USA: SPSS Inc.
Stimson, J., Frape, S., Drimmie, R., & Rudolph, D. (2001). Isotopic and geochemical evidence of regional-scale anisotropy and interconnetivity of an alluvial fan system, Cochabamba Valey, Bolivia. Applied Geochemistry, 16, 1097–1114.
Stites, W., & Kraft, G. J. (2001). Nitrate and chloride loading to groundwater from an irrigated North-Central U. S. Sand- Plain vegetable field. Journal of Environmental Quality, 30, 1176–1184.
Subbarao, C., Subbarao, N. V., & Chandu, S. N. (1996). Characterisation of ground water contamination using factor analysis. Environmental Geology, 28, 175–180.
Subramani, T., Elango, L., & Damodaraswamy, S. R. (2005). Groundwater quality and its suitability for drinking and agricultural use in Chithar River basin, Tamilnadu, India. Journal of Environmental Geology, 47, 1099–1110.
Takem, G. E., Chandrasekharam, D., Ayonghe, S. N., & Thambidurai, P. (2010). Pollution character-ristics of alluvial groundwater from springs and bore wells in semi-urban informal settlements of Douala, Cameroon Western Africa. Environment Earth Science, 61(2), 287–298.
Todd, D. K. (2001) Groundwater hydrology, 2nd edition, Wiley, p. 334.
Tyagi, P. D., Buddhi, R., Chaudhary, K. C., & Sawhney, R. L. (2002). Degradation of ground water quality in industrial area in India. India Journal Environment Protect, 20, 174–181.
Wassenaar, L. (1995). Evaluation of the origin and fate of nitrate in the Abbotsford Aquifer using the isotopes of 15N and 18O in NO3 _. Applied Geochemistry, 10, 391–405.
WHO, (1996) Guidelines for drinking water, Vol. 2, Recommendations, World Health Organization.
Acknowledgements
The authors thank University Grants Commission, New Delhi, India, for funding in the form of a major research project (No:- F.30-101/2004 (SR)).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kannan, N., Joseph, S. & Sheela, A.M. Characterization of Groundwater in the Shallow and Deep Aquifers of an Agriculture-Dominated Tropical Subhumid to Semiarid Region, India: a Multivariate and GIS Approach. J Indian Soc Remote Sens 49, 1853–1868 (2021). https://doi.org/10.1007/s12524-021-01356-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12524-021-01356-z