Abstract
This study investigates how the technology of Coptic Glazed Ware (CGW) – which is one of the earliest examples of Islamic glazed pottery – was developed, allowing for an insight into the mechanisms that contributed to the making of early Islamic material culture. The range of technologies of 20 CGW samples recovered from different sites in Israel was reconstructed, based on the characterisations by thin-section petrography, optical microscopy, and scanning electron microscopy energy-dispersive spectrometry. Our results show that the samples were originated from Aswan, Egypt. The procurement of kaolinitic clay from local deposits to form the ceramic body and slip, as well as the preference of painting as the principal mode of decoration, represents a continuation of the local fine ware tradition (Egyptian red and white slip ware and Coptic painted ware). The use of lead glaze was more akin to the Byzantine glaze technology. The CGW technology is further distinguished by the use of a diverse range of colourants and how the coloured glazes were prepared. Although individual elements of the CGW technology display influences from preceding and contemporaneous pottery technologies, it was not until the production of CGW that all these elements were combined together for the first time, highlighting the innovative character of the CGW technology. We argue that such innovation was born out of a strong local fine ware tradition that was embedded in the landscape of highly specialized craft production, while stimulating by a desire to establish new identities and new material representations by the Arab-Muslim newcomers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emergence of Islamic glazes in the eighth century had sparked a chain of revolutions in ceramic technology, production and consumption habits and artistic representations. As such, Islamic glazes are the focus of many art-historical and technological studies (e.g. Mason 2004; Watson 2004, 2017). Particular emphasis has been placed on the opaque glazed wares because tin-opacified glaze technology was advocated as a genuine Islamic invention originating from Iraq around the ninth century, driven by the desire to imitate Chinese porcelain (Mason and Tite 1997). Recent re-examination of the evidence, however, suggested that the opaque glaze technology was likely developed from the lead-tin pigment used to create yellow decoration of the early Islamic Egyptian-origin Coptic Glazed Ware (CGW) (Matin et al. 2018; Tite et al. 2015; Watson 2014, 2017: 481–83, 486–88). This new link is made based on the analysis of two CGW samples – one recovered from the site of Madaba in Jordan (Matin et al. 2018) and one from the Alexander Kaczmarczyk collection of Egyptian glazed wares (Salinas et al. 2019; Tite et al. 2015) – mostly focusing on the yellow glaze. Thus, we still know very little about the features of the CGW technology and its development, even though CGW is now being placed at the starting point of this drastic change in ceramic technology.
CGW was first identified by Rodziewicz (1976) in the excavation of Alexandria, Egypt. An early eighth century date was designated to CGW as its fragments were found to have deposited on top of some Roman houses dating to the sixth and seventh centuries. CGW is subsequently reported to be present in various locations throughout the Levant (see Matin et al. 2018: 43, section 2.1 for overview; see also Grossmann et al. 2009: 205–208; Konstantinidou 2015; Taxel 2014: 121–22, Table 1). Rodziewicz proposed that Egypt was the original production centre of CGW, but these vessels bear little similarity, especially in terms of their composition, to the products from the workshops in Fustat (Mason 1997: 208; Mason and Keall 1990). CGW is identifiable for its open vessel form (e.g. bowl or plate) and flat base, often with straight walls, plain rim, pink ceramic fabric and multihued painted decoration that was applied either under or over the glaze. In their respective description of the CGW assemblages from Alexandria and Aqaba, Rodziewicz (1976) and Whitcomb (1989) both noticed that these bowls display similar formal and stylistic elements to Egyptian red and white slip wares and Coptic painted ware, which were derived from the Late Roman fine ware of north Africa, Asia Minor and Cyprus. With this observation in mind, one may wonder the extent to which the technology used to make CGW was also similar to these existing ceramic traditions.
To investigate how this early Islamic glaze technology developed, the CGW assemblages from various sites in central and southern Israel are the focus of this study (see Taxel 2014 for a review of CGW finds in historical Palestine). In this study, thin-section petrography, optical microscopy and scanning electron microscopy energy-dispersive spectrometry were used to characterize the CGW technology, including the sources of raw materials, the preparation methods of ceramic body, slip and glaze and the method and order of glaze application. This is followed by a discussion of the CGW technology in relation to existing ceramic traditions – with special attention to local unglazed fine ware and Byzantine and pre-Islamic Mesopotamian (mainly Sasanian) glaze technologies – to establish the potential technological connection. By referring to the sociohistorical context under which the CGW technology was developed, it allows for an insight into the mechanisms that fuelled the change in ceramic technology during the early Islamic period.
Materials
Given the restricted access to the Egyptian material, we turned to the CGW collections from early Islamic Palestine. CGW is said to have a wider distribution than opaque glazed and lustre ware in Palestine with its presence being reported in both urban and rural settlements (Taxel 2014: 133), but its relative quantity within each assemblage is still very low. We have sampled 20 CGW samples that were recovered from five sites (Fig. 1). All samples represent a bowl form with straight wall and plain rim, which are morphological and stylistic features typical of CGW as mentioned above. Some of these CGW samples have already been published in the relevant excavation reports, whereas the publication of other samples is underway. The sites where the samples came from are plotted on Fig. 2, in which the site locations refer to the archaeological sites and the DMS geographical coordinates are based on published data. The description of the sites and samples are as follows:
-
(1)
Ramla was a major inland coastal urban centre and the capital of early Islamic Palestine. Two of the sampled bowls were found in a Tel Aviv University excavation (Tal and Taxel 2008: 128, Fig. 6.83: 1, 2), and eight bowls were retrieved from a Hebrew Union College excavation (Kohn-Tavor 2017: 25, Fig. 2.1). These bowls were originated in mixed contexts, usually not earlier than the ninth century.
-
(2)
Jaffa was an important harbour town. Two of the sampled bowls were originated from a Tel Aviv University excavation at the site.Footnote 1 The context of the sampled bowls dates to the late eighth or ninth century.
-
(3)
Yavneh was an inland coastal town. An Israel Antiquities Authority (IAA) excavation was carried out there, yielding two bowls we sampled.Footnote 2 The first bowl (CG06) came from a context dated to the mid-/late eighth to ninth century, and the second bowl (CG13) was retrieved from a ninth-century context.
-
(4)
Yavneh-Yam was the entrepot of Yavneh and a military stronghold, where two of the sampled bowls were found in a Tel Aviv University excavation (for one of the bowls, see Taxel 2014: 131–132, Fig. 3: 2).Footnote 3 As in the case of the Ramla samples, the Yavneh-Yam bowls were recovered from mixed contexts, usually not earlier than the ninth century.
-
(5)
Khirbat ‘Amra was a rural settlement in the northern Negev (the Beersheba Valley). An IAA excavation was carried out thereFootnote 4 and retrieved three additional bowls we sampled, all of which are tentatively dated to the ninth century.
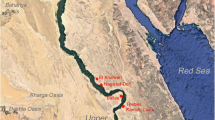
Map showing the sites in southern and central Asia where the samples under studied were recovered and the sites that were mentioned in text. The DMS geographical coordinates are 29 31 50.46601872 N and 034 59 58.35994404E for Aqaba, 31 13 52.78927080 N and 029 58 23.17427940E for Alexandria and 30 05 14.69101308 N and 031 19 40.06580628E for Fustat. The map was prepared by Emil Aladjem
The samples were exported under the permit issued by the IAA. Although no evidence of CGW production was retrieved from these sites, these bowls are ideal for reconstructing the technology or the range of technologies used to make CGW because they have preserved a diversity of surface decorations. The interior surface of these bowls was decorated with a combination of green, honey, yellow, brown and white glazes. The exterior surface of most samples was not covered with slip and glaze, but in some samples, the presence of slip and glaze is identified in the area below the rim, which might have been the result of the slip and glaze being brushed over unintentionally when applying the decoration on the interior surface. We tried to extract more than one glaze colour sample from each vessel, but it was not possible in some cases as this would have destroyed the integrity of the bowls (Table 1).
Analytical methods
Thin-section petrography
As an initial step, petrographic analysis was performed on the samples to identify their mineralogical and textural (e.g. abundance, shape, size of inclusions) characteristics. By comparing with the local geological data, the mineralogical composition of the samples allows for establishing their potential provenance, which is of crucial importance in this case as the CGW samples under studied were not recovered from sites of production. The textural characteristics of the samples, on the other hand, provide useful clues to understand how potters prepared the ceramic paste. All samples were prepared at the UCL Wolfson Archaeological Sciences Laboratories and analysed using the LEICA DM EP Polarization Microscope. For the description of the petrographic data, the percentage charts developed by Matthews et al. (1991) were used to estimate the abundance of inclusions.
Optical microscopy and scanning electron microscopy energy-dispersive spectrometry (SEM-EDS)
All samples were then submitted to examination by optical microscopy, followed by SEM-EDS analysis, to characterize the composition and microstructure of the ceramic body, slip and glaze, constituting the basis of reconstructing different aspects of glaze technology. These aspects include the preparation methods of slip and glaze, the method and order of glaze application and the assembling sequence. The samples were prepared in polished blocks and carbon-coated. Optical microscopy was conducted at the Pitts-River Laboratory for Archaeological Science, University of Cambridge, using the Keyence VHX-6000 digital microscope.
SEM-EDS analysis was conducted at UCL Wolfson Archaeological Science Laboratories using the CARL-ZEISS EVO25 scanning electron microscope, fitted with the Oxford Instruments AZtec energy-dispersive spectrometer. The system was set to 20.0 kV accelerating voltage and collect about 750,000 X-rays, which took about 22 to 25 s total per measurement. The area of analysis is around 10 × 10 μm for the glaze with no particles and crystallines and the ‘matrix’ (i.e. clear area) of the glaze that contain particles and crystallines; and in the latter case, larger area of analysis (around 25 × 50 μm) was also performed to include the particles and crystallines in characterizing the glaze composition. These particles and crystallines were subjected to further spot analyses. The area of analysis for the slip is around 25 × 50 μm, whereas the area of analysis for the ceramic body is around 200 × 300 μm at × 50 magnification. It is worth noting that although a larger area of analysis at low magnification was used to examine the ceramic body, the resultant composition represents an estimate value. That being said, the SEM-EDS data, when interpreted with reference to the petrographic observation, are sufficient to highlight the variation in the composition of the ceramic body. The data presented below represent an average of three to five analyses. Even though three to five analyses might not have reflected the full extent of compositional variability that exists in the glaze, slip and ceramic body, the number of measurements taken in this study is consistent with the analytical protocol used by other researchers in glaze analysis (e.g. Matin et al. 2018; Molera et al. 2018; Salinas and Pradell 2018; Salinas et al. 2019; Tite et al. 2015). The measurements were converted to oxides by stoichiometry and normalized to 100 wt% to account for the fluctuations in beam intensity and sample porosity. The oxides with concentration lower than 0.1 wt% are not reported here as they are below the limits of detection of the instrument. Corning Glass B and C were analysed as the reference materials at the beginning of each analytical session to evaluate the accuracy and precision of the measurements (Table 7). A cobalt standard was analysed at regular intervals to monitor the beam current stability.
Results
Ceramic body
Petrographically, all samples have a pale-coloured fabric (7.5YR 7/4 pink), characterized by similar mineralogical composition, including quartz, plagioclase feldspar, biotite, amphibole, volcanic rock fragments of basaltic composition and iron-rich nodule. Slight variation exists in the relative abundance and grain size of the inclusions. The inclusions are coarser-grained and less frequent in CG03, CG06, CG07, CG08, CG11, CG15, CG16 and CG20. These samples are characterized by around 20% quartz, 10% iron-rich nodule and 5% or less biotite, plagioclase feldspar, amphibole and volcanic rock fragments (Fig. 3a). The inclusions range from 0.08 mm to 0.80 mm in size, with a mode size of 0.32 mm. The remaining samples are characterized by around 10% quartz and iron-rich nodule, 5 to 10% biotite and less than 5% plagioclase feldspar, amphibole and volcanic rock fragments (Fig. 3b). The inclusions in these samples are finer-grained, which measure between 0.08 mm and 0.40 mm, with a mode size of 0.16 mm. SEM-EDS analysis reveals that all samples have a noncalcareous ceramic body, with 0.4 to 1.9 wt% CaO (Table 2). The ceramic body is further characterized by high Al2O3 and Fe2O3 concentration, which measures between 25.7 and 29.0 wt% and 3.8 and 5.8 wt%, respectively.
Slip
Macroscopically, the presence of slip is recorded in all samples. The slip layer is particularly obvious in the area below the rim of the exterior surface of the bowl, which is unglazed in most cases (Table 1). The slip appears to be white or greyish-white in colour, as confirmed by the optical microscopy (Fig. 4a to c). SEM-EDS analysis shows that the slip layers have different texture from their associated ceramic bodies, which is highlighted in the unglazed slip on the exterior surface of CG15 (Fig. 4d). The examination of this sample shows that the slip is 60 μm thick, characterized by the presence of quartz inclusions that are homogeneous in size (around 10 μm) and clay. The composition of this slip has higher Al2O3 and lower CaO and Fe2O3 concentration than its associated ceramic body.
Photomicrographs showing the presence of a white slip layer, as indicated by black arrow, in (a) the green glaze of CG09, (b) the yellow glaze of CG04 and (c) the brown glaze of CG19 by optical microscopy and backscattered electron image of the unglazed slip on the exterior surface of CG15 by SEM-EDS. All special features are indicated by yellow arrows and associated description
These textural and compositional characterizations also apply to the slip of the remaining samples. All slip layers measure between 55 μm and 90 μm in thickness, which are identifiable for the presence of fine-grained quartz inclusions (around 10 μm to 20 μm). The slip has between 23.8 and 33.5 wt% Al2O3, 0.2 and 1.6 wt% CaO and 1.2 and 3.7 wt% Fe2O3 concentration (Table 3). That being said, a major difference is noted in the slip composition between CG15 and the remaining samples, as expressed in the PbO concentration. The slip of CG15 has no PbO concentration or one that is below the limit of detection, whereas the slip of the remaining samples is rich in PbO concentration, measuring between 14.2 and 23.0 wt%. It is argued that lead oxide was sometimes added to the slip to strengthen its adhesiveness to both ceramic body and glaze (Molera et al. 2020); but if this was the case, high PbO concentration should have been detected in both glazed and unglazed surfaces of the bowl, and it was unlikely that a separate slip was prepared just to cover the area below the rim of the exterior surface. It is equally unlikely that such high PbO concentration was achieved through reacting with the glaze alone. Thus, at present, we do not have any plausible explanation to account for the apparent variation in the PbO concentration of the slip between the glazed and unglazed surfaces.
Nonetheless, by comparing these values with the composition of the associated ceramic body and plotting them in a series of biplots, a weak but positive correlation is displayed between the two, suggesting that kaolinitic clays from similar sources were used to make both slip and ceramic body (Fig. 5a and b). It is worth highlighting that the Al2O3: SiO2 ratio changes from around 0.45 in the ceramic body to 0.65 in the slip, both deviating slightly from the ratio of kaolinite clay minerals, which is around 0.85. Such changes in Al2O3: SiO2 ratio might have reflected how the slip was prepared, in which the finest clay fraction was collected from the water surface after allowing a clay-water mixture to settle. In this way, most Fe2O3 and TiO2, which might have also occurred in colloidal size, were collected in the fine clay fraction. This fine clay was then mixed with some quartz inclusions to form the slip.
Glaze
Microstructure
Glaze is found to be present only on the interior surface of all samples, except CG01, CG03, CG09 and CG13 (Table 1). In these four samples, glaze can also be seen on the exterior surface but limited to the area below the rim, suggesting that the glaze was unlikely applied to this surface on purpose. Thus, the following discussion focuses on the glaze on the interior surface, which was intended for decoration. Also noteworthy is that a distinction is made between the particles and crystallites that are present in the glaze. Particles refer to the undissolved materials used to make the glaze and are identifiable for retaining more or less their original shapes, whereas crystallites are formed during firing.
Most glazes display signs of corrosion and little interaction with the underlying slip. The thickness of the glaze varies, ranging from 20 μm to 140 μm. Some green and honey glazes have a few particles. These particles are mostly undissolved quartz grains, which range from 30 μm to 100 μm, as seen in the green glaze of CG03, CG13 and CG14. The green glaze of CG17 stands out for the presence of bright microcrystallites that scatter throughout the glaze. These microcrystallites, which are less than 5 μm in size, are also present in the yellow glaze (Fig. 6a). The two white glaze samples have different microstructure. The glaze of CG14 has undissolved quartz grains that are up to 50 μm in size (Fig. 6b), whereas CG15 has bright microcrystallites dispersing throughout the glaze (Fig. 6c). Dark crystallites and particles are identified in the brown glazes, but these dark crystallites and particles have slightly different characteristics. The dark crystallites in CG04 are uniform in size (around 10 μm) and occur in cluster (Fig. 7a), and the dark particles in CG10 are coarser-grained (40 μm and 60 μm) (Fig. 7b). The dark crystallites and particles in CG04 and CG10 are found in association with quartz and bright equant crystallites that are 10 μm and 20 μm in size. The dark particles of CG12, CG15 and CG19 are coarser-grained and more angular, measuring between 40 μm and 100 μm (Fig. 7c to e). These dark crystallites are found in the glaze with bright microcrystallites, similar to those that are present in the yellow glaze.
Backscattered electron images showing the presence of (a) Pb-Sn microparticles scattering throughout the yellow glaze of CG11; (b) transparent glaze with undissolved quartz grains over slip of the white glaze of CG14; and (c) Pb-Sn-rich microcrystallites scattering throughout the white glaze of CG15. All special features are indicated by yellow arrows and associated description
Backscattered electron images showing the presence of (a) Mn-rich crystallites and equant, well-formed Pb-Mn-Si-rich crystallites in the brown glaze over a transparent glaze of CG04; (b) Mn-rich particle and well-formed Pb-Mn-Si-rich crystallites in the brown glaze of CG10; and angular and coarser-grained Mn-rich particles and Pb-Sn-rich microcrystallites in the brown glaze of (c) CG12, (d) CG15 and (e) CG19. All special features are indicated by yellow arrows and associated description
With the intention of preserving the integrity of the decoration of the sherds in mind, we did not manage to sample the areas where different coloured glazes overlap, which appear to have been mostly along the border of the decorated patterns and lines at the centre of each sample as shown in Fig. 1. Owing to this possible bias caused by our sampling strategy, a single glaze layer is identified in all but two (CG04 and CG08) samples. Double glaze layers are recorded in CG04, which is marked by the presence of cluster of dark crystallites and bright equant crystallites in the brown glaze and bright microcrystallites of the yellow glaze on top of a glaze layer with no particle or crystallite. Similar observation is seen in CG08, in which the bright microcrystallites of the yellow glaze overlie a glaze layer with no particle or crystallite.
Composition
The glaze of most samples is classified as the high lead type, with the PbO concentration measuring between 60.8 and 74.9 wt% (Table 4). The PbO concentration is lower in the green glaze of CG03, CG06, CG12 and CG14, honey glaze of CG03 and CG16 and white glaze of CG14, which ranges from 38.9 to 57.6 wt%. It seems that some kind of correlation exists between the PbO concentration and glaze colours, with the high lead type being linked to the yellow and brown glaze and the lower lead type linked to the green and honey glaze. This correlation will be further explored below. In all cases, the glaze has low alkali value, with the sum of Na2O, MgO and K2O concentration generally less than 1.0 wt%. The Al2O3 and CaO concentration varies, ranging from 0.6 to 5.9 wt% and 0.1 to 3.5 wt%, respectively. An exception is the glaze of CG03, which has higher alkali (> 2.0 wt%) concentration. It has 5.6 to 8.8 wt% Al2O3 and 4.2 to 4.9 wt% CaO, which is also higher than other samples.
Copper alloys were added to make the green glaze, characterized by the presence of 0.9 to 3.9 wt% CuO and less than 1.0 wt% ZnO and SnO2. Higher SnO2 concentration is recorded in the green glaze of CG04 and CG17. The honey colour was achieved through the addition of iron oxide, resulting in an elevated Fe2O3 concentration that measures between 0.6 and 4.0 wt%. Lead-tin oxide was used to make the yellow glaze, confirmed by the presence of bright microcrystallites that are rich in PbO and SnO2 concentration. A comparison of the composition of the yellow glaze matrix (i.e. area clear of any particles and crystallites) and larger area with particles and crystallites shows that systematic variation exists between the two. The composition of the larger area of all yellow glazes tends to have higher SnO2 concentration than the matrix, which attributes to the limited solubility of tin oxides in the glaze and the scattered distribution of the pigment particles (Heck et al. 2003: 39). The identification of the use of lead-tin oxide as colourant may also explain why the yellow glaze tends to have higher PbO concentration because high lead contents (ca. > 60 wt% PbO) would have prevented the dissolution and possible recrystallization of tin oxide crystallites.
The white glaze has different composition. The composition of CG14 resembles a transparent glaze. The white glaze of CG15 is probably due to the use of lead-tin pigment, as evident in the presence of the bright microcrystallites and higher SnO2 concentration in the glaze bulk. Whereas the transformation of the lead-tin pigment from yellow to white is often facilitated through the addition of alkali (Matin et al. 2018), such transformation could still occur if the pigment was fired at higher temperature without adding alkali (Molera et al. 1999; Tite et al. 2008). The brown glaze has 0.6 to 3.6 wt% MnO in the matrix and 1.8 to 4.9 wt% MnO in the larger area, which is related to the presence of the dark crystallites and particles with high MnO concentration (Table 5). The well-formed, equant crystallites that are found together with the manganese-rich crystallites and particles in CG04 and CG10 have high PbO, MnO and SiO2 concentration, resembling the composition of kentrolite (Pb2Mn2Si2O9). The bright microcrystallites in CG12, CG15 and CG19 are rich in PbO and SnO2. In the case of CG10, copper oxide is also measured in the brown glaze, suggesting that more than one type of colourants might have been used at the same time. For the samples with double glazes, the glaze that underlies the coloured glaze only contains traces of oxides used for colourants, indicating that the glaze was likely transparent.
Discussion
Reconstructing the technology of the Coptic Glazed Ware
Based on the results of the petrographic and SEM-EDS analysis, the CGW technology will be discussed in terms of the procurement of raw materials for the ceramic body, slip and glaze, the methods used to prepare these raw materials and the assembling sequence of the final product (e.g. firing frequency and method and order of glaze application). The mineralogy of the CGW ceramic body displays striking similarity with the composition of the so-called Egyptian pink clay. A recent petrographic analysis on the pink clay pottery from Syene and Elephantine shows that the ‘pink clay’ represents a series of fabrics, all of which were made of kaolinitic clay deposits but with varying amount of quartz, reddish brown shale fragment, feldspar, amphibole, and volcanic rock fragments (Katzjäger et al. 2016: 732, Fig. 1; Ownby et al. 2016: 5–6). Thus, the slight variation seen in the relative abundance of inclusion types may simply reflect the natural variation in the clay sources, and a single recipe was used to make the CGW ceramic body. Deposits of kaolin clays are present in Carboniferous Ataqa sands and Nubia sandstone on both sides of the central-upper region of the Gulf of Suez, as well as the Nubia sandstone at Aswan (Katzjäger et al. 2016; Tobia and Sayre 1974: 6). The co-existence of iron-rich nodules and volcanic rock fragments in the CGW samples suggests that the clays were likely procured from area close to the banks of the Nile in the Aswan region. The chemical composition of the samples has high alumina and low lime, which is characteristic of the kaolinitic clay from the Aswan region (Matin et al. 2018: 48, Table 5; Salina et al. 2019; Waksman et al. 2017). By combining these two lines of evidence, an Egyptian origin, specifically the Aswan region, is postulated for CGW under studied.
Once the ceramic body was form, it was covered by a thin layer of slip. Despite having higher alumina and low lime and iron oxide, the slip has more or less similar composition to the ceramic body. The lower lime and iron oxide in the slip were probably caused by the removal of iron-rich nodules and other coarse-grained inclusions from the clay. The refined kaolin clay was then mixed with quartz grains that are homogeneous in size and shape to form a single, standardized recipe for the slip. All slipped vessels were fired before the glaze application, resulting in the development of a thin interaction layer between the glaze and slip.
As for glaze making, lead oxide and silica were the two main types of raw materials. The use of a lead-silica mixture rather than the direct application of lead oxide on the ceramic body is confirmed by the lack of discernible correlation between the glaze and slip by comparing the composition between the two. For this purpose, the glaze composition is renormalized to 100 wt% after removing PbO and the oxides that were used for colourants (CuO, ZnO and SnO2). Although iron oxide was used to produce the honey glaze, Fe2O3 is not removed in this case as it may have incorporated into the glaze through other raw materials. By plotting the renormalized glaze composition, a positive correlation exists between Al2O3 and CaO and between MgO and K2O among the samples (Fig. 8), even though the yellow and brown glaze samples tend to deviate for their higher alumina as a result of how the lead-tin pigment was formed (see below). Thus, the glaze was likely derived from a common source.
Furthering on the renormalized glaze composition, its CaO concentration measures between 0.4 and 8.7 wt% with a mean of 2.5 wt%. This value is higher than the renormalized CaO concentration of the slip, which averages 0.5 wt%. The renormalized glaze is further marked by relatively high but varying amount of alumina and iron oxide. It is likely that these oxides were incorporated into the glaze through the silica source, which was one of the two major constituents of the glaze mix. A low lime value is distinctive of the composition of the newly refined Early Islamic Egypt IA and IB glass types (Schibille et al. 2019), which are equivalent to the Egypt I glass type (Freestone et al. 2000) and Group 8 and 9 of Umayyad period glass (Foy et al. 2003). In the case of glass production, the association between low lime (with corresponding low strontium oxide) and high alumina and iron oxide is argued to be indicative of using the shell-containing coastal sand from Wadi Natrun and possibly from the Egyptian Delta (Freestone et al. 2000: 72; Phelps et al. 2016: 62). Based on this interpretation, it is probable that the coastal sand from the said sources was also procured to produce the glaze, even though the strontium value of the glaze is not measured owing to the limits of detection of the SEM-EDS. The lead sources are yet to be determined as further isotopic analysis is warranted.
The raw materials used for colourants were also largely available from local sources. The zinc- and tin-containing copper alloys for the green glaze were typical alloy types in the Islamic Middle East (Craddock 1979: 69–73; Ponting 2003: 95–96). The manganese-rich crystallines and particles in the brown glaze point to the possible use of pyrolusite (MnO2), which would have transformed into other phases, such as bixbyite, haussmanite, braunite or kentrolite, after firing; and the determination of these changes in mineral phases will require the use of X-ray diffraction. Pyrolusite occurs in large deposits in Sinai (Freestone 2006), which might have been acquired to produce the brown glaze. The identification of the bright microparticles in the yellow glaze and some examples of brown, green and white glaze, coupled with the overall higher alumina of these glazes, suggests that the lead-tin pigment was a preformed material. The pigment was prepared by heating the lead-tin calx with small amount of silica, which would transform into lead-tin oxide in a lead-silica glass by reacting with the crucible fabric (Heck et al. 2003; Peake and Freestone 2014: 18–19). The pigment was then crushed and added to the glaze. Lead-tin pigment was manufactured in various parts of the mediaeval world (see Matin 2018 for overview; Tite et al. 2008), although no such evidence has been recovered in Egypt and the eastern Mediterranean.
Although the same types of raw materials were used for the glaze and colourant, the glaze of the same colour was prepared in different ways. The green glaze was mostly produced by adding the copper alloys as the only source of colourant, but the copper alloys were mixed with the lead-tin pigment in CG17. The presence of the manganese-rich crystallites and particles and well-formed, equant crystallites (possibly kentrolite) in CG04 and CG10 is similar to the microstructure of the brown glaze of some eleventh-century glazed ceramics from Mallorca (Molera et al. 2013: 88–89, Fig. 2). Such combination of microstructure and microcrystallites is argued to have reflected the use of a roasted manganese oxide-bearing pigment, which was applied on top of the raw glaze and subsequently fired at temperatures that were not too high. The brown glaze of CG12, CG15 and CG19, on the other hand, was formed by mixing larger chunks of the manganese-rich crystallites with lead-tin pigment. The white glaze was achieved by either applying the transparent glaze over white slip or firing the lead-tin pigment at higher temperatures. Variation is also noted in how to decorate the vessels with glaze. Although the majority of samples were only covered with a single layer of coloured glazes, a few samples were adorned with a transparent glaze followed by decoration by coloured glazes.
The making of an early Islamic glaze technology
Our findings correspond with the recent observation made by Salinas et al. (2019) and confirm that Egypt was the original production centre of CGW, a hypothesis that was initially proposed by Rodziewicz (1976). The next step is to investigate whether or not CGW shares similar technological traits with the local fine ware traditions, as represented by Egyptian red and white slip wares and Coptic painted ware. The potential technological connections between CGW and Byzantine and Sasanian glaze traditions – which are the glazing techniques that prevailed in the eastern Mediterranean around the time of the appearance of CGW – are also considered. Previous characterizations of these ceramic traditions are reorganized in the same sequence of production (Table 6).
The raw materials and preparation method of the CGW ceramic body are consistent with the pink clay tradition characteristic of the pottery production originated in Aswan. The pink clay fabrics are said to have been extensively used to make pottery during late antiquity, especially tablewares of Egyptian red and white slip wares (Katzjäger et al. 2016: 731). What makes CGW stand out is that the ceramic body was covered with a white slip, which does not seem to have been identified in the examples of preceding and contemporaneous glaze wares. The use of slip is known to have been a common practice in the production of Egyptian red and white slip wares and Coptic painted ware, which were the derivatives of Late Roman fine ware. Although parallel evidence of the raw materials and preparation method used in the production of these slips is not available, the high level of standardization seen in the composition and texture of the slip of CGW samples points to sophistication in slip production, reflecting a high level of technical knowledge and skills possessed by the potters. Thus, it is logical to assume that the use of slip for CGW was developed out of these earlier slipped and painted wares.
The antecedent of glazing does not seem to have existed in the local fine ware technological repertoires, but the use of lead glaze exhibits stronger influence from the Byzantine tradition, as opposed to the Sasanian tradition that used alkali glaze (Pace et al. 2008). Although lead glaze was used in the production of CGW, the glaze was prepared in a different way from the Byzantine tradition. The glaze of CGW was formed by mixing lead oxide with silica, whereas previous analyses of some fifth-century Italian glazed wares, sixth-century Serbian glazed wares and seventh- to late eighth/early ninth-century Byzantine Glazed White Ware I showed that lead oxide was applied directly onto the ceramic body (Damjanovic et al. 2014; Vroom 2017: 176–78; Waksman et al. 2007: 130–32; Walton and Tite 2010: 738–39, Table 2). On the other hand, the use of a lead-silica mixture was noted in the production of the Islamic glazed pottery recovered from Saint Symeon dating to the eighth and ninth centuries – which are more or less contemporaneous to CGW – although in this case such practice was associated with a calcareous ceramic body and no underlying slip (Waksman et al. 2007: 132).
The stronger Byzantine influence seen in the CGW glazing technology is probably due to the fact that Egypt was under the Roman and Byzantine control before the Arab conquest and continued to maintain commercial contacts with the Byzantine-controlled territories (notably Cyprus and southern Anatolia) after the conquest (see Brubaker and Haldon 2011: 493–506, 510–11; Haldon 2012; Zavagno 2017: 161–66; for other ceramic evidence for commercial contacts between early Islamic Egypt and Palestine and parts of the Byzantine Empire, see Taxel and Cohen-Weinberger 2019). The Byzantine (Constantinopolitan) Glazed White Ware I – especially its later forms, which include open tablewares dating to the eighth century – overlaps with the appearance of CGW. According to the published evidence, this ware was distributed primarily in Asia Minor, the Aegean and Cyprus, with a negligible representation in north Africa (e.g. Carthage) and apparently none in the southeast Mediterranean (see Vroom 2017: 178, Fig. 13.1). Interestingly though, according to Reynolds (2016: 145), Glazed White Ware also occur at Alexandria, demonstrating links between Byzantium and Umayyad Egypt; yet, he did not provide a clear reference to this find. In any rate, the apparent inspiration of Glazed White Ware I on Egyptian potters could have occurred either in Egypt itself, in the form of occasional imports or gifts arrived from Byzantine lands, or overseas when Egyptian merchants and craftsmen became acquainted with this ware while trading with Cyprus or southern Anatolia.
The potters of CGW had also revolutionized the production of lead glaze by introducing new glaze colours and new decorative technique. In addition to using the common colourants such as copper and iron oxide, new colourants were used to create a wider range of glaze colours. Manganese oxide and lead-tin pigment – which had been used as colourant and decolourant in glass production – are recorded, for the first time, to produce brown, yellow and white glazes. Accompanying the appearance of new glaze colours was the introduction of new decorative technique that was painting. Previously, whether it was the Roman, Byzantine or Sasanian glazed wares, monochrome or bichrome glazes were applied to cover the surface of the ceramic body, which was incised or adorned with applique designs in some cases. Multihued painting, on the other hand, had been used by the potters in Egypt to decorate the Coptic painted ware (Schrunk 2003: 89). It seems that the potters had drawn from a decorative technique that they were familiar with but experimented with different medium by using glaze rather than clay-based paint or slip. This exploratory phase is seen in the diversity of practices used by the potters to colour and apply the glazes, which mark a stark contrast to the standardized approach in preparing the recipes for the ceramic body, slip and even the base glaze.
By examining the elements of CGW individually, it is evident that they were mostly derived from the local unglazed fine ware and Byzantine glazing traditions; but, a combination of these elements has made the technologies used to make CGW truly innovative. This then raises the question of what caused this change in ceramic technology. Although the appearance of CGW dated to the mid-eighth and ninth centuries, we argue that the mechanisms that fuelled this change seem to be in place earlier than this time. Egypt was brought under the Arab control after the AD 640–641 conquest. This, together with the Arab conquest of the rest of the Middle East and north Africa, had effectively disrupted the circulation of Late Roman fine ware in the eastern Mediterranean. This had resulted in a shift in the fine ware ceramic patterns from the domination of imports to the intensification of local production (Fischer 2011: 186–91; Konstantinidou 2015; Morony 1995: 10–13).
A rich tradition of fine ware production was thus nurtured in Egypt (Watson 2014: 127–28). Aswan, in particular, is described to have been the largest fine ware producer, with at least four production sites co-existing in the region (Ballet et al.1991). Evidence indicating the presence of division of labour was also reported, in which some producers specialized in the production of certain types of fine ware (Schrunk 2003: 87). The picture that emerged from the Aswan region in the seventh and eighth centuries was a concentration of potters with a high level of craftsmanship entrenched in a specialized structure of craft organization (McNally and Schrunk 2000: 91). This specialized craft structure might have also encouraged interaction and knowledge exchange between artisans from different crafts. Of particular interest is the inter-craft connection between the potters and glassmakers, a hypothesis that was put forth by Whitcomb (1989: 175). In fact, in their study of the Aswan Pink Clay pottery, Katzjäger et al. (2016: 734) pointed out that some locally made white-slipped small bowls displayed close formal and stylistic affinities to glass vessels. It is highly possible that the CGW potters acquired the knowledge of how to colour glaze from the glassmakers, resulting in the unprecedented use of manganese oxide and lead-tin pigment, although we lack the textural, archaeological and technological evidence to prove the existence of such interaction. In all, these ‘internal’ factors serve to pave the way for the change in ceramic technology to take place.
On the other hand, the Arab control, mainly the Umayyad government, seems to have had little impact on many aspects of the economic, social and cultural life in Egypt and other areas that were under its control, at least in the decades immediately followed the conquest (Sijpesteijn 2007: 444). By the late seventh and early eighth centuries, two major developments were noted. The first one was a series of reforms implemented by the Caliph ‘Abd al-Malik (r. 685–705) and his successors, which had resulted in the creation of a unified monetary system, the building of new roads and the founding of new cities and new urban market places (Robinson 2005: 72–80; Walmsley 2000: 270–71). The second development was the construction of the Dome of the Rock in Jerusalem, which also took place during the reign of the Caliph ‘Abd al-Malik (Milwright 2010a: 25; 2010b 665). The impact of the first development was considerable growth in local and regional production and trade, whereas the second development demonstrated the desire by the Arab rulers to establish new representations of their identities. In Egypt, parallel developments were recorded (Kennedy 1998: 71–73). The Arab governors are said to have become increasingly efficient in collecting tax, commissioned new infrastructure such as mosque and palace in Fustat and improved the irrigation systems. All these had the effect of stimulating the local economies and leading to a surge in the urban elites, who were the ones with the purchasing powers and the demands for new material representations. These advances in the social, political and economic systems might have acted as the catalyst that set the change in ceramic technology in motion. In this way, our observation is consistent with the other lines of evidence, for example, recent studies on the well-dated Egyptian glass weights showed that the changes in glass composition and associated technology only occurred in the eighth century (Schibille et al. 2019: 17).
Conclusion
Our analysis corresponds with the results of the previous characterisations, as well as generates some new observations, which together contribute to a better understanding of the CGW technology. The ceramic body and slip was made of the kaolin clay deposits, possibly from the Aswan region. The clay for the slip was refined by removing the iron-rich pellets and other coarser-grained inclusions. A lead-silica mixture was used to form the glaze, which was coloured by copper alloys, iron oxide, lead-tin pigment and manganese pellet. The multihued glazes were then painted onto the surface of the slipped ceramic body, which was biscuit-fired. The high level of standardization in the recipes for the ceramic body, slip and base glaze suggests that the potters who were involved in the production of the CGW possessed a high level of craftsmanship. The diversity seen in coloured glaze composition and microstructure can be interpreted as the potters experimenting with glaze colouring and using glaze as a medium to decorate.
Based on these characterisations, we postulate that the local Egyptian fine ware and possibly glass technologies and the Byzantine glaze technology were the precursors of the CGW technology. But, it was not until the production of CGW that all these existing elements were combined for the first time. This finding has not only highlighted the innovative character of the CGW technology but also revealed that the change in ceramic technology was a gradual process rather than an abrupt invention. This new technology was born out of a strong local fine ware tradition that was embedded in the landscape of high level of specialization in craft production and stimulated by the desire to establish new identities and new material representations by the Arab-Muslim newcomers. This finding corresponds with other lines of evidence that suggests a slow and gradual transformation during the early Islamic period (e.g. Avni 2014; Foss 1997; Milwright 2010a, b; Walmsley 2007).
Notes
The Tel Aviv University excavations in Jaffa were directed by Alexander Fantalkin in 2000–2001. The final report is in preparation by Fantalkin and Taxel.
The IAA excavations in Yavneh were directed by Eli Yannai in 2010–2012. The final report is in preparation by Yannai and Taxel.
The Tel Aviv University excavations in Yavneh-Yam were directed by Moshe Fischer in 1992–1999 and by Fischer and Taxel in 2005–2011. The final report is in preparation by Fischer and Taxel.
The IAA excavations in Khirbat ‘Amra were directed by Gil Tahal in 1993–1994. The final report is in preparation by Noé David Michael and Taxel.
References
Adlington LW (2017) The corning archaeological reference glasses: new values for ‘old’ compositions. Papers Institute Archaeol 27:1–8. https://doi.org/10.5334/pia-515
Armstrong P, Hatcher H, Tite M (1997) Changes in byzantine glazing technology from the ninth to thirteenth centuries. In: d’Archimbaud GD (ed) La Céramique Médievale en Méditerranée, Actes du 6e Congrès. Narration Éditions, Aix-en-Provence, pp 225–229
Avni G (2014) The Byzantine-Islamic transition in Palestine: an archaeological approach. Oxford University Press, Oxford
Ballet P, Mahmoud F, Vichy M, Picon M (1991) Artisanat de la céramique dans l’Égypte romaine tardive et byzantine: prospections d’ateliers de poteiers de Minia à Assuan. Cah Céramique Égyptienne 2:129–143
Brukbaker L, Haldon JF (2011) Byzantium in the iconoclast era c. 680-850: a history. Cambridge University press, Cambridge
Craddock PT (1979) The copper alloys of the medieval Islamic world – inheritors of the classical tradition. World Archaeol 11:68–79. https//doi.org/https://doi.org/10.1080/00438243.1979.9979750
Damjanovic L, Bikic V, Saric K, Eric S, Holclajtner-Antunovic I (2014) Characterisation of the early Byzantine pottery from Varivin Grad (South Serbia) in terms of composition and firitng temperature. J Archaeol Sci 46:156–172. https://doi.org/10.1016/j.jas.2014.02.031
Fischer G (2011) Between empires: Arabs, Romans and Sasanians in late antiquity. Cambridge University Press, Cambridge
Foy D, Picon M, Vichy M (2003) Verres Omeyyades et Abbasides d’origine Egyptienne: les temoignages de l’archéologie et de l’archéometrie. In: Annales du 15e Congrès de l’Association Internationale pour l’Histoire du Verre, vol 15. AIHV, New York and Corning, pp 138–143
Foss C (1997) Syria in transition, a.D. 550–750: an archaeological approach. Dumbarton Oaks Papers 51:189–269. https://doi.org/10.2307/1291765
Freestone IC (2006) Glass production in late antiquity and the early Islamic period: a geochemical perspective. In: Magetti M, Messiga B (eds) Geomaterials in cultural heritage, geological society special publications 257. Geological Society, London, pp 201–216
Freestone IC, Goren-Rosen Y, Hughes MJ (2000) Primary glass from Israel and the production of glass in late antiquity and the early Islamic period. In: Nenna M-D (ed) La route du verre: ateliers primaires et secondaires du second millenaire Av. J.-C. au moyen age. Masion de l’Orient Méditerranéen, Lyon, pp 65–83
Freestone IC, Politis KD, Stapleton CP (2001) The byzantine glazed pottery from Deir ‘Ain ‘Abata, Jordan. In: Villeneuve E, Watson PM (eds) Le céramique Byzantine et proto-Islamique en Syrie-Jordanie (IVe – VIIIe siècles apr. J.-C.), Actes du Colloque tenu à Amman les 3, 4 et 5 décembre, vol 1994. Bibliothèque archéologique et historique, Beirut, pp 197–205
Grossman P, Brooks Hedstrom DL, Osman AMM, Noeske H-C, Al-Rahim AA, Al-Fatah TSA, Al-Mugdi MA, Wolfgang J (2009) Second report on the excavation in the monastery of Apa Shenute (Dayr Anba Shinuda) at Suhag. Dumbarton Oaks Papers 63:167–219
Haldon JF (2012) Commerce and exchange in the seventh and eighth centuries: regional trade and the movement of goods. In: Morrisson C (ed) Trade and markets in Byzantium. Dumbarton Oaks Research Library and Collection, Washington DC, pp 99–122
Heck M, Rehren T, Hoffmann P (2003) The production lead-tin yellow at Merovingian Schleitheim (Switzerland). Archaeometry 45:33–44. https://doi.org/10.1111/1475-4754.00095
Katzjäger D, Peloschek L, Rembart L (2016) The multiplicity of Aswan pink clay pottery (Roman times to late antiquity): synchronising shape repertoire, clay pastes and firing properties. REI Certeraiæ Romanæ Favtorvm Acta 44:731–736
Kennedy H (1998) Egypt as a province in the Islamic caliphate, 641-868. In: Petry CF (ed) The Cambridge history of Egypt, vol 1. Cambridge University Press, Cambridge, pp 62–85
Kohn-Tavor A (2017) Excavations at Ramla (white mosque street): ceramic finds. NGSBA Archaeology 4:23–129
Konstantinidou A (2015) The monasteries of the Wadi-al-Natrun between Alexandria and Fustat: a long transitional period viewed from the pottery (sixth to tenth centuries). In: Hoyland R (ed) The late antique world of early Islam: Muslims among Christians and Jews in the East Mediterranean. The Darwin Press Inc., Princeton, pp 231–257
Mason RB (1997) Medieval Egyptian lustre-painted and associated wares: typology in a multidisciplinary study. J Am Res Centre Egypt 34:201–242
Mason RB (2004) Shine like the sun: lustre-painted and associated pottery from the medieval Middle East. Mazda Publishers, Costa Mesa
Mason RB, Keall EJ (1990) Petrography of Islamic pottery from Fustat. J Am Res Centre Egypt 27:165–184
Mason RB, Tite MS (1997) The beginnings of tin-opacification of pottery glazes. Archaeometry 39:41–58. https://doi.org/10.1111/j.1475-4754.1997.tb00789.x
Matin M (2018) Tin-based opacifiers in archaeological glass and ceramic glazes: a review and new perspectives. Archaeol Anthropol Sci 11:1155–1167. https://doi.org/10.1007/s12520-018-0735-2
Matin M, Tite M, Watson O (2018) On the origins of tin-opacified ceramics glazes: new evidence from early Islamic Egypt, the Levant, Mesopotamia, Iran, and Central Asia. J Archaeol Sci 97:42–66. https://doi.org/10.1016/j.jas.2018.06.011
Matthews AJ, Woods AJ, Oliver C (1991) Spots before the eyes: new comparison charts for visual percentage estimation in archaeological material. In: Middleton A, Freestone I (eds) Recent developments in ceramic. British Museum Research Laboratory, London, pp 211–264
McNally S, Schrunk I (2000) The impact of Rome on the Egyptian pottery industry. J Am Res Centre Egypt 37:91–114
Milwright M (2010a) An introduction to Islamic archaeology. Edinburgh University Press, Edinburgh
Milwright M (2010b) Archaeology and material culture. In: Robinson C (ed) Islam, the formation of the Islamic world, sixth to eleventh centuries. Cambridge University Press, Cambridge, pp 664–682
Molera J, Carvajal López JC, Molina G, Pradell T (2018) Glazes, colourants and decorations in early Islamic glazed ceramics from the Vega of Granada (9th to 12th centuries CE). J Archaeol Sci reps 21:1141–1151. https://doi.org/10.1016/j.jasrep.2017.05.017
Molera J, Coll J, Labrador A, Pradell T (2013) Manganese brown decorations in the 10th to 18th century Spanish tin glazed ceramics. Appl Clay Sci 82:86–90. https://doi.org/10.1016/j.clay.2013.05.018
Molera J, Pradell T, Salvadó N, Vendrell-Saz M (1999) Evidence of tin oxide recrystallisation in opacified lead glazes. J Am Ceram Soc 82:2871–2875. https://doi.org/10.1111/j.1151-2916.1999.tb02170.x
Molera J, Martinez Ferreras V, Fusaro A, Gurt Esparraguera JM, Gaudenzi Asinelli M, Pidaev SR, Pradell T (2020) Islamic glazed wares from ancient Termez (southern Uzbekistan): raw materials and techniques. J Archaeol Sci Rep 29. https://doi.org/10.1016/j.jasrep.2019.102169
Morony M (1995) Material culture and urban identities: the evidence of pottery from the early Islamic period. In: Bierman I (ed) Identity and material culture in the early Islamic world. Centre for Near East Studies. University of California Los Angeles, Los Angeles, pp 2–46
Ownby MF, Giomi E, Williams G (2016) Glazed ware from here and there: petrographic analysis of the technological transfer of glazing knowledge. J Archaeol Sci reps 16:616–626. https://doi.org/10.1016/j.jasrep.2016.04.019
Pace M, Bianoc Prevot A, Mirti P, Venco Ricciardi R (2008) The technology of production of Sasanian glazed pottery from Veh Ardashir (Central Iraq). Archaeometry 50:591–605. https://doi.org/10.1111/j.1474-4754.2007.00369.x
Peake JRN, Freestone IC (2014) Opaque yellow glass production in the early medieval period: new evidence. In: Keller D, Price J, Jackson C (eds) Neighbours and successors of Rome: tradition of glass production and use in Europe and the Middle East in the later 1st millennium AD. Oxbow Books, Oxford, pp 15–21
Pelphs M, Freestone IC, Gorin-Rosen Y, Gratuze B (2016) Natron glass production and supply in the late antique and early medieval near east: the effect of the Byzantine-Islamic transition. J Archaeol Sci 75:57–71. https://doi.org/10.1016/j.jas.2016.08.006
Ponting MJ (2003) From Damascus to Denia: scientific analysis of three groups of Fatimid period metalwork. Hist Metall 37:85–105
Reynolds P (2016) From Vandal Africa to Arab Ifrīqiya: tracing ceramic and economic trends through the fifth to the eleventh centuries. In: Stevens ST, Conant JP (eds) North Africa under Byzantium and early Islam. Dumbarton Oaks Research Library and Collection, Washington DC, pp 129–171
Robinson Ch F (2005) Abd al-Malik. Oneworld, Oxford
Rodziewicz M (1976) Alexandrie I: La céramique Romaine tardive d’Alexandrie. Editions Scientifiques de Pologne, Warsaw
Salinas E, Pradell T (2018) The transition from lead transparent to tin-opacified glaze productions in the western Islamic lands: al-Andalus, c. 875–929 CE. J Archaeol Sci 94:1–11. https://doi.org/10.1016/j.jas.2018.03.010
Salinas E, Pradell T, Matin M, Tite S (2019) From tin- to antimony-based yellow opacifiers in the early Islamic Egyptian glazes: regional influences and ruling dynasties. J. Arch. Sci.: reps 26. https://doi.org/10.1016/j.jasrep.2019.101923
Schibille N, Gradtuze B, Ollivier E, Blondeau É (2019) Chronology of early Islamic glass compositions from Egypt. J Archaeol Sci 104:10–18. https://doi.org/10.1016/j.jas.2019.02.001
Schrunk ID (2003) Spiritual economy and spiritual craft: monastic pottery production and trade. In: Sellew P (ed) living for eternity: the white monastery and its neighbourhood. Proceedings of a symposium at the University of Minnesota, Minneapolis, march 6–9 2003. http://egypt.umn.edu/Egypt/1-pb%20pdfs/steart.pdf
Sijpesteijn PM (2007) The Arab conquest of Egypt and the beginning of Muslim rule. In: Bagnall RS (ed) Egypt in the byzantine world, 300–700. Cambridge University Press, Cambridge, pp 437–459
Tal O, Taxel I (2008) Ramla (south). An early Islamic industrial site and remains of previous periods (salvage excavation reports 5). Emery and Claire Yass publications in archaeology of the Institute of Archaeology, Tel Aviv University, Tel Aviv
Taxel I (2014) Luxury and common wares: socio-economic aspects of the distribution of glazed pottery in early Islamic Palestine. Levant 46:118–139. https://doi.org/10.1179/0075891413Z.00000000036
Taxel I, Cohen-Weinberger A (2019) A newly-identified type of late antique Palestinian amphora: production, evolution and use of the Mediterranean globular amphora. J Mediterr Archaeol 32(1):3–31
Tite M, Pradell T, Shortland A (2008) Discovery, production and use of tin-based opacifiers in glasses, enamels and glazes from the late Iron age onwards: a reassessment. Archaeometry 50:67–84. https://doi.org/10.1111/j.1475-4754.2007.00339.x
Tite MS, Watson O, Pradell T, Matin M, Molina G, Domoney K, Bouquillon A (2015) Revisiting the beginnings of tin-opacified Islamic glazes. J Archaeol Sci 57:80–91. https://doi.org/10.1016/j.jas.2015.02.005
Tobia S, Sayre EV (1974) An analytical comparison of various Egyptian soils, clays, shales and some ancient pottery by neutron activation. In: Bishay A (ed) Recent advances in science and technology of materials III. Plenum Press, New York, pp 99–128
Vroom J (2017) Ceramics. In: Niewohner P (ed) The archaeology of Byzantine Anatolia: from the end of late antiquity until the coming of the Turks. Oxford University Press, Oxford, pp 176–193
Waksman SY, Bouquillon A, Cantin N, Katona I (2007) The first byzantine ‘glazed white wares’ in the early medieval technological context. In: Waksman Y (ed) Archaeometric and archaeological approaches to ceramics: papers presented at EMAC’05, 8th European meeting on ancient ceramics, Lyon 2005. Archaeopress, Oxford, pp 129–135
Waksman SY, Capelli C, Cabella R (2017) Études en laboratoire de céramiques islamiques du Caire: l’aaport des fouilles recentes. In: Gayraud R-P, Vallauri L (ed) Fustat II. Fouilles d’Istl ‘Antar. céramiques d’ensembles de IXe et Xe siècles. Institute Français d’archaeologie Orientale, Cairo, pp 383-414
Walmsley A (2000) Production, exchange and regional trade in the Islamic East Mediterranean: old structures, new systems? In: Hansen IL, Wickham C (eds) The long eighth century. Brill, Leiden, pp 265–344
Walmsley A (2007) Early Islamic Syria: an archaeological assessment. Gerald Duckworth & Co. Ltd., London
Walton MS, Tite MS (2010) Production technology of Roman lead-glazed pottery and its continuance into late antiquity. Archaeometry 52:733–759. https://doi.org/10.1111/j.1476-4754.2009.00506.x
Watson O (2004) Ceramics from Islamic lands. Thames and Hudson, London
Watson O (2014) Revisiting Samarra: the rise of Islamic glazed pottery. In: Gonnella J, Abdellatif R, Struth S (ed) Beiträge zur Islamischen Kunst und Archäologie 4:125–44
Watson O (2017) Ceramics and circulation. In: Flood FB, Necipoğlu G (eds) A companion to Islamic art and architecture. Wiley, Hoboken, pp 478–500
Whitcomb D (1989) Coptic glazed ceramics from the excavations at Aqaba, Jordan. J Am Res Centre Egypt 26:167–182
Zavagno L (2017) Cyprus between late antiquity and the early middle ages (ca. 600-800). An island in transition. Routledge, London and New York
Acknowledgements
This research was carried out with funding provided by the European Commission Horizon 2020 Marie Skłodowska Curie Actions Individual Fellowship 2016 (Grant agreement no.: 750904; Project acronym: GLAZE). We would like to thank Professor Ian Freestone for this expertise and insightful comments on the interpretation of the data used to reconstruct the CGW glaze technology. We are grateful to the directors of excavation, who have kindly granted us with the permission to sample the materials from their respective project. We are equally grateful to the Israel Antiquities Authority for granting us with the permission to extract and export the materials for further analyses. We would also like to thank Tom Gregory at the UCL Wolfson Archaeological Sciences Laboratories for his technical support, Catherine Kneale at the Pitts-River Laboratory for Archaeological Science, University of Cambridge, for the training on digital microscopy, and Emil Aladjem of the Israel Antiquities Authority for preparing Fig. 2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Appendix 1
Appendix 1
Rights and permissions
About this article
Cite this article
Ting, C., Taxel, I. Indigeneity and innovation of early Islamic glaze technology: the case of the Coptic Glazed Ware. Archaeol Anthropol Sci 12, 27 (2020). https://doi.org/10.1007/s12520-019-01007-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-019-01007-y