Abstract
Background
Antibiotics are widely prescribed among children and pregnant women, but their safety profile is controversial. This study aimed to summarize and appraise current evidence for the potential impact of antibiotic exposure on pregnancy outcomes and children’s health.
Methods
PubMed, Embase, Web of Science and the Cochrane Database of Systematic Reviews were searched from inception to June 2022. Meta-analyses of any study design comparing the impact of antibiotic exposure with nonexposure among children, pregnant women and prepregnant women on adverse health outcomes of children and pregnancy were retrieved. The quality of evidence was assessed by a Measurement Tool to Assess Systematic Reviews 2 (AMSTAR2) and the Grading of Recommendations, Assessment, Development and Evaluation (GRADE). Data were reanalyzed, and the credibility of the evidence was determined.
Results
Out of 2956 studies identified, 19 articles with 39 associations were included. Totally 19 of the associations (48.72%) were statistically significant with a P value ≤ 0.05, while only six were supported by highly suggestive evidence. Children with postnatal antibiotic exposure had a higher risk of developing asthma odds ratio (OR): 1.95, 95% confidence interval (CI): 1.76–2.17, wheezing (OR: 1.81, 95% CI 1.65–1.97) and allergic rhinoconjunctivitis (OR: 1.66, 95% CI 1.51–1.83), with prediction intervals excluding the nulls. Quality assessed by both AMSTAR2 and GRADE of included meta-analyses were very low in general.
Conclusions
Antibiotic exposure in early life was associated with children’s long-term health, especially in cases of allergic diseases. Prenatal exposure might also influence children’s health in some aspects but requires more high-quality evidence. Potential adverse effects of antibiotics on pregnancy outcomes were not observed in our study. Studies with higher quality and better quantification of antibiotic exposure are needed in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antibiotics have greatly improved health outcomes in many aspects, leading to their widespread use [1]. Previous surveys estimated that the overall daily doses of antibiotics consumed have increased by 65% from 2000 to 2015, and their consumption could double in 2030 if no policy changes were made [2, 3]. However, antibiotic exposure is not that safe, considering the rising level of antimicrobial resistance and other potential adverse health outcomes [4, 5].
Appropriate antibiotic use is particularly important in pediatrics. Researches revealed that the antibiotic prescription rate was highest among children under two years old [6], and antibiotic exposure in early life was related to an increased risk of some childhood medical conditions [6, 7]. In addition, children are vulnerable to antibiotic exposure in childhood, and prenatal antibiotic exposure may cause both short-term and long-term effects on pregnancy outcomes and children’s health [8]. Although many previous studies have assessed the safety of antibiotic use among pregnant women and children from different aspects, some of the results are inconsistent [7, 9]. Several meta-analyses and systematic reviews have assessed the safety of antibiotic exposure on pregnancy outcomes and children’s health; however, they usually only cover a single aspect of adverse health outcomes of various systems, and the results are easily biased and lack quantified credibility [10, 11].
Umbrella review summarizes evidences from multiple meta-analyses of the same topic, providing more comprehensive quality assessment and credibility of evidence, which will better inform guidelines and clinical practice [12, 13]. Therefore, we performed this umbrella review to summarize the results from meta-analyses and appraise current evidence for the potential associations of antibiotic exposure and adverse outcomes in children and pregnant women to provide evidence for future policy making.
Methods
Protocol
The umbrella review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA 2020 statement) checklist [14], with the protocol registered on PROSPERO, CRD42022299246.
Literature search, study selection and data extraction
PubMed, Embase, Web of Science and Cochrane Database of Systematic Reviews were searched from inception to June 2022 for eligible meta-analyses of any study design. Medical subject heading (MeSH) terms and keywords were used in the search, including (“antibiotics”) AND (“pregnancy outcome” OR “childhood”) AND (“meta-analysis” or “systematic review”), without language limitations (Supplementary Table 1). References of included studies were also searched manually.
Two authors (YL and LL) independently screened the titles and abstracts and went for full-text review for eligibility. For any discrepancy, discussion with another reviewer (ZJ) was conducted. Studies were initially included if they met the following criteria: (1) population: prepregnant/pregnant women or children under 18 years old; (2) intervention: antibiotics of any regimen administered orally or by injection for any purpose; (3) control: no antibiotics or placebo; (4) outcomes: pregnancy outcomes and adverse health outcomes of children related to antibiotic exposure; and (5) study design: systematic reviews with meta-analyses of either observational studies (cohort, case‒control, nest case–control or cross-sectional studies) or interventional studies [randomized controlled trials (RCTs)]. We excluded (1) systematic reviews without meta-analysis; (2) studies with insufficient data for reanalysis; (3) network meta-analyses or conference abstracts; (4) studies comparing different types of antibiotics or different doses of antibiotics, and (5) studies reporting unspecified adverse events. Where two or more meta-analyses examined the same population and outcome, the most comprehensive one was selected (considering both the number of original studies and their publication years included in the meta-analysis) [10, 15].
Data were extracted by two authors (YL and PL) independently and verified by another two authors (XJ and HL). For each included meta-analysis, we recorded (1) first author, (2) publication year, (3) PICOS (population, intervention, control, outcome and study design) elements, (4) number of included original studies, (5) types of antibiotics used, (6) number of cases/total population, (7) control event rate, (8) follow-up time, (9) effect size [i.e., relative risk (RR), odds ratio (OR) with 95% confidence interval (CI)] and type of pooling model. For each original study included in the meta-analysis, we recorded study design, effect size with 95% CI or number of events and total population in both exposure and control groups if given.
Quality assessment of included studies
The methodological quality for each included meta-analysis was assessed by A Measurement Tool to Assess Systematic Reviews 2 (AMSTAR2), which categorizes evidence into “high”, “moderate”, “low”, or “critically low” [16]. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) working group classification was employed for quality assessment of evidence for each outcome, which categorizes evidence into “high”, “moderate”, “low”, or “very low” [17].
Data analysis
We reanalyzed the pooled effect size and its 95% CIs by the DerSimonian and Laird (DL) random-effect model to ensure the real effect of exposure for each meta-analysis [18]. Since the DL model may overestimate the effect in meta-analyses including a small number of studies, we also calculated the effect size by the modified Hartung–Knapp–Sidik–Jonkman (HKSJ) method in meta-analyses with ≤ 5 original studies [19, 20]. Heterogeneity was assessed using I2 and τ2 statistics. The I2 statistic indicates the proportion of variance observed in the estimated effect reflecting true differences in effect size (I2 > 50% indicates large heterogeneity) [21, 22]. The τ2 statistic quantifies the true variance in pooled estimates [22]. We also calculated the 95% prediction intervals, providing the possible range of effect sizes for an individual study [23]. Small study effects (i.e., studies with smaller sample size show different, often larger, effect size than large ones, which may threaten the validity of the pooled estimate) was assessed by Egger regression asymmetric test. A small study effect was indicated when a P value ≤ 0.10 was observed in Egger’s test, while the effect size of the largest study was also smaller than the pooled estimate in the meta-analysis [10, 24]. Excess significance bias of studies with statistically significant findings was assessed to detect whether the number of studies observed to have statistically significant results (O) differed from the expected number (E). A χ2-based test was conducted, and a P value ≤ 0.10 was considered to indicate excess significance bias [25]. Publication bias was assessed by Egger’s regression asymmetric test, and for associations with Egger’s P < 0.05, trim and fill analysis with a funnel plot was further conducted [26].
Determining the credibility of evidence
We categorized the evidence from these meta-analyses into five classes according to previous umbrella reviews [10, 11, 27,28,29,30]: convincing (class I), highly suggestive (class II), suggestive (class III), weak (class IV), and not significant (NS) (criteria in details are in Supplementary Table 2). An evidence map was created to show the potential risk of antibiotic exposure and the certainty of evidence [31]. For meta-analyses classified as class I-III evidence, we performed sensitivity analysis by including prospective studies only to further assess the robustness and reliability of the results.
Results
Characteristics of the included studies
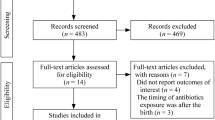
We initially identified 2956 articles after removing duplicates, and 89 of them underwent full-text review. Ultimately, we included 19 studies with 39 groups of exposure population outcomes in this umbrella review (Fig. 1, list of 19 included studies is presented in Supplementary Table 3, and the list of 70 excluded studies is presented in Supplementary Table 4) [7, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. According to the period of antibiotic exposure, the outcomes were presented in two groups: outcomes of prenatal exposure and outcomes of postnatal exposure.
Quality of evidence and methodological quality
Of 39 meta-analyses going for methodological quality assessment by AMSTAR2, 2 (5.13%) meta-analyses were graded as moderate, four (10.26%) as low and the remaining 33 (84.62%) as critically low quality (Supplementary Table 5, details of AMSTAR2 assessment are presented in Supplementary Table 6). The overall methodological quality of the included meta-analyses was unsatisfying mainly for no protocol registration, no list of excluded studies, no report on the sources of funding of individual studies, or no satisfactory explanation for observed heterogeneity.
For the GRADE classification for quality of evidence, eight (20.51%) out of 39 meta-analyses were graded as low quality, while the other 31 (79.49%) were graded as very low quality (Supplementary Table 5, details of GRADE classification are presented in Supplementary Table 7). Poor quality of evidence was mainly due to the mixed study design of the included studies, serious inconsistency and serious imprecision.
The credibility of evidence of included studies
We used OR as an effect metric to reanalyze each meta-analysis, except for one study exploring the toxicity of fluconazole in children, which recruited four RCTs where RR was employed in the estimate [49]. Nineteen out of 39 associations (48.72%) were statistically significant with a P value < 0.05. Of them 24 (61.5%) had a P value < 0.001. Totally 16 out of 39 (41.03%) reported more than 1000 cases, and 28 (71.79%) showed large heterogeneity. A small study effect was noted in eight associations (20.51%), and excess significance bias were detected in two (5.13%). Three associations (7.69%) presented 95% prediction intervals excluding the nulls (Supplementary Table 5). Sensitivity analysis was conducted by including prospective studies only in eight (20.51%) meta-analyses (Supplementary Table 8, one association supported by class III evidence included only one prospective study; thus, sensitivity analysis was not performed). Publication bias was observed in seven (17.95%) associations with funnel plots (Supplementary Table 9). Fourteen associations were re-analyzed by modified HKSJ methods (Supplementary Table 10).
In total, six out of 39 associations (15.38%), namely, the associations between postnatal antibiotic exposure and early life eczema, asthma, allergic rhinoconjunctivitis, atopic dermatitis, food allergy and wheezing, were classified into Class II evidence (highly suggestive), 3 (7.69%) were Class III evidence (suggestive), 10 (25.64%) were Class IV evidence (weak) and the others (51.28%) were not significant (Supplementary Table 5, Fig. 2).
Prenatal antibiotic exposure
We summarized the results of different outcomes of antibiotic exposure during or before pregnancy from 20 meta-analyses embodying 121 original studies (Fig. 3) [32,33,34,35,36,37,38,39,40,41]. Weak evidence has shown that antibiotic use of any regimen during pregnancy may be related to the development of wheezing/asthma (OR: 1.29, 95% CI 1.17–1.42) [32], eczema/atopic dermatitis (OR: 1.60, 95% CI 1.21–2.13) [32] and childhood attention-deficit/hyperactivity disorder (ADHD) (OR: 1.14, 95% CI 1.10–1.19) [34] in offspring in early life. Prenatal antibiotic exposure seemed to be safe in terms of the development of acute lymphoblastic leukemia (ALL) (OR: 1.09, 95% CI 0.97–1.21) [36] and childhood overweight/obesity (OR: 1.08, 95% CI 0.97–1.21) [33].
Summary estimates of meta-analyses of prenatal antibiotic exposure and adverse health outcomes. Annotation: Solid lines present effect sizes with 95% CI of each meta-analysis, and dotted lines present scales of 95% prediction intervals of each analysis. Arrows indicate that the exact scale is larger than the defined scale given in the graph. ○○○ indicates I2 ≤ 25%, ●○○ indicates 25% < I2 ≤ 50%, ●●○ indicates 50% < I2 ≤ 75%, ●●● indicates I2 ≥ 75%. AMSTAR2 a Measurement Tool to Assess Systematic Reviews 2, GRADE the Grading of Recommendations, Assessment, Development and Evaluation, CI confidence interval
Suggestive evidence indicated the possible association between antibiotic exposure during pregnancy and spontaneous abortion (OR: 1.39, 95% CI 1.24–1.55) [35]. However, the association became insignificant after either removing retrospective/cross-sectional studies or trim and fill analysis. In addition, in another analysis focused on quinolone exposure during the first trimester [40], the association of spontaneous abortion (OR: 1.19, 95% CI 0.93–1.52) was insignificant. Prenatal antibiotic exposure seemed to be safe in terms of other pregnancy outcomes.
There are some gynecological diseases that may affect pregnancy outcomes and require antibiotic treatment. In this condition, whether antibiotic treatment has a positive or negative effect on pregnancy outcomes is crucial. Three meta-analyses investigated associations between antibiotic use before pregnancy in patients with chronic endometritis and the implantation rate, intrauterine pregnancy rate and live birth rate later; and none of the differences were significant [41].
Postnatal antibiotic exposure
Nineteen meta-analyses embodying 309 original studies have assessed the potential risk of antibiotic exposure in early life (Fig. 4) [7, 34, 37, 42,43,44,45,46,47,48,49]. Evidences have indicated an association between antibiotic exposure in childhood and the development of allergic diseases [7, 42], including eczema (OR: 1.26, 95% CI 1.15–1.37), allergic rhinoconjunctivitis (OR: 1.66, 95% CI 1.51–1.83), asthma (OR: 1.95, 95% CI 1.76–2.17), atopic dermatitis (OR: 1.40, 95% CI 1.30–1.52), food allergy (OR: 1.35, 95% CI 1.20–1.52) and wheezing (OR: 1.81, 95% CI 1.65–1.97). Suggestive evidence also supported the associations between childhood antibiotic exposure and inflammatory bowel disease (IBD) (OR: 1.50, 95% CI 1.22–1.85) [45], which is also related to the immune system. Weak evidence supported childhood antibiotic exposure and risk of autism spectrum disorder (ASD) (OR: 1.13, 95% CI 1.07–1.21) [43], childhood overweight/obesity (OR: 1.19, 95% CI 1.12–1.25) [44] and hypomineralized second primary molar (HSPM) (OR: 1.47, 95% CI 1.15–1.87) [46].
Summary estimates of meta-analyses of postnatal antibiotic exposure and adverse health outcomes. Annotation: Solid lines present effect sizes with 95% CI of each meta-analysis, and dotted lines present scales of 95% prediction intervals of each analysis. Arrows indicate that the exact scale is larger than the defined scale given in the graph. ○○○ indicates I2 ≤ 25%, ●○○ indicates 25% < I2 ≤ 50%, ●●○ indicates 50% < I2 ≤ 75%, ●●● indicates I2 ≥ 75%. AMSTAR2 a Measurement Tool to Assess Systematic Reviews 2, GRADE the Grading of Recommendations, Assessment, Development and Evaluation, CI confidence interval
For antibiotics of a specific type, weak evidence showed that macrolide exposure before one year of age was associated with infantile hypertrophic pyloric stenosis (IHPS) (OR: 1.98, 95% CI 1.12–3.50) [37]. For some special populations, antibiotic exposure of preterm infants in the first three days could increase the risk of necrotizing enterocolitis (NEC) (OR: 2.35, 95% CI 1.54–3.57) [48], and hospitalized children exposed to antibiotics had a higher risk of Clostridioides difficile infection (CDI) than those without exposure (OR: 2.14, 95% CI 1.31–3.52) [47].
Discussion
Antibiotics are widely prescribed among children and pregnant women [6, 8]. Our review aimed to provide a comprehensive overview of the potential adverse effects of antibiotic exposure on pregnancy outcomes and children’s health.
We reviewed 19 publications with 39 unique exposure outcomes, comprising 430 original studies. We found six associations between postnatal exposure and adverse effects on children’s health supported by highly suggestive evidence and another 13 associations supported by weak to suggestive evidence. Generally, current evidence supports the association between postnatal antibiotic exposure and the development of allergic diseases and other immune-related diseases. The associations were especially important between postnatal exposure and asthma, allergic rhinoconjunctivitis and wheezing with 95% prediction intervals excluding the nulls, which means that future studies are unlikely to yield contrary results. The potential of antimicrobial resistance to antibiotic exposure in childhood cannot be ignored either. Antibiotic use in pregnancy seemed to be safe in terms of pregnancy outcomes such as birth defects and stillbirth, but the drug should still be given carefully until more confirmed evidence comes out. Obvious heterogeneity was observed in most outcomes. We explored the source of heterogeneity by trim and fill and sensitivity analysis in some outcomes. The association of prenatal exposure and spontaneous abortion became insignificant after either adjusting for missing studies or sensitivity analysis. In addition, the economy and healthcare resources of different regions will affect antibiotic prescriptions and health outcomes and therefore may be an important source of heterogeneity. The types and doses of antibiotics may also make a difference. Furthermore, an exact exposure period is crucial for the development of these adverse outcomes. The factors above are possible sources of heterogeneity and should be better determined in future meta-analyses.
Mechanisms underlying postnatal antibiotic exposure and children’s health are complex and may vary in different conditions [7]. One of the most accepted and widely applied hypotheses is the perturbation of microbiota [50]. The early life period, especially the first three years, is crucial for the colonization and maturation of microbes [51, 52]. Childhood antibiotic exposure, especially during infancy, can decrease the species richness and diversity of gut microbiota. Dysbiosis can alter intestinal permeability, inflammation, metabolism and the immune state and therefore plays an important role in the pathological process of many diseases [53,54,55,56].
In our study, the risk of asthma almost doubled in the exposure group. Antibiotics may induce dysbiosis in both the lung and gut, and the consequent dysregulation of immune cells and cytokines can possibly cause inflammation and hypersensitivity of the airway and thus lead to the onset of asthma [57, 58]. Apart from the immune system, the brain-gut-microme axis has also been studied widely in recent years and is related to neurodevelopmental disorders and metabolic disorders such as obesity [59,60,61,62]. Meanwhile, antibiotic exposure can destroy the natural gut microbiota and create a favorable environment for Clostridioides difficile, as well as lead to an expansion of antibiotic resistance genes. These factors may contribute to the increased risk of CDI among the pediatric inpatient population [47, 48]. Apart from microbiota, some specific types of antibiotics may have other direct effects. In the case of erythromycin, it can act as a motilin receptor agonist and then stimulate phase III migrating motor complexes in the stomach, which is related to the development of IHPS [37].
Despite the possible cues indicating the associations, differences in the risks of ADHD, celiac disease, allergic sensitization and type 1 diabetes between the postnatal exposure and nonexposure groups were not observed in our study. Considering the contrary results of original studies and the presence of heterogeneity in the pooled estimates, high-quality cohort studies with larger sample sizes and better study designs are needed for further assessment.
The effects of prenatal antibiotic exposure are more complicated. Antibiotics of almost any type administered to pregnant women can cross the placental barrier and reach the fetus [63]. Antibiotic exposure of the fetus can either have direct toxicity and teratogenicity or disrupt microbiota, which is important in the maturation of their immune system [64, 65]. In addition, recent studies also discovered the presence of microbiota or their related components in the uterus, which may be potentially involved in the modulation of immune cell subsets that are essential for implantation and the fetus’s immunological development [66]. Meanwhile, the time and type of antibiotic exposure during pregnancy will make a difference. The first trimester is the most sensitive period to teratogenic agents, while the potential effects of microbiota perturbation are more likely to be observed in the second and third trimesters [8]. The toxicity and teratogenicity of antibiotics differ between different antibiotic regimens, while the effects on microbiota can be observed in antibiotics of almost any type [8].
In our review, the incidence of wheeze/asthma, atopic dermatitis/eczema and ADHD increased in the exposure group despite weak credibility. Regarding toxicity and teratogenicity, antibiotic use seemed to be safe except for an overall slight increase in spontaneous abortion risk in the exposure group. Since the quality of these included studies was unsatisfying, we still believed antibiotics should be prescribed very cautiously in gestation, while more high-quality studies are needed to confirm the effects of different types of antibiotics given in different periods of gestation on pregnancy outcomes.
In addition, there is concern about prenatal antibiotic exposure and carcinogenesis since the reproductive toxicity and genotoxic potential of antibiotics were observed in a few animal studies and in vitro studies [36]. A possible association between antibiotic consumption and colorectal cancer in adulthood was also reported in previous studies [67,68,69]. From our results, however, no association was observed between maternal antibiotic use and child ALL. Further research assessing the influence of different types of antibiotics, different times of exposure and different tumor types is needed.
To our knowledge, this is the first umbrella review systematically assessing the potential adverse effects of antibiotic exposure on pregnancy outcomes and children’s health. Umbrella review is a good tool to study the associations between uncertain and complex adverse health outcomes and medical variables. We used an optimized search strategy and followed a strict selection process to ensure that the included meta-analyses provided the most comprehensive and up-to-date information. We assessed the methodological quality of the included studies by GRADE and AMSTAR2 and provided the effect size of each meta-analysis after reanalysis. We also presented an evidence map to intuitively show the risk of exposure and the credibility of the evidence. More importantly, our review also objectively described the overall status of research on prenatal and postnatal antibiotic exposure. The high heterogeneity in the existing meta-analyses and low quality of current evidence indicated an evidence gap in this field, which requires more high-quality research in the future.
Nevertheless, there are several limitations. The overall quality of evidence is low due to the limitations of original studies and this topic. First, the definition of antibiotic exposure is obscure. Information on whether the patient was exposed was usually collected retrospectively by asking guardians or referring to medical records; therefore, a history of antibiotic use can be omitted, and recall bias cannot be avoided. In addition, antibiotic exposure does not only come from drugs. Antibiotics are involved in animal feed production for treating or preventing diseases and growth promotion, which may lead to antibiotic residues in food such as milk, eggs and meat, causing various health problems [70]. Antibiotic use by breastfeeding mothers may also have an impact on infant health [71]. Second, most original studies did not provide information on exposure time, doses, courses and reasons for antibiotic use, which were important to adverse effects. Third, large heterogeneity was observed in most meta-analyses. Several associations of adverse health outcomes were insignificant despite being supported by microbiota theory. Considering the existence of the confounding factors mentioned above, we were not able to confirm the true relationship between antibiotic exposure and these health outcomes. Last, the short-term and long-term adverse effects of antibiotic use are very complex and comprehensive topics. Here, we only studied pregnancy outcomes and children’s health and mainly discussed long-term adverse health outcomes related to the microbiota.
In future studies, we should determine more objective and precise methods to determine the true history of antibiotic exposure. Antibiotic use in pregnant women and children should be better recorded prospectively, including indications, precise time of use, types of antibiotics, doses and courses. We hope there are more studies providing data on quantified exposure levels to enable dose‒response analysis, which is important for establishing the causal relationship. The detailed mechanism underlying the potential harm of antibiotic exposure should also be clarified in the future.
In conclusion, this umbrella review assessed potential adverse effects of antibiotic exposure on pregnancy outcomes and children’s health, which is important in the context of widespread use of antibiotics in these populations. Associations were found for multiple adverse outcomes, especially for those related to the immune system, including postnatal exposure and asthma, allergic rhinoconjunctivitis and wheezing. The association for antimicrobial resistance-related conditions was also significant despite the weak evidence. Antibiotic prescriptions should be administered cautiously in clinical practice, especially for those under three years, since they are in the key period for gut microbiota colonization and immune system development. Although a potential mechanism may explain the associations, the causal relationship cannot yet be confirmed. Future studies should make more efforts to measure and quantify antibiotic exposure precisely and address whether true causality exists.
Data availability
All data generated or re-analyzed during this study come from included meta-analyses.
References
Browne AJ, Chipeta MG, Haines-Woodhouse G, Kumaran EPA, Hamadani BHK, Zaraa S, et al. Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study. Lancet Planet Health. 2021;5:e893–904.
Klein EY, Milkowska-Shibata M, Tseng KK, Sharland M, Gandra S, Pulcini C, et al. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: an analysis of pharmaceutical sales data. Lancet Infect Dis. 2021;21:107–15.
Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115:E3463–70.
Qiao M, Ying GG, Singer AC, Zhu YG. Review of antibiotic resistance in China and its environment. Environ Int. 2018;110:160–72.
Hofer U. Rise in global antibiotic use. Nat Rev Microbiol. 2022;20:63.
Good A, Olans RCE. Pediatric antibiotic stewardship. Am J Nurs. 2021;121:38–43.
Duong QA, Pittet LF, Curtis N, Zimmermann P. Antibiotic exposure and adverse long-term health outcomes in children: a systematic review and meta-analysis. J Infect. 2022;85:213–300.
Bookstaver PB, Bland CM, Griffin B, Stover KR, Eiland LS, McLaughlin M. A review of antibiotic use in pregnancy. Pharmacotherapy. 2015;35:1052–62.
Kamphorst K, Van Daele E, Vlieger AM, Daams JG, Knol J, van Elburg RM. Early life antibiotics and childhood gastrointestinal disorders: a systematic review. BMJ Paediatr Open. 2021;5:e001028.
Dragioti E, Solmi M, Favaro A, Fusar-Poli P, Dazzan P, Thompson T, et al. Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiat. 2019;76:1241.
Kim JH, Kim JY, Lee J, Jeong GH, Lee E, Lee S, et al. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: an umbrella review. Lancet Psychiatry. 2020;7:955–70.
Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13:132–40.
Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21:95–100.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Salvo EA-O, Ferko NC, Cash SB, Gonzalez A, Kahrilas PA-O. Umbrella review of 42 systematic reviews with meta-analyses: the safety of proton pump inhibitors. Aliment Pharmacol Ther. 2021;54:129–43.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Foroutan F, Guyatt G, Zuk V, Vandvik PO, Alba AC, Mustafa R, et al. GRADE guidelines 28: use of GRADE for the assessment of evidence about prognostic factors: rating certainty in identification of groups of patients with different absolute risks. J Clin Epidemiol. 2020;121:62–70.
DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–45.
Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, et al. Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med. 2014;160:267–70.
Li X, Celotto S, Pizzol D, Gasevic D, Ji MM, Barnini T, et al. Metformin and health outcomes: an umbrella review of systematic reviews with meta-analyses. Eur J Clin Invest. 2021;51:e13536.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Borenstein M. Research Note: In a meta-analysis, the I(2) index does not tell us how much the effect size varies across studies. J Physiother. 2020;66:135–9.
Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549.
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002.
Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. 2007;4:245–53.
Moreno SG, Sutton AJ, Ades AE, Stanley TD, Abrams KR, Peters JL, et al. Assessment of regression-based methods to adjust for publication bias through a comprehensive simulation study. BMC Med Res Methodol. 2009;9:2.
Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035.
Li X, Meng X, Timofeeva M, Tzoulaki I, Tsilidis KK, Ioannidis JP, et al. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ. 2017;357:j2376.
Belbasis L, Bellou V, Evangelou E, Ioannidis JPA, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14:263–73.
Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: an umbrella review of systematic reviews and meta-analyses. Alzheimers Dement. 2017;13:406–18.
Wu M, Yang X, Tian J, Fan H, Zhang Y. Antibiotic treatment of pulmonary infections: an umbrella review and evidence map. Front Pharmacol. 2021;12:680178.
Zhong Y, Zhang Y, Wang Y, Huang R. Maternal antibiotic exposure during pregnancy and the risk of allergic diseases in childhood: a meta-analysis. Pediatr Allergy Immunol. 2021;32:445–56.
Wan S, Guo M, Zhang T, Chen Q, Wu M, Teng F, et al. Impact of exposure to antibiotics during pregnancy and infancy on childhood obesity: a systematic review and meta-analysis. Obesity. 2020;28:793–802.
Ai Y, Zhao J, Shi J, Zhu TT. Antibiotic exposure and childhood attention-deficit/hyperactivity disorder: systematic review and meta-analysis. Psychopharmacology. 2021;238:3055–62.
Omranipoor A, Kashanian M, Dehghani M, Sadeghi M, Baradaran HR. Association of antibiotics therapy during pregnancy with spontaneous miscarriage: a systematic review and meta-analysis. Arch Gynecol Obstet. 2020;302:5–22.
Zhu X, Meng Y, Yang Y, Feng N. Maternal antibiotics exposure during pregnancy and the risk of acute lymphoblastic leukemia in childhood: a systematic review and meta-analysis. Eur J Pediatr. 2022;181:471–8.
Abdellatif M, Kamel M, Ghozy S, Elawady S, Ghorab M, Attia A, et al. Association between exposure to macrolides and the development of infantile hypertrophic pyloric stenosis: a systematic review and meta-analysis. J Pediatr Gastroenterol Nutr. 2018;66:329.
Li P, Qin X, Tao F, Huang K. Maternal exposure to sulfonamides and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS ONE. 2020;15:e0242523.
Goldberg O, Moretti M, Levy A, Koren G. Exposure to nitrofurantoin during early pregnancy and congenital malformations: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2015;37:150–6.
Ziv A, Masarwa R, Perlman A, Ziv D, Matok I. Pregnancy outcomes following exposure to quinolone antibiotics—a systematic-review and meta-analysis. Pharm Res. 2018;35:109.
Kato H, Yamagishi Y, Hagihara M, Hirai J, Asai N, Shibata Y, et al. Systematic review and meta-analysis for impacts of oral antibiotic treatment on pregnancy outcomes in chronic endometritis patients. J Infect Chemother. 2022;28:610–5.
Ahmadizar F, Vijverberg SJH, Arets HGM, de Boer A, Lang JE, Garssen J, et al. Early-life antibiotic exposure increases the risk of developing allergic symptoms later in life: a meta-analysis. Allergy. 2018;73:971–86.
Yu HY, Zhou YY, Pan LY, Zhang X, Jiang HY. Early life antibiotic exposure and the subsequent risk of autism spectrum disorder and attention deficit hyperactivity disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2022;52:2236–46.
Meng X, Zhu Y, Di H, Zhang M, Feng J, Xu M, et al. Dose-response association of early-life antibiotic exposure and subsequent overweight or obesity in children: a meta-analysis of prospective studies. Obes Rev. 2021;22:e13321.
Zou Y, Wu L, Xu W, Zhou X, Ye K, Xiong H, et al. Correlation between antibiotic use in childhood and subsequent inflammatory bowel disease: a systematic review and meta-analysis. Scand J Gastroenterol. 2020;55:301–11.
Garot E, Rouas P, Somani C, Taylor GD, Wong F, Lygidakis NA. An update of the aetiological factors involved in molar incisor hypomineralisation (MIH): a systematic review and meta-analysis. Eur Arch Paediatr Dent. 2022;23:23–38.
Anjewierden S, Han Z, Deshpande A. Risk factors for clostridium difficile infection among pediatric inpatients: a meta-anlaysis. J Investig Med. 2018;66:852.
Rina P, Zeng Y, Ying J, Qu Y, Mu D. Association of initial empirical antibiotic therapy with increased risk of necrotizing enterocolitis. Eur J Pediatr. 2020;179:1047–56.
Egunsola O, Adefurin A, Fakis A, Jacqz-Aigrain E, Choonara I, Sammons H. Safety of fluconazole in paediatrics: a systematic review. Eur J Clin Pharmacol. 2013;69:1211–21.
Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. 2020;10:572912.
Derrien M, Alvarez AS, de Vos WM. The gut microbiota in the first decade of life. Trends Microbiol. 2019;27:997–1010.
Akagawa S, Akagawa Y, Yamanouchi S, Kimata T, Tsuji S, Kaneko K. Development of the gut microbiota and dysbiosis in children. Biosci Microbiota Food Health. 2021;40:12–8.
Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 2020;113:2019–40.
Cait A, Wedel A, Arntz JL, Duinkerken J, Datye S, Cait J, et al. Prenatal antibiotic exposure, asthma, and the atopic march: a systematic review and meta-analysis. Allergy. 2022;77:3233–48.
Biedermann L, Rogler G. The intestinal microbiota: its role in health and disease. Eur J Pediatr. 2015;174:151–67.
Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81:e36-117.
Patrick DM, Sbihi H, Dai DLY, Al Mamun A, Rasali D, Rose C, et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir Med. 2020;8:1094–105.
Barcik W, Boutin RCT, Sokolowska M, Finlay BB. The role of lung and gut microbiota in the pathology of asthma. Immunity. 2020;52:241–55.
Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6:133–48.
Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol. 2017;2:747–56.
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–63.
Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71.
Pacifici GM. Placental transfer of antibiotics administered to the mother: a review. Int J Clin Pharmacol Ther. 2006;44:57–63.
Grech A, Collins CE, Holmes A, Lal R, Duncanson K, Taylor R, et al. Maternal exposures and the infant gut microbiome: a systematic review with meta-analysis. Gut Microbes. 2021;13:1–30.
Macpherson AJ, de Agüero MG, Ganal-Vonarburg SC. How nutrition and the maternal microbiota shape the neonatal immune system. Nat Rev Immunol. 2017;17:508–17.
Benner M, Ferwerda G, Joosten I, van der Molen RG. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum Reprod Update. 2018;24:393–415.
Sanyaolu LN, Oakley NJ, Nurmatov U, Dolwani S, Ahmed H. Antibiotic exposure and the risk of colorectal adenoma and carcinoma: a systematic review and meta-analysis of observational studies. Colorectal Dis. 2020;22:858–70.
Armstrong D, Dregan A, Ashworth M, White P, McGee C, de Lusignan S. The association between colorectal cancer and prior antibiotic prescriptions: case control study. Br J Cancer. 2020;122:912–7.
Simin J, Fornes R, Liu Q, Olsen RS, Callens S, Engstrand L, et al. Antibiotic use and risk of colorectal cancer: a systematic review and dose–response meta-analysis. Br J Cancer. 2020;123:1825–32.
Bacanlı M, Başaran N. Importance of antibiotic residues in animal food. Food Chem Toxicol. 2019;125:462–6.
van Wattum JJ, Leferink TM, Wilffert B, Ter Horst PGJ. Antibiotics and lactation: an overview of relative infant doses and a systematic assessment of clinical studies. Basic Clin Pharmacol Toxicol. 2019;124:5–17.
Acknowledgements
The authors would like to thank authors and publishers of all meta-analyses and the original studies.
Funding
This work was supported by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (Grant no. ZYJC18015, Grant no. ZYGD18011) and the Post-Doctor Research Project, West China Hospital, Sichuan University (Grant no. 2020HXBH016). These funders had no role in the study design, writing of the manuscript, or decision to submit this or future manuscripts for publication. KW is funded by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (Grant no. ZYJC18015). HL is funded by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (Grant no. ZYGD18011). ZJ is funded by the Post-Doctor Research Project, West China Hospital, Sichuan University (Grant no. 2020HXBH016).
Author information
Authors and Affiliations
Contributions
LY: data curation, formal analysis, project administration, methodology, visualization, and writing–original draft. LLH: conceptualization, formal analysis, visualization, and writing–original draft. JZY: conceptualization, formal analysis, methodology, funding acquisition, and writing–review and editing. LPH and JX: data curation and validation. LH: supervision, funding acquisition, and writing–review and editing. WKJ: conceptualization, funding acquisition and writing–review and editing. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. LY, LLH and JZY contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethical approval
Not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Liu, LH., Jian, ZY. et al. Association between antibiotic exposure and adverse outcomes of children and pregnant women: evidence from an umbrella review. World J Pediatr 19, 1139–1148 (2023). https://doi.org/10.1007/s12519-023-00711-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-023-00711-z