Abstract
Groundwater is an essential freshwater resource and contributes about two-thirds of the world’s freshwater reserves. Groundwater contamination can occur due to faulty construction or leakage in pipes and somehow through surface contaminants like a human or animal fecal matter or other foreign substances. The present study was conducted to assess the physicochemical as well as the bacteriological quality of groundwater in the rural areas of the Kurukshetra district, northern India. The samples were collected and analyzed for various physicochemical (pH, EC, TDS, Cl, TH, Ca, and Mg) and the bacteriological [total coliforms (TC) and fecal coliforms (FC)] parameters. The samples were then treated and re-analyzed after applying various household practices like boiling, solar disinfection or SODIS, chlorination, and reverse osmosis (RO) technique. After treatment, the trend obtained from the analysis for removing bacteriological contaminants follows as RO ≈ boiling > chlorination ≈ SODIS. It indicates that RO followed by boiling is the best method for drinking water treatment at home which removes most of the microbes as well as EC and TDS to a large extent. However, chlorination and SODIS methods are also effective in removing contaminants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drinking water can be called as ‘safe’ when it does not pose any significant health risk over a lifetime of consumption [World Health Organization (WHO), 2008]. Generally, borehole water or groundwater has fine bacterial quality. However, pathogens can be noticed due to infiltration of surface water, septic discharges, or leakage in sewer lines (Ahmed et al. 2011). Water scarcity has become a major problem, especially in the arid and semi-arid regions of India, which are receiving < 500 mm of the average annual precipitation (Keesari et al. 2014). The dependency on groundwater resources increased mainly because of the failure of monsoonal rains and the limited availability of surface water.
In India, water-borne diseases are responsible for the death of over 100,000 people annually. Providing safe water to such an increasing population is a major challenge (WaterAid India 2019). India is at first rank worldwide with over 386,000 annual deaths attributable to diarrheal diseases [United Nations Children’s Fund (UNICEF) and World Health Organization (WHO), 2009]. According to WHO/UNICEF (2010) Joint Commission Report, out of the 1.2 billion population in India, now 88% of people have access to improved water sources, as compared to 72% in 1990. Although, the report also says the presence of improved water sources does not ensure that the water is safe for drinking. Water experts have universally accepted that the significant risk related to the intake of water is the presence of microbial contamination by human or animal feces (WHO, 2004). Waterborne diseases such as diarrhea, cholera, and typhoid become an enormous burden due to a lack of safe water. Diarrhea itself is a prime reason for morbidity and mortality among children < five years of age and, mostly in low-income countries, diarrhea was the third prime cause of death in 2004 (WHO, 2009).
Piped water supplies used for drinking purposes should be provided with water protection and centralized treatment. However, worldwide about 780 million people are not having access to an “improved” drinking water source, and many available water sources are unsafe and at a far distance from the houses [Centers for Disease Control and Prevention (CDC), 2016]. Although households can access piped water at home, it may also get contaminated by some faults present in the distribution system. Drinking water can also get contaminated due to improper handling practices and unsafe storage in the household (Nath et al. 2006) and increased distance from the treatment reservoir to the distribution points (Sharma et al. 2013).
Water treatment at the household level is promoted widely as a suitable intervention, especially in developing countries, to reduce the waterborne disease burden in poor and remote rural areas (Islam et al. 2015; Boisson et al. 2013). Generally, water treatment is intended to improve the water source to make it and suitable for the end-user. Any system used for water treatment can remove organic or inorganic matter, which can be insoluble or particulate form, including microbes. The household water treatment (HWT) system imparts a medium to improve the drinking water quality. Today’s desired treatment technologies comprise chemical, ceramic filters, disinfectants, solar disinfection (SODIS), or ultraviolet (UV) disinfection, as well as boiling process (Clasen 2009). If electricity is available in continuous supply at the household level, numerous technologies are available for water treatment. Examples of such treatment brands available in India include water purification by reverse osmosis (RO), like Kent Osmosis or other that uses a combination of several treatments like UV and boiling treatment, and sediment filtration, for example, Aqua Guard (Jain 2009). Although, in developing countries, especially rural and low-income people are often deprived of continuous electricity supply, and mostly these people are at higher risk of having an unsafe supply of water.
Many chemical disinfectants are available for water disinfection, such as chlorine dioxide, ozone, chlorine, and hydrogen peroxide. Globally, chlorination treatment is the most widely used technique to disinfect water. In many countries, it is used enormously because of its lower price, effectiveness against microbes, and implementation in any size operations (Tomás-Callejas et al. 2012; Luo et al. 2011). Chlorine is also associated with some disadvantages like the developing disinfection by-products (DBPs) in the water, residual chlorine with harmful impacts on aquatic life, and its low effectiveness against some viruses and protozoan parasites (Tomas-Callejas et al. 2012). Chlorine is most efficacious against bacteria such as E. coli as compared to parasites (Arnold and Colford 2007). In India, several studies showed resistance among users to use water treated with chlorine due to the unpleasant smell and taste, which are perceived longer (Firth et al. 2010; Gopal et al. 2009). Application of chlorine in low concentration may cause ineffective disinfection of water and in high dosage, chlorine may change the taste and smell resulting in a high concentration of DBPs. These complexities lead to ineffective chlorination at the household level. Therefore, household chlorination is generally performed by tablets or a dilute solution with a fixed concentration of chlorine and these are mostly provided by government supplies.
Boiling water is probably the simplest and oldest method, which is mostly used to remove pathogens from water. Some authorities recommend that the water should be boiled for 1‒5 min (CDC 2017). The water is brought to a rolling boil to kill the pathogens in the most effective way, especially in turbid waters or even at high altitudes (WHO, 2018). Field studies in India proved to be effective the boiling process in improving the microbiological quality of water (Clasen et al. 2008; Freeman et al. 2012). Approximately, 21.6% population in 67 middle- and low-income countries reported water boiling at home before drinking, which is four times higher than those reported drinking water chlorination or filtration (Rosa et al. 2010).
Natural solar (UV) radiation can be an effective method to heat water up to temperatures lower than boiling temperature. Especially the UV-A spectrum (320 to 400 nm) is the most essential component of solar radiation for inactivating microorganisms. The UV light damages the nucleic acids of the microbes and gets inactivated. It also prevents replication [United States Environment Protection Agency (USEPA), 2003]. Temperatures of 55 °C to more than 60 °C killed most of the pathogens rapidly due to the thermal effect. This high temperature represents pasteurization capable of inactivating nearly all enteric pathogens (enteric viruses, bacteria and parasites) within several minutes to hours (Skinner and Shaw 2004). The present study has the following objectives:
-
1.
To analyze the untreated household water samples for physicochemical and bacteriological parameters.
-
2.
To check the efficiency of various household treatment practices including boiling, chlorination, SODIS, and reverse osmosis in treating the raw water at home.
Materials and methods
Study area
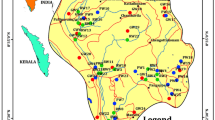
Kurukshetra district is present in Haryana north‒eastern region with an area covering 1530 km2. It is demarcated latitudinally in North with 29o 53′ 00″ and 30o 15′ 02″ and longitudinally in East with 76o 26′ 27″ and 77o 07′ 57″. In Haryana, 3.46 percent area is covered by the Kurukshetra district. The population of Kurukshetra is 9,64,231 as per the 2011 census, which is distributed in six blocks, namely Thanesar, Babain, Shahabad, Ladwa, Ismailabad, and Pehowa. Kurukshetra is covered by arid brown soil. The Markanda river mainly irrigates the study area, and the river Sarasvati have also joined the river Ghaggar in Kurukshetra (Pehowa) [Ministry of Water Resources (MOWR), 2016]. The average annual rainfall of about 582 mm is recorded over the study area. However, the annual rainfall during the study (in 2015) was 390.8 mm (India Meteorological Department, 2015). According to the estimates of 2011 [Central Ground Water Board (CGWB), 2013], in major parts of the Kurukshetra district, the water level lies at > 30 m below ground level (bgl) and ranges from 20.18 to 32.64 m bgl during the pre-monsoon and from 21.80 to 34.41 m bgl in the post-monsoon season. The major concern for the Kurukshetra district is the depletion of its groundwater resources, as the water table now lies under the dark zone (CGWB, 2013). This study’s purpose was to analyze the microbiological quality of groundwater in nine villages of Kurukshetra district and to check the efficiency of different HWT practices to improve the quality of groundwater. The villages were selected from the study blocks Thanesar (T), Shahabad (S), and Ladwa (L).
Sample collection
The sampling sites were finalized based upon the physicochemical and bacteriological analysis of the 81 groundwater samples from different villages of the Kurukshetra district during pre-monsoon, monsoon, and post-monsoon season from 2015 to 2016. The results of one year study were already published elsewhere (Malan et al. 2020; Malan and Sharma 2018). However, the nine samples that were found to be most contaminated during the one year study were finally selected for this treatment experiment. The water samples were collected from the household borewells of different villages located in three blocks of Kurukshetra (Fig. 1) during September 2016 (monsoon season). The borewells depth ranged between 36 and 94 m bgl. Clean and sterilized glass bottles were used for sampling of groundwater. The sample bottles were filled after running the water for five minutes to avoid any external contamination. Sterile conditions were sustained throughout the collection and analysis of the samples. Sampling and analysis were performed as per the guidelines given by Bureau of Indian Standards (BIS 2012) and (WHO, 2011). The samples were analyzed for TC and FC within six hours of collection using the most probable number (MPN) method. The samples were then treated with various HWT practices like boiling, chlorination, SODIS, and reverse osmosis technique and analyzed again after treatment. The chemicals and media were acquired from Hi-Media, Mumbai, India.
Physicochemical analysis
The untreated and treated water samples were examined for different physicochemical parameters, including pH, total dissolved solids (TDS), electrical conductivity (EC), total hardness (TH), bicarbonate, chloride, calcium, and magnesium contents using the methods of American Public Health Association (APHA) (2005).
Enumeration of TC and FC (MPN index/100 ml)
The TC and FC count in the samples were enumerated by MPN method as per APHA (2005). The TC were enumerated using the “five-tube assay” of the MPN technique by using MacConkey broth. The MPN index of water samples was evaluated from the MPN table (APHA 2005). The presence of coliforms in water samples was confirmed by the transfer of culture (a loopful) from a positive presumptive tube into a tube of “Brilliant Green Lactose Bile” (BGLB) broth with Durham tubes for the production of gas. Further, the pure colonies of the bacteria were isolated by streaking a loopful of broth from a positive BGLB tube onto “Eosine Methylene Blue” (EMB) agar plate. The identification of colonies on EMB agar were based on “Gram’s staining test” and some other biochemical tests, including indole, methyl-red, Voges-Proskauer (VP), and citrate utilization test, collectively known as IMViC test.
The positive BGLB tubes obtained after incubating at 44.5 ˚C confirmed the FC in groundwater samples. Eijkman test was also used for FC confirmation in which the MPN positive tubes from the presumptive test were inoculated in “Tryptone broth” tube for an indole production test.
Treatment procedure
The HWT techniques like boiling, chlorination, solar disinfection (SODIS), and reverse osmosis (RO) were used to treat groundwater samples. One liter of water was boiled to a rolling boil in a metal container for approximately 10 min and then filtered before drinking (WHO, 2004). In the chlorination treatment method, the chlorine tablets supplied by Primary Health Centers (PHCs) to the villagers free of cost through Accredited Social Health Workers (ASHA) workers by the Government of India (GoI) were used. About 2 mg of chlorine tablet was used to treat 1 L of water and kept for half an hour to complete the reaction. In SODIS method, direct sunlight is used to treat the water at home. Polyethylene tetraphthalate (PET) bottles as specified/recommended by WHO/UNICEF (2012), usually of 1-L capacity, were used to collect water. They were exposed to sunlight for a minimum of 6 h for total disinfection under optimum weather conditions (less than 50% cloudy). If the sky is more than 50% cloudy, the bottle water temperature does not exceed 42 °C, the bottle should be exposed for two consecutive days. The water with high turbidity should be filtered before using the SODIS method as it can affect the UV radiation. In reverse osmosis (commonly referred as RO), water is de-mineralized by flowing under pressure through a semi-permeable membrane. The treated water was collected from household RO systems of different brands and then analyzed in the laboratory to know the efficiency of various RO systems in the removal of contaminants as compared to other HWT practices.
Results and discussion
Physicochemical analysis of water sample before and after treatment
Based on physicochemical parameters, groundwater suitability for drinking in some of these villages has been discussed elsewhere (Malan and Sharma 2018) and the bacterial isolates and source identification was reported in another study (Malan et al. 2020). However, the present paper investigates the efficiency of various treatment facilities used by rural households. The result of the physicochemical analysis of groundwater samples was mentioned in Table 1. In raw water samples, the pH was ranged between 7.41 and 8.11 (Fig. 2), EC 337‒1308 µS/cm (Fig. 3), TDS 202‒623 mg/l (Fig. 4), HCO3− 200‒416 mg/l (Fig. 5), Cl− 6.01‒142.16 mg/l (Fig. 6), TH 44‒252 mg/l (Fig. 7, Ca2+ 24‒224 mg/l (Fig. 8), and Mg2+ between 4.86 and 28 mg/l (Fig. 9). After applying various treatment practices, the pH was ranged between 6.88 and 8.84, EC 39.6‒1400 µS/cm, TDS 19.7‒696 mg/l, HCO3− 16‒420 mg/l, Cl− 0‒186.20 mg/l, TH 24‒372 mg/l, Ca2+ 0‒200 mg/l, and Mg2+ between 2.92 and 57.35 mg/l (Figs. 2, 3, 4, 5, 6, 7, 8, 9).
On mean value basis, all the physicochemical parameters were observed within WHO's permissible limits (2011) except Ca2+ ion and TDS before disinfection. The studied parameters of treated water samples varied differently after treatment with different HWT practices. Maximum reduction in all physicochemical parameters was observed with RO treatment. There is no reduction in parameters values after treatment with chlorine except Ca2+. The various treatment practices showed the overall trend of RO > boiling ≈ SODIS > chlorination for most of the physicochemical parameters except calcium ion. Studies have shown effective removal of the physico-chemical parameters by household treatment practices. A report from CDC (2008) stated that RO is effective in the removal of some aqueous salts like sodium, chloride, and common metal ions. A significant decrease was found in the total hardness of the samples after boiling at 100 ∘C for 10 min in Jaffna Peninsula of Sri Lanka (Wijeyaratne and Subanky 2017).
Bacteriological analysis of water samples before and after treatment
TC and FC count results in drinking groundwater samples before and after treatment are depicted in Tables 2 and 3, respectively. All study sites were found contaminated with bacteria. Among nine samples tested, all the groundwater samples showed TC count ranged between 11 and 48 with a mean value of 26.22 ± 12.31, which was above the permissible limits of zero or nil as prescribed by WHO (2011). The FC were comparatively observed in very less numbers and varied between 2‒6.8 with a mean value of 4.84 ± 1.39 and were also above the permissible limits (nil/100 ml of water) of WHO (2011) used for drinking purposes (Fig. 10). In general, no particular trend was observed between the water samples of different villages for physico-chemical as well as bacteriological parameters. However, in groundwater samples of Ladwa and Thanesar blocks, the coliform count was found more which may be either due to the improper sanitary conditions observed in the periphery of the households or may be due to underground leakage in septic tanks and from human or animal fecal matter contamination. In general, drinking water samples from households with animal and solid wastes in their surroundings and poor sanitation practices were found to have more TC and FC count.
Various HWT practices used for total coliforms removal were found to be effective in order of RO ≈ boiling > chlorination ≈ SODIS. Both RO system and boiling were found to successfully remove about 87.0% of TC from the groundwater samples. However, chlorination and SODIS treatments removed 76.94% and 76.00% TC.
In the case of FC, all the treatment practices were shown to remove up to < 1.8 to 2, which is considered almost nil as per MPN table APHA (2005); however, no specific trend was observed between different HWT practices. In a similar study by Clasen et al. (2008), the boiling method successfully reduced 99% of fecal coliforms from the Vasai and Nalasopara regions of India. The WHO (2016) in India reported the effectiveness of boiling followed by filtration, chlorination and SODIS treatment processes in removing viruses, bacteria, and protozoa from drinking water.
Boiling treatment of water samples
Following the present findings, heating water to a rolling boil is sufficient to inactivate or kill the pathogens (WHO, 2011). Boiling has reduced the concentration of most of the ions except chloride, magnesium, and TDS, which were found to be more in most of the samples after treatment. The increased concentration may be due to bicarbonate salts left after boiling, thereby increasing TDS (WHO, 2004). Moreover, the non-carbonate hardness caused by the excess magnesium could not be removed by boiling (Sengupta 2013). Also, at acidic pH, the reaction between Cl− and HO·, results in the formation of HOCl·− which promotes the production of Cl· (Jayson et al. 1973). In certain Asian countries, more than 90% of the population preferred boiling as a household drinking water treatment method (WHO, 2009). However, boiling is dependent on the availability of fuel required to achieve the boiling temperature up to 100 °C, which is added to its cost. In rural India, boiling water costs up to approximately US $10.56 per annum (about 471.40 INR) by using liquid petroleum gas (LPG) gas for treating six liters of water per household/day (Firth et al. 2010). While wood biomass uses, the estimated annual cost of treating water through boiling per household in India remained only US $1.66 (74.10 INR) per annum.
Chlorination treatment of water samples
Chlorination is also much more effective in the removal of pathogens than physicochemical parameters, as investigated in the current study depicted in Table 2. The WHO standards (WHO, 2003) stated 2‒3 mg/L and 5 mg/L as the minimum and maximum limits for adding chlorine in water, respectively, for satisfactory disinfection and to maintain the minimum residual chlorine. All parameters had shown a significant increase after chlorine disinfection except calcium ion. It may be due to the chemical nature of the chlorine, which adds a disadvantage to this method. After a retention time of approximately 30 min, some byproducts formation occurred after various chemical reactions of chlorine with the organic substances naturally present in water. These compounds are known as disinfection byproducts (DBPs), such as trihalomethanes (carcinogenic) and halogenated acetic acids, which affect the chemical quality and somewhat physical properties like the taste and odor of water (Washington State Department of Health 2004). Some of the DBPs investigated as carcinogens and may cause an adverse impact on the reproductive and developmental health of humans (Chowdhury et al. 2009; Wei et al. 2010).
The chlorine left after complete disinfection and does not react with the water components is known as free or residual chlorine (RC) (0.3‒0.5 mg/l) (Oram 2014a) which must be present in treated water to avoid the growth of pathogens (Bertelli et al. 2018). In the present study, RC concentration in treated water ranged from 1.42 to 4.25 mg/l and was nil in two samples, thereby not meeting the minimum criteria of 0.2‒0.5 mg/l RC (WHO, 2011). It may be due to the maximum use of chlorine during the disinfection process. In Gwalior city (Madhya Pradesh, India), the average concentration of RC from all sampling locations of municipal piped water supply ranged from 0.08 to 0.98 mg/l, which was within the limits of WHO (Sharma and Rather 2015). While in rural Bangladesh, the average RC concentration was measured as 0.06 mg/l among the water samples of the source pond, and all samples were below the WHO standard of 0.2–0.5 mg/l (Ahsan et al. 2017). Similarly, in piped distribution supply of France, RC was absent in 50% of the water samples (Bertelli et al. 2018). Hence, it is depicted from this study that the concentration of chlorine tablets added to treat water is not sufficient due to higher bacterial count and RC was not left behind in about 44% of samples. However, chlorination is a convenient method to treat a large amount of water if added to proper concentration. In India, chlorine tablets are distributed free of cost in the rural area of Haryana and can be a better option.
SODIS treatment of water samples
SODIS is a treatment method that treats raw water by solar energy (ultraviolet and infrared radiations). The duration of sun exposure for effective water treatment depends on factors such as season, cloud coverage, latitude, altitude, size of the bottle, and the turbidity of water. However, there is the risk of migration of plasticisers from the plastic bottle into the water. Schmid et al. (2008) revealed maximum concentrations of 0.046 and 0.71 mg/L, respectively of di(2-ethylhexyl)phthalate (DEHP) and di(2-ethylhexyl)adipate (DEHA) plasticisers and were in the same range as reported in the commercially bottled water and hence safe to use. In the present study, SODIS has effectively reduced the EC and TDS, but the concentration of total hardness and cations and anions remained somewhat similar or unchanged in some treated samples. It is a low-price technology for sunny regions where electricity is unavailable (Ray and Jain 2011). In India, about 40‒75% reduction in estimated diarrheal cases has been observed among children after SODIS drinking water treatment (Rose et al. 2006; Rai et al. 2010). The major disadvantage is that a large amount of water cannot be treated at a time because it depends on the availability of PET bottles and the household’s storage capacity. However, it is a cheaper method as compared to other HWT techniques because it is not dependent on electricity and fuel consumption.
RO treatment of water samples
The RO process uses the reverse osmosis phenomenon and depends on the osmotic pressure difference between the pure water and the salt water, which removes the salts from water (Younos and Tulou 2005). RO system had reduced almost all ions to a very low level and maintained an optimum balance between cations and anions. This treatment process demineralizes water to a large level. Pre-treatment from RO can utilize various options, like microfiltration (MF), ultrafiltration (UF), and nanofiltration (NF). The basic difference in the membranes is based on the pore size, with NF being the smallest pore size and MF having the largest (Ray and Jain 2011). The RO systems generally remove heavy metals, phosphate, fluoride, nitrates, sodium, TDS, volatile organic compounds, and other pharmaceutical or agrochemical contaminants in a one-step procedure. Besides, this method also removes biological contaminants because of the very small pore sizes in the membranes without extra time or cost.
Although the RO method is very effective in treating water, there are some drawbacks like potentially high start-up prices, electricity requirements, effluent water handling, and the need to replace filters and membranes at a regular interval (Wimalawansa 2013). Another disadvantage of the RO system is that it uses large amounts of water. About 75% or more of water is discarded along with the contaminants (Oram 2014b). Due to these reasons, three out of nine households in this study did not install RO system in their houses. Each of the two households was found to have different brands of RO systems like Brand 1 (RO + UV + UF), Brand 2 (RO + UV) and Brand 3 (RO only). All RO’s were equally effective in treating bacteriological contaminants, but the system with brand 1 had reduced the coliforms up to < 2 MPN/100 ml. It indicates that UF membrane is more functional in the removal of microbes as compared to other RO and UV systems. However, the estimated cost for RO treatment is based on the location (geography, quality of water and economic status of the households, etc.) and volume of water to be treated. In India, the cost of RO systems with the capacity of 10–15 L generally ranges from 8000 to 20,000 INR based on the functions available like simple reverse osmosis, UV, ultrafiltration, and taste enhancers etc. Based on electricity requirements, RO is the most expensive method, whereas boiling is dependent on fuel availability. Chlorination relies on the availability of chlorine tablets or solutions, while SODIS is weather dependent. Therefore, the adoption of the treatment method also depends on the accessibility of resources in rural settings.
In the current study, it was found that RO followed by boiling is the best household method to treat drinking water at home, which removes most of the microbes and EC and TDS to a large extent. In a similar study on water treatment practices conducted in an urban area of Burla in Odisha, India, and majority (63%) of the participants responded boiling to be the prime method for disinfecting the drinking water at home, accompanied by a membrane filter (13%) and chlorine tablets (6.8%) (Pradhan et al. 2018). Similar findings were reported in urban slums by Joshi et al. 2014 in Delhi and Beistline (2016) in Kolkata in India. In Gwalior city in Madhya Pradesh, India, boiling was used by 32% of households used, aqua guard (RO brand) by 18% and 16.5% adopted alum treatment of municipal supplied drinking water (Sharma and Rather 2015). In India’s rural population (in states namely West Bengal, Uttar Pradesh, Tamil Nadu, Rajasthan, and Andhra Pradesh), approx. 28.5% of people used boiling (traditional method), about 81% filtered by cloth or RO/Purifier (modern method) and about 15.2% had awareness regarding the addition of bleaching powder for water treatment (Rural marketing survey on safe water supply, 2012). The report further mentioned that most of the people preferred filtration with RO/local purifier or with simple cloth filtration and it was observed as the finest method for removing toxic contaminants from water. In a report from Zambia, a lower-middle-income country, HWTS was followed by only 34.9% of households and/out of which 25.9% households were using chlorination disinfection while 15.2% depended on boiling for the purification of their water (Rosa et al. 2016). Similar case studies were observed from semi-urban areas of Vasai and Nalasopara of India and peri-urban Cambodia (Clasen et al. 2008; Brown and Sobsey 2012). From the above discussion, boiling and filtration by RO are more practiced in rural areas than SODIS and chlorination.
Statistical analysis
The significance between values of different treated parameters was calculated by one-way ANOVA and differences were considered significant at p < 0.05. The concentration of all the parameters was observed significant between different treatments except HCO3 ion. The values of different parameters after treatment were not observed significant along with different sites (Table 4).
The correlation analysis of parameters with different treatments is given in Table 4. It was found that EC and TDS were in high positive correlation with, HCO3, Cl, TH, Ca, and with each other. Bicarbonate ion was positively correlated with TH and Ca. Total hardness was in positive correlation with Ca and Cl ions. A strong positive correlation was observed between TC and FC counts but they did not show any correlation with other physicochemical parameters (Table 4).
Conclusions
All the analyzed rural groundwater samples were contaminated with the coliforms whereas except calcium ion, all physico-chemical parameters remained within the permissible limits. Various household treatment practices viz. boiling, chlorination, SODIS and RO were effective in reducing coliforms in groundwater samples. In comparison, boiling and RO were the most efficient treatment methods for microbe’s removal from drinking groundwater. Further, boiling practice is more preferred by the villagers because of its low cost as compared to RO systems. Also, it is not dependent on the availability of electricity which is a big problem in rural areas. After chlorination, chlorine residual should be checked regularly to know the proper working of the disinfection system. The use of the method depends on the availability of resources like fuel, electricity, chlorine tablets, weather, and the treatment method’s cost per household. Therefore, the present study results depicted that all household methods are effective in treating drinking water and water should be treated at home before drinking to prevent various water-borne diseases, especially in rural settings.
References
Ahmed W, Gardner T, Toze S (2011) Microbiological quality of roof harvested rainwater and health risks: a review. J Environ Qual 40:3–21
Ahsan MS, Akber MA, Islam MA, Kabir MP, Hoque MI (2017) Monitoring bacterial contamination of piped water supply in rural coastal Bangladesh. Environ Monit Assess 189(11):1–3
APHA (2005) Standard methods for examination of water and wastewater. Twentieth ed. Washington DC, USA
Arnold BF, Colford JM (2007) Treating water with chlorine at point-of-use to improve water quality and reduce child diarrhea in developing countries: a systematic review and meta-analysis. Am J Trop Med Hyg 76(2):354–364
Beistline HA (2016) Understanding the barriers of clean water access in urban slums of Kolkata. India Christ J Glob Health 3(1):46–56
Bertelli C, Courtois S, Rosikiewicz M, Piriou P, Aeby S, Robert S, Loret JF, Greub G (2018) Reduced chlorine in drinking water distribution systems impacts bacterial biodiversity in biofilms. Front Microbiol 23(9):2520
BIS (Bureau of Indian Standards) (2012) Indian drinking water specification- second revision, Manak Bhavan, New Delhi. http://cgwb.gov.in/documents/wq-standards.pdf Accessed 30 October 2017
Boisson S, Stevenson M, Shapiro L, Kumar V, Singh LP, Ward D, Clasen T (2013) Effect of household-based drinking water chlorination on diarrhoea among children under five in Orissa, India: a double-blind randomised placebo-controlled trial. PLoS Med 10(8):e1001497
Brown J, Sobsey MD (2012) Boiling as household water treatment in Cambodia: a longitudinal study of boiling practice and micro- biological effectiveness. Am J Trop Med Hyg 87:394–398
CDC (Centers for Disease Control and Prevention) (2008) Drinking water treatment technologies for household use. Fact sheet for heathy drinking water. https://www.cdc.gov/healthywater/pdf/drinking/Household_Water_Treatment.pdf Accessed 20 March, 2020
CDC (Centers for Disease Control and Prevention) (2017) Water, sanitation, and hygiene (WASH)-related emergencies and outbreaks. https://www.cdc.gov/healthywater/emergency/drinking/making-water-safe.html. Accessed 11 July 2019
CGWB (Central Ground Water Board) (2013) Ground water information booklet, Kurukshetra district, Haryana. Ministry of Water Resources, Government of India, North Western region, Chandigarh. http://cgwb.gov.in/District_Profile/Haryana/Kurukshetra.pdf Accessed 25 September 2016
Chowdhury S, Champagne P, McLellan PJ (2009) Models for predicting disinfection byproduct (DBP) formation in drinking waters: a chronological review. Sci Total Environ 407(14):4189–4206
Clasen T (2009) Scaling up household water treatment among low-income populations. (WHO/HSE/WSH/09.02). World Health Organization, Geneva. http://whqlibdoc.who.int/hq/2009/WHO_HSE_WSH_09.02_eng.pdf. Accessed 15 September 2018
Clasen T, McLaughlin C, Nayaar N, Boisson S, Gupta R, Desai D, Shah N (2008) Microbiological effectiveness and cost of disinfecting water by boiling in semi-urban India. Am J Trop Med Hyg 79(3):407–413
Firth J, Balraj V, Muliyil J, Roy S, Rani LM, Chandresekhar R, Kang G (2010) Point-of-use interventions to decrease contamination of drinking water: a randomized, controlled pilot study on efficacy, effectiveness, and acceptability of closed containers, Moringa oleifera, and in-home chlorination in rural South India. Am J Trop Med Hyg 82(5):759
Freeman MC, Trinies V, Boisson S, Mak G, Clasen T (2012) Promoting household water treatment through women’s self help groups in rural India: assessing impact on drinking water quality and equity. PLoS ONE 7(9):e44068
Gopal S, Sarkar R, Banda K, Govindarajan J, Harijan BB, Jeyakumar MB, Mitta P, Sadanala ME, Selwyn T, Suresh CR, Thomas VA (2009) Study of water supply & sanitation practices in India using geographic information systems: some design & other considerations in a village setting. Indian J Med Res 129:233–241
India Meteorological Department (Ministry of Earth Sciences) (2016) Hydromet Division, Rainfall statistics of India-2015. REPORT NO. ESSO/IMD/HS R. F. REPO RT/04(2016)/22 http://hydro.imd.gov.in/hydrometweb/(S(ah0kjd55lgvs5siowd4f0jvl))/PRODUCTS/Publications/Rainfall%20Statistics%20of%20India%20%202015/Rainfall%20Statistics%20of%20India%20-%202015.pdf Accessed 15 July 2018
Islam M, Azad AK, Akber M, Rahman M, Sadhu I (2015) Effectiveness of solar disinfection (SODIS) in rural coastal Bangladesh. J Water Health 13(4):1113–1122
Jain M (2009) Status of household water treatment and safe storage in 45 countries and a case study in Northern India. Master's Dissertation, Massachusetts Institute of Technology, Boston, Massachusetts. https://dspace.mit.edu/handle/1721.1/58082. Accessed 30 Dec 2022
Jayson GG, Parsons BJ, Swallow AJ (1973) Some simple, highly reactive, inorganic chlorine derivatives in aqueous solution. Their formation using pulses of radiation and their role in the mechanism of the Fricke dosimeter. J Chem Soc Faraday Trans 1: Phys Chem Condens Phases 69:1597–1607
Joshi A, Prasad S, Kasav JB, Segan M, Singh AK (2014) Water and sanitation hygiene knowledge attitude practice in urban slum settings. Glob J Health Sci 6(2):23
Keesari T, Kulkarni UP, Deodhar A, Ramanjaneyulu PS, Sanjukta AK, Kumar US (2014) Geochemical characterization of groundwater from an arid region in India. Environ Earth Sci 71:4869–4888
Luo Y, Nou X, Yang Y, Alegre I, Turner E, Feng H, Abadias M, Conway W (2011) Determination of free chlorine concentrations needed to prevent Escherichia coli O157: H7 cross-contamination during fresh-cut produce wash. J Food Protect 74(3):352–358
Malan A, Kumar V, Sharma HR (2020) Bacteriological evaluation of groundwater in open-defecation-free villages of Kurukshetra district, Haryana, India. Int J Environ Stud 77:928–941
Malan A, Sharma HR (2018) Groundwater quality in open‒defecation‒free villages (Nirmal grams) of Kurukshetra district, Haryana, India. Environ Monit Assess 190:472 (1–17)
MOWR (Ministry of Water Resources) (2016) Palaeochannels of North West India: Review and assessment, Report of the Expert Committee to review available information on palaeochannels http://mowr.gov.in/writereaddata/PalaeochannelExpertCommittee_15thOct2016.pdf Accessed 11 July 2017
Nath KJ, Bloomfield S, Jones M (2006) Household water storage, handling and point-of-use treatment. A review commissioned by International Scientific Forum on Home Hygiene (IFH), Vol. 34. http://www.ifh-homehygienshare.org, 2006. Accessed 10 March 2020
Oram B (2014a) Chlorination of drinking water. Water Research Center. https://water-research.net/index.php/water-treatment/tools/chlorination-of-water Accessed 13 July, 2019
Oram B (2014b) Reverse Osmosis. Water Research Center. https://water-research.net/index.php/water-treatment/tools/chlorination-of-water Accessed 13 July, 2019
Pradhan SK, Sinha U, Satapathy DM, Swain AP, Mishra RP (2018) Assessment of household water treatment and storage practices. Int J Community Med Public Health 5(3):1060–1063
Rai BB, Pal R, Kar S, Tsering DC (2010) Solar disinfection improves drinking water quality to prevent diarrhea in under-five children in Sikkim. India J Glob Infect Dis 2(3):221
Ray C, Jain R (2011) Drinking water treatment-focusing on appropriate technology and sustainability, http://www.springer.com/978-94-007-1103-7, p–264
Rosa G, Kelly P, Clasen T (2016) Consistency of use and effectiveness of household water treatment practices among urban and rural populations claiming to treat their drinking water at home: a case study in Zambia. Am J Trop Med Hyg 94(2):445
Rosa G, Miller L, Clasen T (2010) Microbiological effectiveness of disinfecting water by boiling in rural Guatemala. Am J Trop Med Hyg 82(3):473
Rose A, Roy S, Abraham V, Holmgren G, George K, Balraj V, Abraham S, Muliyil J, Joseph A, Kang G (2006) Solar disinfection of water for diarrhoeal prevention in southern India. Arch Dis Child 91(2):139–141
Rural marketing survey on safe water supply (final report) (2012) Gfk mode Pvt Limited, BL House, New Delhi, India. https://www.jica.go.jp/india/office/information/event/2012/ku57pq00000qgi0natt/121113_02.pdf Accessed 5 June 2019
Schmid P, Kohler M, Meierhofer R, Luzi S, Wegelin M (2008) Does the reuse of PET bottles during solar water disinfection pose a health risk due to the migration of plasticisers and other chemicals into the water? Water Res 42(20):5054–5060
Sengupta P (2013) Potential health impacts of hard water. Int J Prev Med 4:866–875
Sharma HK, Rather MA (2015) Assessment of chlorination efficiency and quality of municipal drinking water in Gwalior city, Madhya Pradesh, India. Int J Sci Res 4:1699–1707
Sharma HR, Worku W, Hassen M, Tadesse Y, Zewdu M, Kibret D, Gashe A, Meseret M, Gessesse D, Kebede A, Gondar PO (2013) Water handling practices and level of contamination between source and point-of-use in Kolladiba Town, Ethiopia. Environ We Int J Sci Technol 8:25–35
Skinner B, Shaw R (2004) Technical brief: Household water treatment. Part 1. Water and environmental health at London and Loughborough (Well). Consultation 27/12/2004. http://www.lboro.ac.uk/well/resources/technical-briefs/58-household-water-treatment1.pdf Accessed 25 July 2017
Tomás-Callejas A, López-Gálvez F, Sbodio A, Artés F, Artés-Hernández F, Suslow TV (2012) Chlorine dioxide and chlorine effectiveness to prevent Escherichia coli O157: H7 and Salmonella cross-contamination on fresh-cut Red Chard. Food Control 23(2):325–332
Tomas-Callejas A, Lopez-Velasco G, Valadez AM, Sbodio A, Artes-Hernandez F, Danyluk MD, Suslow TV (2012) Evaluation of current operating standards for chlorine dioxide in disinfection of dump tank and flume for fresh tomatoes. J Food Protect 75(2):304–313
UNICEF/WHO (2009) Diarrhoea: Why children are still dying and what can be done. Geneva, WHO. https://apps.who.int/iris/bitstream/handle/10665/44174/9789241598415_eng.pdf;jsessionid=296EE5A49179EEDA645JointCommissionReport,C43401937AAEF?sequence=1. Accessed 23 August 2018
USEPA (United States Environment Protection Agency) (2003) Draft Report on The Environment Protection Technical Document. https://cfpub.epa.gov/ncea/cfm/eroe/recordisplay.cfm?deid=56830 Accessed 10 November 2017
Washington State Department of Health (2004) Chlorination of drinking water. https://www.doh.wa.gov/Portals/1/Documents/Pubs/331-253.pdf Accessed 15 July 2019
WaterAid India (2019) http://wateraidindia.in/what-we-do/safe-water/. Accessed 16 September 2019
Wei J, Ye B, Wang W, Yang L, Tao J, Hang Z (2010) Spatial and temporal evaluations of disinfection by-products in drinking water distribution systems in Beijing. China Sci Total Environ 408(20):4600–4606
WHO (World Health Organization) (2004a) Desalination guidelines development for drinking water: background. https://www.who.int/water_sanitation_health/dwq/nutdesalination.pdf Accessed 10 July 2018
WHO (World Health Organization) (2004b) Guidelines for drinking water quality. Third ed. World Health Organization, Geneva, Switzerland
WHO (World Health Organization) (2008) Guidelines for drinking-water quality, third edition, incorporating first and second addenda (3rd ed., Vol. 1). Geneva, Switzerland: WHO. http://www.who.int/water_sanitation_health/dwq/gdwq3rev/en/, 2008. Accessed 5 December 2017
WHO (World Health Organization) (2009) Scaling up household water treatment among low-income populations. https://www.who.int/household_water/clasen_scalingup_clean_jun2009.pdf. Accessed 18 July 2018
WHO (World Health Organization) (2011) Guidelines for drinking water quality. Recommendations.4 Ed. ISBN 9789241548151. Geneva, World Health Organization http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/ Accessed 19 November 2018
WHO (World Health Organization) (2016) Results of round I of the WHO international scheme to evaluate household water treatment technologies. https://apps.who.int/iris/bitstream/handle/10665/204284/9789241509947_eng.pdf;jsessionid=CA7FF29D4CE9AA8C12D9EB0EBCE45858?sequence=1 Accessed 5 May, 2019
WHO and UNICEF (2012), Progress on Drinking Water and Sanitation, World Health Organization, Retrieved from: 978-924-1503279 Accessed 15 July 2017
WHO/UNICEF, Joint monitoring programme for water supply and sanitation estimates for the use of improved drinking-water sources, India (p. 9). Geneva, Switzerland, 2010
Wijeyaratne WM, Subanky S (2017) Assessment of the efficacy of home remedial methods to improve drinking water quality in two major aquifer systems in Jaffna Peninsula, Sri Lanka. Scientifica https://doi.org/10.1155/2017/9478589
Wimalawansa SJ (2013) Purification of contaminated water with reverse osmosis: effective solution of providing clean water for human needs in developing countries. Int J Emerg Technol Adv Eng 3(12):75–89
World Health Organization (2003) Chlorine in drinking-water background document for development of WHO guidelines for drinking-water quality. Switzerland, Geneva
Younos T, Tulou KE (2005) Overview of desalination techniques. J Contemp Water Res Educ 132(1):3–10
Acknowledgements
The authors would like to acknowledge the Director of the Institute of Environmental Studies for providing all the laboratory facilities to complete this work and to the households for allowing water sampling.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Broder J. Merkel
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malan, A., Sharma, H.R. Assessment of drinking water quality and various household water treatment practices in rural areas of Northern India. Arab J Geosci 16, 96 (2023). https://doi.org/10.1007/s12517-022-11153-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-11153-8