Abstract
This paper presents results of laboratory investigation of alkali–surfactant–polymer (ASP) flooding technology for improving recovery of crude oil from Upper Assam oilfields. Oil displacement efficiency of two-component ASP formula consisting of polymer–surfactant, hydrophobically modified by added alkali was tested by conducting core flood studies. Contact angle and wettability is an important criterion for alkali flooding. In chemical enhanced oil recovery, reservoir should be strong water wet. The reservoir under study was initially intermediate wet, but after alkali and surfactant flooding, it altered to strong water wet. Ultra low interfacial tension in the range of 10−3 mN/m can be achieved by surfactant flooding. Salinity plays an important role in ASP flooding. It should be compatible with reservoir. During the course of the experiments, 5 pore volumes (PV) concentrated aqueous solutions of ASP slugs were injected into the core samples as a result of which oil displacement increased to maximum 34% after secondary water flooding. This extra recovery is due to microscopic displacement and macroscopic sweep efficiency. This technology has potential to become an alternative to the other chemical recovery methods especially in cases where application of these methods is restricted by geological, economical, or ecological factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Upper Assam basin is one of the most petroliferous and old oil reservoirs in India. It was discovered in 1901. Most of the wells are producing from the Tipam, Barail, and Eocene formation (Gogoi 2009). Typical recovery factor from primary drive mechanism is about 30%, depending on the properties of oil and the characteristics of the reservoir rock. On average, the recovery factor after primary and secondary oil recovery operations is between 35 and 45%. Remaining 55% of initial oil in place is unrecoverable by secondary flooding. Significant recovery can be achieved by using proper slug in chemical enhanced oil recovery (CEOR). This process involves injection of exotic fluids like alkali, surfactant, polymer, and combination of these (Gogoi 2010). This method mobilizes and recovers the oil that has been left behind or cannot produce economically by conventional means; i.e., it reduces the residual oil saturation. To sustain global production of energy resources, it is imperative to recover more conventional reserves. EOR methods are among the key ways of achieving this goal. Using EOR, 30–60% or more of the reservoirs original oil can be extracted compared with 35–45% using primary and secondary recovery. The selection of EOR method is a challenging approach (Mandal and Bera 2015). All types of EOR methods are not feasible in all types of reservoir. For that characterization of reservoir rock as well reservoir fluid has great importance. Alkali–surfactant–polymer (ASP) flooding is a promising method under CEOR. The recovery of oil depends on the identification of alkali, surfactant, and polymer in ASP flooding. Since it is a cost-sensitive business, proper selection and formulation of slug is an important criterion (Hazarika and Gogoi 2014; Gogoi 2009). ASP flooding deserves special attention. This method was developed in 1984 by R. C. Nelson, Shell (Kudaibergenov et al. 2015), but only in recent years, it started to be applied throughout the world. In ASP flooding alkali, surfactant and polymer are injected into the reservoir simultaneously or as separated slugs. Addition of surfactant, alkali, and polymer in various combinations improves displacement properties of injected water. Presence of surfactant in injected brine causes decrease of IFT at oil–water interface down to ultra-low values 0.05–0.01 mN/m that allows mobilization of residual discontinuous oil droplets, which coagulate forming oil bank. Addition of high molecular weight polymers causes thickening of the aqueous phase, which leads to more even distribution of the displacement front and, thus, improvement of macroscopic sweep efficiency. Application of alkali is motivated by its interaction with oil and rock. Almost all-natural oils contain active components—organic acids—though their amount and composition vary. Reactions that take place in situ between alkali and organic acids occurring in oil produce surfactants, which cause reduction of IFT at oil water interface (Gogoi 2011). Increase of the amount of organic acids in crude oil increases the efficiency of alkali flooding.
High-molecular-weight polyacrylamide (PAM) has been widely used in chemically enhanced oil recovery (EOR) processes under mild conditions. PAM shows excellent viscosifying criteria. It can significantly increase the viscosity of the displacing fluid and hence decrease the mobility ratio of the process even at very low concentrations. It has resistance to shear degradation and exhibits pseudo-plastic behavior. It is stable over large ranges of pH and less sensitive to mechanical shearing and to divalent ion concentration (hardness) due to its rigid polysaccharide chains. In un-hydrolyzed form, it is generally nonionic in nature. Non-ionic PAM has high adsorption in the porous media, so it is not used for EOR applications (Shah and Schechter 1977). However, most of the currently used CEOR polymers are modified forms of PAM. The amide group present in PAM may undergo hydrolysis at higher reservoir temperature and high pH (Ramirez 1987). The hydrolysis of PAM at high temperature and pH was first observed by Muller in 1980. But at very harsh reservoir condition it may suffer from thermal degradation. Therefore, at mild reservoir condition, PAM is efficient to recover more oil during CEOR process.
Na2CO3 buffering action can reduce alkali retention in the rock formation as well as adsorption of SDS in the porous media. Low tensions required for EOR by alkaline flooding can be achieved with alkaline solutions at pH < 11. Na2CO3 is effective in lowering IFT. Oxidative degradation of polymers may become a serious concern in EOR (Marszall 1977, Alagic and Skauge 2010). Sodium carbonate has been demonstrated to play a key role in stabilizing polymers against multiple sources of degradation. The interfacial viscosity drastically decreases in the presence of Na2CO3. Under favorable conditions, the change may exceed three or four orders of magnitude. Simultaneously, Na2CO3 effectively suppresses the non-Newtonian flow behavior of the interfacial layer (Nagarajan 2002).
The conventional CEOR methods are mainly polymer flooding, and surfactant and alkaline flooding (Samanta et al. 2012). The mechanism of polymer flooding is to reduce the mobility ratio and hence increase the macroscopic sweep efficiency. In some cases the efficiency of polymer flooding may be reduced due to viscosity reduction in presence of reservoir brine and high reservoir temperature. In case of surfactant flooding, recovery can be enhanced by reducing IFT and hence increase the microscopic displacement efficiency. Wettability alteration, IFT reduction by producing in situ surfactant, and reduction of chemical adsorption in CEOR are the main objectives of alkali flooding. The combination of the abovementioned methods for example alkali–surfactant (AS), surfactant/polymer (SP), alkaline/polymer (AP), and alkaline/surfactant/polymer (ASP) slug have proven to be most efficient methods in CEOR. Recently, the use of foam-assisted surfactant and polymer, for improved stability of the injected slug and mobility control, has been studied and found to enhance oil recovery for CEOR processes; nanofluid flooding has been evaluated and explored as a chemical EOR process with field application reported in Colombia (Franco et al. 2017). The mechanism of the improved oil recovery was identified as structural disjoining pressure, wettability alteration, IFT reduction, and improved viscosity of injectant (Nikolov et al. 2019). More recently, the use of nanoparticles to conventional CEOR has been studied and reported to yield novel materials with excellent and improved properties. For example, polymer slug stability can be increased by adding polymeric nanofluids, which can improve rheological properties and stability of the slug for application in the presence of high reservoir temperature and high reservoir brine salinity conditions (Gbadamosi et al. 2018). Furthermore, the adsorption of surfactant can be reduced and efficiency of the system can be improved by the synergistic application of nanoparticles (Yekeen et al. 2018).
In this study, IFTs for different samples were determined by spinning drop Tensiometer using byVonnegut’s formula (Vonnegut 1942). Contact angles of different core samples were determined in KRUSS DSA100 instrument, and hence, wettability was determined from the contact angle values (Gogoi 2007). Relative permeability of wetting and non-wetting phase was measured by JBN method. Core flooding experiment was done to calculate the recovery efficiency for secondary brine flooding, alkali, surfactant, polymer, and combination of these.
Experimental
Materials
The list of materials is shown in Table 1.
Two anionic surfactants namely SDS and SDBS were used, the polymer used was PAM, and the alkali was NaOH, NaHCO3, and Na2CO3. The core samples were collected from a depth of 2315 to 3600 m of the producing horizon of Upper Assam Basin. Liquid paraffin light was selected instead of crude oil because in the existing core flood apparatus, crude oil cannot be flooded. Moreover, liquid paraffin light resembles crude oil in terms of API and viscosity (Hadia et al. 2007). Since the size of core sample is 1.5-inch diameter and 3.5-inch length, it is homogenous in nature. Effective porosity, permeability, and saturation properties are constant throughout the core sample. Since the properties of liquid paraffin which is used in core flooding process resemble the crude oil under study area, there is very negligible effect on Sw, So, recovery efficiency, and viscosity. The list of equipment used in this work is given in Table 2.
Methodology
Interfacial tension (IFT)
The IFT of the aqueous phases, which comprises surfactants or their mixtures and oleic phase which was NH, was determined in spinning drop tensiometer. Calculations were done byVonnegut’s formula (Vonnegut 1942).
In this formula.
\(\sigma\) = IFT in (N/m),
\({R}_{\mathrm{m}}\)=mean radius in (m),
\(\Delta \rho\)= density difference in (kg/m3),
ω = angular velocity in (rad/S).
\({R}_{\mathrm{m}}\) is the radius of the drop at equator as indicated in Fig. 1. This formula has been shown to be valid within 0.1% if the length of the drop exceeds 4 times its diameter. Since the centrifugal acceleration is not constant along the elongated drop, the radius at the tip is not equal to that in center as indicated in Fig. 1 (\({R}_{\mathrm{o}}\) = 2/3 \({R}_{\mathrm{m}}\)).
Surfactants were SDS (S1) or SDBS (S2) or SDS + NaOH (S3) or SDS + Na2CO3 (S4) or SDS + NaHCO3 (S5) or SDBS + NaOH (S6) or SDBS + Na2CO3 (S7) or SDBS + NaHCO3 (S8). The steps for determining IFT are as follows:
-
(a)
IFTs were measured between the aqueous phase comprising SDS dissolved in brine (0 to 4000 ppm) (0.1–0.7% by volume of 0.1 M SDS) and NH and the CMCs were determined at minimum IFTs.
-
(b)
The CMC value of (S1) was added to different concentrations (0.25–1.15% by weight) of NaOH to determine the CMC at minimum IFT.
-
(c)
The CMC value of (S1) was added to different concentrations (0.25–1.15% by weight) of Na2CO3 to determine the CMC at minimum IFT.
-
(d)
The CMC value of (S1) was added to different concentrations (0.25–1.15% by weight) of NaHCO3 to determine the CMC at minimum IFT.
-
(e)
IFTs were measured between the aqueous phase comprising SDBS dissolved in brine (0 to 4000 ppm) (0.1–0.7% by volume of 0.1 M SDBS) and NH and the CMCs were determined at minimum IFTs.
-
(f)
The CMC value of (S2) was added to different concentrations (0.25–1.15% by weight) of NaOH to determine the CMC at minimum IFT.
-
(g)
The CMC value of (S2) was added to different concentrations (0.25–1.15% by weight) of Na2CO3 to determine the CMC at minimum IFT.
-
(h)
The CMC value of (S2) was added to different concentrations (0.25–1.15% by weight) of NaHCO3to determine the CMC at minimum IFT.
Contact angle and wettability
Contact angles of C1-C4 samples were measured in KRUSS DSA100 instrument. From the contact angle values, wettability was determined (Gogoi 2007). Contact angle and wettability is an important criterion for alkali flooding. For chemical EOR process, the reservoir rock should be water wet; otherwise, it should be altered from oil wet to water wet by alkali flooding (Anderson 1987). Wettability depends on contact angle. Before measuring the contact angle, the sample was saturated under vacuum in 3000 ppm brine solution for 3 h; then, the core was centrifuged with crude oil for 30 min (3000 rpm); after that, it was soaked in toluene for 2 s to remove the surface oil under atmospheric pressure. Toluene has the capability of removing oil by dissolving. During this process, toluene could not flush out the oil in the core because core was saturated under vacuum and soaking was done under atmospheric condition. After removing the surface oil, the core was placed in the centrifuge at 3000 rpm for overnight in surfactant solution. Core was placed in core holder of contact angle measuring instrument. One drop of oil from reservoir under study was injected through the needle. The oil drop makes an angle when it has touched the core sample surface. The picture of the oil drop has been taken with the microscope associated with the instrument and tangent was drawn to find the contact angle. The wettability obtained from DSA 100 was validated with the relative permeability graph obtained from JBN method. In relative permeability curve if the crossover point between non-wetting phase and wetting phase is 50% of water saturation (Sw), then the rock is said to be water wet; otherwise, it is oil wet (Anderson 1987). Alkali has a strong role over the alteration of wettability. It changes the wettability from oil wet to water wet which is desirable for ASP flooding (Samanta et al. 2012; Das and Gogoi 2015) (Tables 3, 4, and 5).
Relative permeability
Relative permeability of wetting phase and non-wetting phase can be measured by JBN method. In this method, the capillary effect is neglected; therefore, in case of low flow, this method cannot be used. The experimental data was generated in core flooding instrument made by Gon engineering works (Core flooding Instrument). The experimental data generally recorded includes:
\({Q}_{\mathrm{i}}\)= quantity of displacing phase injected.
Δp = pressure differential
\({\Delta p}_{\mathrm{i}}\)= pressure differential at initial conditions.
\({Q}_{\mathrm{o}}\)= volume of oil produced.
\({Q}_{\mathrm{w}}\)= volume of water produced.
The above data can be analyzed by the technique described by Johnson, Bossler and Nauman and are used to calculate the relative permeability ratio Kro/Krw, the values of Kro and hence Krw, with respect to water saturation (Lui et al. 2010). This method is aimed at giving almost constant outflow values, which is essential for this method. The fractional flow of oil at the core outlet can be calculated by
Relative permeability of oil can be calculated by
Core flooding
Core flooding experiment was done to calculate the recovery efficiency by secondary brine flooding (3000 ppm) and alkali, surfactant, polymer, and combination of these. Pore volume of the rock sample was calculated by saturation method in vacuum chamber. The core was placed in the Hassel core holder in core flooding instrument made by Gon Engineering Works (Core flooding Instrument). Overburden pressure of almost 1000 psi was given to the core to restrict the annulus flow and injection pressure less than overburden was applied. 3000 ppm brine was flooded until a steady flow occurs (10 PV). At that stage we get the absolute permeability of water by collecting the water through outlet for 60 s,; next, we flood the liquid paraffin of viscosity 15 cp until a steady flow of paraffin occurs at the outlet; at that stage, we get the initial water saturation (\({S}_{\mathrm{wi}}\)) and initial oil saturation (\({S}_{\mathrm{oi}}\)). Secondary brine flooding (5 PV) was done to recover the oil; this is known as secondary recovery. This secondary recovery is less than initial oil saturation volume. To recover the residual oil, we have flooded 5 PV of formulated alkali, surfactant, polymer, and combination of these slugs as shown in Table 6. The sequence of flooding was alkali, surfactant, polymer, AS, SP, and ASP one after another. Permeability impairment has detrimental effect on oil recovery. So after each flooding, the core was cleaned in Soxhlet apparatus and made ready for next flooding.
Results
Interfacial tension (IFT)
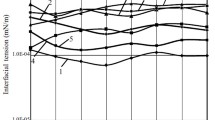
IFT study was done to formulate the concentration of surfactant and alkali for the CEOR slug. We have selected SDS and SDBS as surfactant and Na2CO3, NaOH, and NaHCO3 as alkali. The samples were prepared with different salinities ranging from 1000 to 4000 ppm, because most of the reservoir salinity in this basin is in that range. The CMC value for SDS was determined to be 0.4% (by volume) of 0.1 M SDS solution (Fig. 2). After fixing the SDS concentration added alkali further reduces the IFT. Among the three different alkali, Na2CO3 shows the best result at concentration of 0.85% (by weight) as shown in Fig. 2. This 0.4% (by volume) of 0.1 M SDS + 0.85% (by weight) of Na2CO3 was selected for core flooding experiment. Similarly for SDBS the CMC value was determined to be 0.5% (by volume) of 0.1 M SDBS solution as shown in Fig. 3. Again SDBS with Na2CO3 shows the better result among the three alkalis; the concentration of Na2CO3 at lowest IFT was 1% (by weight) (Tables 7 and 8). Therefore, 0.5% (by volume) of 0.1 M SDBS + 1% (by weight) of Na2CO3 was selected for flooding experiment.
The CMC value of SDS is less than SDBS; this may be due to the high solubility of SDS in the slug than SDBS. The solubility of surfactant whether it is soluble in aqueous or oleic phase will be determined by HLB value. The higher the HLB value is, the more water-soluble the surfactant and the reverse is true for oil soluble. The normal range of HLB value is from 0 to 20 (Wang 2009), but in some cases due to the high solubility of surfactant in water, this value goes to 40 (Tichelkamp et al. 2014). High value of HLB favors the formation of o/w emulsions while low value is for w/o emulsions. Further studies have shown that the HLB for SDS is 40 (Shukla and Rehag 2008) and 10.6 for SDBS (Hunter 1981). The HLB values are in agreement with theory, as SDBS has a longer tail than SDS (more lipophilic than SDS). The type of emulsion formed by the surfactant can be estimated by Bancroft’s Rule, which is based on HLB: “When an interfacial active agent is present along with two immiscible liquids, then after agitation the liquid that is the better solvent appears as the continuous phase” (Brown et al. 2016). The type of emulsion is another criterion for lowering of CMC value. o/w emulsion is more suitable than w/o emulsion in CEOR technique because in case of w/o emulsion there is loss of surfactant and also it changes the system viscosity (Rosen 2004). Critical packing parameter (CPP) is a factor that affects the CEOR process. CPP determines the shape of the surfactant aggregate structure whether it is spherical (CPP < 1) or rod (CPP > 1) in the emulsions. The value of the CPP has been found by Wang and Mittal, for SDS it is 1/3 and for SDBS it is nearly 1.
Contact angle and wettability
Contact angles of N core samples saturated with N crude were determined in KRUSS DSA100. In all the cores, it was observed that the contact angle was more than 50°. Which shows that the reservoir is towards water wet. Contact angles between cores saturated with same crude and SDS + 3000 ppm brine were found to be more than initial contact angles. Added alkali has further increased the contact angles. This shows that using Na2CO3 the reservoir has changed the wettability from intermediate wet to strongly water wet. This is essential for enhanced oil recovery (Saikia et al. 2018). Contact angles of core sample with brine, SDS, and Na2CO3 are shown in Table 3.
SO42− ion of NaC12H25SO4 (SDS) can enhance the surface hydrophilicity of sandstone reservoir, thus enhancing the imbibition capacity of sandstone reservoirs (Rezaeidoust et.al. 2009). pH of the injected CEOR slug determines the presence of chemical groups on the porous media and the polar groups of organic acids and bases (Hua et al. 2015), and then the adsorbing capacity of counter ions on the rock surface and crude oil/CEOR slug interface. The forces responsible for alteration of wettability are mainly Van der Waals’ force, hydrogen bond, Coulomb force, and surface force between groups of rock surface and crude oil. The neutral groups (≡Si—OH) translate into electronegative groups (≡Si—O −) on rock surface on the addition of Na2CO3. The protonation and deprotonation process of organic acids and bases are controlled by pH. The increase of pH that is addition of Na2CO3 to the injected CEOR slug can induce proton transfer reaction. Increase in pH reduces the attractive interaction between the polar groups and rock surface; also, it reduces the Coulomb force between organic bases and rock surface (Austad et al. 2010). Hydrogen-bond interaction between organic acids of crude oil and rock surface translates into electrostatic repulsive force. In due course, hydrophilicity of the rock surface increases due to the desorption of the organic acids and bases from rock surface and hence alters the wettability from less water wet to strong water wet (Al-Khafaji and Wen 2019).
Relative permeability
Relative permeability calculation was done by using the JBN method. Based on the data generated from the relative permeability studies under water–oil system for the core plug of C1, it has been observed that, for this sample, the value of residual oil saturation is 33.38% and value of irreducible water saturation is 24.73%. This indicates that the high value of irreducible water saturation is attributed to the low value of residual oil saturation. The reservoir rock is water wet because the two curves intersect each other at a water saturation of about 63% (beyond 50%). The total oil recovery with brine flooding is about 55.3%. The oil recovery at 0% water cut is 29.4%. Oil recovery is about 43.53% with negligible water cut. So, water flooding is very efficient for this particular core plug. The effective permeability of any reservoir rock to a fluid is a function of the reservoir fluid saturation and the wetting characteristics of the formation. The wetting properties of a reservoir rock that is wettability have a marked effect on the wetting and non-wetting phase relative permeability characteristics of the rock. When both wetting and a non-wetting phases flow together that is at steady state flow condition through porous media, each phase follows separate and distinct paths. Because of attractive forces and the action of capillarity, the wetting phase fluid (water in this case) will preferentially cover the entire solid surface of the reservoir rock and will be held (or tends to occupy) in the smaller pore spaces of the rock. On the other hand, the non-wetting phase (light paraffin) will tend to be expelled/ repelled from contact with the surface of the rock. Thus, at small saturations non-wetting phase will tend to collect in the larger pore openings of the reservoir rock. The wetting phase tends to occupy the smaller pore spaces at small saturation, and these small pore spaces contribute a very little towards total flow; it follows that the non-wetting phase relative permeability has been affected a little extent by the presence of small wetting phase saturation (Heaviside et al. 1987). On the other hand, non-wetting phase occupies the central or large pore spaces which contribute majority to flow through the porous media; smaller non-wetting phase saturation will drastically reduce the wetting phase permeability (Land 1968; Johnson et al. 1958). This is evident from the water–oil relative permeability curve as shown in Fig. 4 as well as from Table 5. The average core and fluid properties of the reservoir under study are shown in Table 4. Same core sample was used for different flooding processes. Core samples were cleaned in the Soxhlet Apparatus for 48 h by liquid–liquid extraction. Toluene and xylene were used to dissolve oil, water, and salt present in the core sample. The Soxhlet apparatus consists of an extraction glassware unit and a heating mantle with thermostatic controller. The core samples are placed in the extractor and cleaned by refluxing solvent. The solvent is heated and vaporized in boiling flasks and cooled at the top by condenser. The cooled solvent liquid falls into the extractor chamber. The cleanliness of the sample is determined from the color of the solvent that siphons periodically from the extractor. This reflux process is continued until no more color change can be seen in the condensed solvent mixture.
Wettability can be interpreted from the relative permeability vs saturation curve. The crossover point between the two relative permeability curves intersects at Sw > 50% as shown in Fig. 4, which indicates that it is water wet (Craig 1971). When the end point relative permeability of water at Sor was determined, it was found to be Sor slightly greater than 30% which can be considered mixed wet or slightly water-wet; initial water saturation (Swi) after the drainage process for the samples was found to be in between 15 and 30% which can again be considered mixed wet (Craig 1971).
Core flooding
In core flooding experiment, after the core being saturated with oil first brine was flooded to recover the oil, which is similar to secondary oil recovery process. The secondary recovery percentage was calculated and is tabulated in Tables 7 and 8. After secondary flooding, the core was flooded with alkali, surfactant, polymer, AS, SP, and ASP slug one after another. Two surfactant SDS and SDBS, one alkali Na2CO3 and PAM as polymer were selected for core flooding operation. Since only one alkali and one polymer were selected for flooding operation, in Table 7 and 8, the recovery percentages for alkali and polymer were the same. Since comparison study of recovery was done in this paper by taking two surfactant SDS and SDBS, so in Tables 7 and 8 same recovery percentage for secondary recovery, alkali flooding and polymer flooding was taken. Permeability reduction during the flooding process was not studied because after each flooding (individual slug) the core was cleaned in Soxhlet apparatus. Since most of the reservoir salinity of Upper Assam basin is in the range of 3000–4000 ppm, so we selected 3000 ppm as the brine salinity for all the slugs. During flooding operation alkali changed the wettability from intermediate to strongly water wet and also reduced IFT. Ultra low IFT was observed in the range of 10−3mN/m when it was measured in between reservoir crude and surfactant. Surfactant increases the microscopic sweep efficiency of the reservoir. Polymer enhances the recovery by increasing the macroscopic displacement efficiency of the reservoir (Needham and Doe 1987). During secondary flooding, we have obtained mobility ratio of about 5 but in case of polymer flooding it was 0.5; hence, macroscopic sweep efficiency was enhanced by polymer flooding (Table 9). Both microscopic displacement efficiency and macroscopic sweep efficiency increase in ASP flooding. The slugs selected for the CEOR core flooding are shown in Table 6.
In all the flooding experiments, we have seen that the recovery was maximum for ASP slugs (Tables 7 and 8). If we compare SDS and SDBS, we have observed that SDS shows better result. Therefore, the best slug for this oil field is 0.4% of 0.1 M SDS + 0.85% of Na2CO3 + 1500 ppm PAM. The initial core properties are shown in Table 4.
Maximum recovery of about 34% initial oil in place was observed for C3 sample. Both the surfactants are anionic in nature. When a charged particle is present in a formulated chemical slug containing large amount of ions, the ions in the slug orient themselves around the charged particle in such a way that they tend to neutralize the electrostatic force. Oppositely charged ions are accumulated as a layer closest to the charge particle. On the other hand, further out from the charged particle both same and opposite ions will accumulate in a layer. The size of the outer layer is larger than the inner layer. The system of formation of two layer around a charge particle is known as electrical double layer (EDL) (Berg 2009, Hunter 1981, Shukla and Rehage 2008). The EDL formed between two SDS or SDBS monomers of same charge at the interface will repeal each other. When NaCl and Na2CO3 were added to the slug, positive and negative ions interact with the double layer and due to the attraction of opposite charges the size of the EDL decreases (Brown et al. 2016). The size of EDL is directly proportional to the CMC as well as IFT of the slug. This is the reason why CMC and IFT values decrease in a chemical slug containing surfactant when salt and alkali are added to the system and hence increase the recovery efficiency.
Discussion
The results of the experimental work highlighted the successful application of the synergic combination of alkali, surfactant, and polymer for improving recovery of residual oil after secondary recovery. In CEOR flooding, water wet condition is more favorable. This is because both capillary and gravity force aid in oil recovery in water-wet pores during CEOR recovery, thus explaining the higher recovery rate. The higher the pH of the slug, the quicker the alteration from less water wet to more water wet condition. The reservoir under study was initially intermediate wet and after alkali flooding it was altered to strongly water wet condition. The primary function of surfactant flooding is to reduce the IFT and hence increase the microscopic displacement efficiency. The selection of surfactant has prime importance in surfactant flooding. Since the reservoir under study is anionic in nature, anionic surfactant is suitable for the study (Hazarika and Gogoi 2021). This will reduce the adsorption of surfactant during flooding and increase the efficiency of the process (Sarmah et al. 2019). In this study comparison was done between SDS and SDBS as two anionic surfactants. For both the surfactants, ultra-low IFT in the range of 10−3 mN/m was achieved, but during core flooding experiments, SDS showed better result than SDBS. Ultra-low IFT was achieved due to the formation of spherical shape aggregation. Hydrophile–lipophile balance (HLB) value is another one most useful parameter for selecting surfactant in CEOR. HLB value gives an idea about the solubility of the surfactant in aqueous and oleic phase (Housaindokht and Nakhaei 2012). Higher HLB value refers to more water soluble and the reverse is true for oil soluble. o/w emulsions can be achieved with high HLB value surfactant while the reverse favors for w/o emulsions. It has been observed that the HLB for SDS is 40 (Kim and Allee 2001). SO42− ion of SDS can enhance the surface hydrophilicity of sandstone reservoir rock, thus enhancing the imbibition capacity of sandstone reservoir and improving the recovery efficiency (Rezaeidoust et.al. 2009). The experimental results tabulated in Tables 7 and 8 show the comparison between different chemical injection processes and their recovery efficiencies. From the abovementioned table and Fig. 5, it is clear that the recovery efficiency is highest for ASP flooding. This may be due to the combined effect of the microscopic displacement efficiency (ED) of the surfactant, and macroscopic sweep efficiency of polymer (Patel et al. 2019). SDS proved to be more efficient than SDBS; this was earlier observed in the scientific literature by Bera et al. (2011; Yu et al. 2011). Though the primary function of alkali flooding is to alter the wettability of the reservoir, alkali can also enhance the microscopic displacement efficiency similar to co-surfactant (Yu et al. 2011). This was reflected in Tables 7 and 8. In this study, an attempt was done to compare three different alkalis with respect to change in wettability and IFT. The effect of Na2CO3 was found to be more effective compared to NaHCO3 and NaOH in terms of IFT (Figs. 2 and 3) and wettability (Table 3). Addition of alkali further reduced the IFT values due to the less adsorption of surfactant on reservoir rock. Alkali can alter the adsorption mechanism from multilayer adsorption to monolayer adsorption (Hazarika et al. 2018). Further alkali can produce in situ surfactant by reacting with naphthenic acid which will further reduce the IFT (Aveyard et al. 1986). Na2CO3 solutions are less corrosive to sandstone. Na2CO3 buffering action can reduce alkali retention in the rock formation. Low tensions required for EOR by alkaline flooding can be achieved with alkaline solutions at pH < 11 (Sheng 2010). When two surfactant monomers with the same electrical charge of the head group are present at an interface, their EDL will interact and repel each other. However, when alkali or salt is introduced to the system, opposite electrical ions will interact with the double layer, decreasing the length of the EDL, and hence the repulsion between the two monomers will reduce (Brown et al. 2016). This is the reason why CMC and IFT as well as microscopic displacement efficiency increase in a surfactant-containing system when alkali is introduced to the system (Hazarika and Gogoi2019). The only polymer used in this study was PAM as PAM was found to be effective in EOR recovery in earlier studies by Raffa et al. (2016; Maia et al. 2009). This may be due to the improvement of the macroscopic sweep efficiency (ES) during the ASP flooding (Mandal 2015). Salinity is one of the most important parameters in chemical flooding; ASP slug salinity should be compatible with reservoir salinity. Interestingly the reservoirs of Upper Assam showed low salinity; in this study, it was found to be 3000 ppm.
Conclusion
Upper Assam basin is one of the most oil-producing provinces in India. It is a good candidate for CEOR. The results of the experimental findings highlighted the successful implementation of the synergic combination of alkali, surfactant, and polymer for improving recovery efficiency of CEOR process. One interesting fact observed was the coincidence of the alteration of wettability from less water wet to more water wet and lowest oil–CEOR slug IFT values due to the addition of alkali to the slug. Ultra-low IFT in the range of 10−3mN/m was observed with SDS and SDBS. Maximum recovery of about 34% initial oil in place was obtained by ASP flooding. The best suited chemical slug for this reservoir is 0.4% of 0.1 M SDS + 0.85% of Na2CO3 + 1500 ppm PAM. The higher recoveries by CEOR slug could be attributed to the reduction of IFT, alteration of wettability, reduction of mobility ratio, enhancement of macroscopic sweep efficiency and microscopic displacement efficiency, etc.
References
Alagic E, Skauge A (2010) A combined low salinity brine injection and surfactant flooding in mixed-wet sandstone Cores. Energy Fuels 24:3551–3559
Al-Khafaji A, Wen D (2019) Quantification of wettability characteristics for carbonates using different salinities. J Pet Sci Eng 173:501–511
Anderson WG (1987) Wettability Literature Survey-Part 5 The effects of wettability on relative permeability. JPT 39:1453–1468
Austad T, Rezaeidoust A, Puntervold T (2010) Chemical mechanism of low salinity water flooding in sandstone reservoirs. Paper presented at the SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA. https://doi.org/10.2118/129767-MS
Aveyard R et al (1986) Interfacial tension minima in oil-surfactant systems: behaviour of alkane-aqueous NaCl systems containing Aerosol OT. J Chem Soc Faraday Trans I 82(1):125–142
Bera A, Ojha K, Mandal A et al (2011) Interfacial tension and phase behavior of surfactant- brine oil system. Colloids Surf A 383(1–3):114–119. https://doi.org/10.1016/j.colsurfa.2011.03.035
Berg JC (2009) An Introduction to Interfaces & Colloids - The Bridge to Nanoscience. World Scientific Publishin Co. Pte. Ltd., pp 804. https://doi.org/10.1142/7579
Brown MA, Goel A, Abbas Z (2016) Effect of electrolyte concentration on the stern layer thickness at a charged interface. Angew Chem Int Ed 55(11):3790–3794
Core flooding Instrument, Gon Engineering Works, Dhanbad, India- 826001.
Craig FF (1971) The reservoir engineering aspects of waterflooding, SPE Monograph 3, Richardson, TX
Das BM, Gogoi SB (2015) Relating IFT with oil recovery with special reference to Bhogpara porous media of Upper Assam basin. J Pet Eng Technol 5:1–8
Franco CA, Zabala R, Cortés FB (2017) Nanotechnology applied to the enhancement of oil and gas productivity and recovery of Colombian fields. J Pet Sci Eng 157:39–55. https://doi.org/10.1016/j.petrol.2017.07.004
Gbadamosi AO, Junin R et al. (2018) Recent advances and prospects in polymeric nanofluids application for enhanced oil recovery. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2018.05.020
Gogoi SB (2007) Study on emulsion flow through porous media in relation to enhanced oil recovery (EOR) process of O.I.L., Duliajan. PhD Thesis.
Gogoi SB (2009) Revisiting Naharkatiya core sample for enhanced oil recovery. Indian J Pet Geol 18:51–61
Gogoi SB (2010) Adsorption of a lignin-based surfactant on Naharkatiya Porous Media. Indian Chem Eng J 52(4):325–335. https://doi.org/10.1080/00194506.2010.616321
Gogoi SB (2011) Adsorption–desorption of surfactant for enhanced oil recovery. Transp Porous Media 90:589–604. https://doi.org/10.1007/s11242-011-9805-y
Grace M 3600 Viscometer, 9434 Katy Freeway, Suite 300, Houstan, TX, USA, 77055
Hadia N, Chaudhari L, Aggarwal A et al (2007) Experimental and numerical investigation of one-dimensional waterflood in porous reservoir. Exp Thermal Fluid Sci 32:355–361. https://doi.org/10.1016/j.expthermflusci.2007.04.009
Hazarika K, Gogoi SB (2014) Comparative study of an enhanced oil recovery process with various chemicals for Naharkatiya oil field. Int J Appl Sci Biotechnol 2(4):432–436. https://doi.org/10.3126/ijasbt.v2i4.11047
Hazarika K, Gogoi SB (2019) Effect of alkali on alkali–surfactant flooding in an Upper Assam oil field. J Pet Explor Prod Technol 10(4):1591–1601. https://doi.org/10.1007/s13202-019-00794-3
Hazarika K, Gogoi SB (2021) Clay analysis of Upper Assam basin for chemical enhanced oil recovery. J Geol Soc India 97:138–144. https://doi.org/10.1007/s12594-021-1644-z
Hazarika K, Kumar R, Gogoi SB et al (2018) Characterization of crude oil for enhanced oil Recovery: study with anionic surfactant. Int J Ambient Energy 38(1):1–12. https://doi.org/10.1080/01430750.2017.1421573
Heaviside J, Brown CE, Gamble IJA (1987) Relative permeability for intermediate wettability reservoirs. SPE Annu Techn Conf Exhib, 27–30 September, Dallas, Texas. SPE 16968 https://doi.org/10.2118/16968-MS
Housaindokht MR, Nakhaei A (2012) Pour, study the effect of HLB of surfactant on particle size distribution of hematite nanoparticles prepared via the reverse micro emulsion. Solid State Sci 14(5):622–625
Hua Z, Mingyuan L, Meiqin L et al (2015) Evaluation of sandstone surface wettability by surface potential. J China Univ Pet (Edition Nat Sci) 39(2):142–150
Hunter RJ (1981) Chapter 2 - Charge and potential distribution at interfaces. Zeta Potential in Colloid Science. Academic Press, pp 11–58
Johnson EF, Bossler DP, Naumann VO (1958) Calculation of relative permeability from displacement experiments. Trans Soc Pet Eng AIME 216:370–372
Kim IB, Allee GL (2001) Effect of carbohydrate sources in phase i and phase ii pig starter diets. Asian-Australas J Anim Sci 14(10):1419–1424
Kudaibergenov S, Akhmedzhanov TK, Zhappasbayev BZH et al (2015) Laboratory study of ASP flooding for viscous oil. Int J Chem Sci 13(4):2017–2025
Land CS (1968) Calculation of imbibition relative permeability for two- and three-phase flow from rock properties. SPE 8(2):149–156. https://doi.org/10.2118/1942-PA
Lui R, Lui H, Li X et al. (2010) Calculation of oil and water relative permeability for extra low permeability reservoir. SPE 131388, CPS/SPE Int Oil Gas Conf Exhib China. https://doi.org/10.2118/131388-MS
Maia AMS, Borsali R, Balaban RC (2009) Comparison between a polyacrylamide and a hydrophobically modified polyacrylamide flood in a sandstone core. Mater Sci Eng C –Biomim Supramol Syst 29:505–09. https://doi.org/10.1016/j.msec.2008.09.018
Mandal A (2015) Chemical flood enhanced oil recovery: a review. Int J Oil Gas Coal Technol 9(3):241–64. https://doi.org/10.1504/IJOGCT.2015.069001
Mandal A, Bera A (2015) Modeling of flow of oil-in-water emulsions through porous media. Pet Sci 12:273–81. https://doi.org/10.1007/s12182-015-0025-x
Marszall L (1977) Bancroft’s rule and emulsion inversion. J Colloids Interf Sci 61(1):202–203
Nagarajan R (2002) Molecular packing parameter and surfactant self-assembly: the neglected role of the surfactant tail. Langmuir 18(1):31–38
Needham RB, Doe PH (1987) Polymer Flooding Review J Pet Technol 39:1503–1507. https://doi.org/10.2118/17140-PA
Nikolov A, Wu P, Wasan D (2019) Structure and stability of nanofluid films wetting solids: an overview. Adv Colloid Interface Sci 264:1–10. https://doi.org/10.1016/j.cis.2018.12.001
Patel HV, Kuipers JAM, Peters E (2019) Effect of flow and fluid properties on the mobility of multiphase flows through porous media. Chem Eng Sci 193:243–254
Raffa P, Broekhuis AA, Picchioni F (2016) Polymeric surfactants for enhanced oil recovery: a review. J Petrol Sci Eng 145:723–733. https://doi.org/10.1016/j.petrol.2016.07.007
Ramirez WF (1987) Application of optimal control theory to enhanced oil recovery. In: Developments in petroleum science, 1st edition. Elsevier, Amsterdam
Rezaeidoust A, Puntervold T, Strand S et al (2009) Smart water as wettability modifier in carbonate and sandstone: a discussion of similarities/differences in the chemical mechanisms. Energy Fuels 23(9):4479–4485
Rosen MJ (2004) Surfactants and interfacial phenomena, 3rd edition. Wiley-Interscience, Hoboken, US
Saikia BD, Mahadevan J, Rao DN (2018) Exploring mechanisms for wettability alteration in low-salinity water floods in carbonate rocks. J Pet Sci Eng 164:595–602
Samanta A, Bera A, Ojha K et al (2010) Effects of alkali, salts, and surfactant on rheological behavior of partially hydrolyzed polyacrylamide solutions. J Chem Eng Data 55:4315–4322. https://doi.org/10.1021/je100458a
Samanta A, Bera A, Ojha K, Mandal A (2012) Comparative studies on enhanced oil recovery by alkali–surfactant and polymer flooding. J Pet Explor Prod Technol 2:67–74. https://doi.org/10.1007/s13202-012-0021-2
Sarmah S, Gogoi SB et al (2019) Formulation of a combined low saline water and polymer flooding for enhanced oil recovery. Int J Ambient Energy. https://doi.org/10.1080/01430750.2019.1683068
Shah DO, Schechter RS (eds) (1977) Improved Oil Recovery by Surfactant and Polymer Flooding. In: AIChE Symposium on Improved Oil Recovery by Surfactant and Polymer Flooding (1976 : Kansas City, Kan.). American Institute of Chemical Engineers, Academic Press, New York
Sheng J (2010) Modern chemical enhanced oil recovery: theory and practice. Gulf Professional Publishing, Texas, USA
Shukla A, Rehage H (2008) Zeta potentials and debye screening lengths of aqueous, viscoelastic surfactant solutions (cetyltrimethylammonium bromide/sodium salicylate system). Langmuir 24(16):8507–8513
Tichelkamp T, Vu Y, Nourani M, Oye G (2014) Interfacial tension between low salinity solutions of sulfonate surfactants and crude and model oils. Energy Fuels 28:2408–2414
Vonnegut B (1942) Rotating bubble method for the determination of surface and interfacial tension. Rev Sci Instrum 13:6–9. https://doi.org/10.1063/1.1134309
Wang Z (2009) Interactions between an anionic fluorosurfactant and a PEO-PPO-PEO triblock copolymer in aqueous solutions. J Surfactants Deterg 13(1):97–102
Yekeen N, Manan MA et al (2018) A comprehensive review of experimental studies of nanoparticles-stabilized foam for enhanced oil recovery. J Pet Sci Eng 164:43–74. https://doi.org/10.1016/j.petrol.2018.01.035
Yu H, Wang Y, Zhang Y et al (2011) Effects of displacement efficiency of surfactant flooding in high salinity reservoir: interfacial tension, emulsification, adsorption. Adv Pet Explor Dev 1(1):32–39. https://doi.org/10.3968/j.aped.1925543820110101.004
Acknowledgements
The authors would like to express their sincere thanks to Dibrugarh University in carrying out the experiments.
Funding
The study was financially supported by DST Project No SB/S3/CE/057/2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Amjad Kallel
Rights and permissions
About this article
Cite this article
Hazarika, K., Borgohain Gogoi, S. Laboratory investigation of chemical enhanced oil recovery, with special reference to Upper Assam Basin. Arab J Geosci 15, 984 (2022). https://doi.org/10.1007/s12517-022-10268-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-10268-2