Abstract
Soil fertility has become a major issue in the Loess Plateau, China. The present study explored the effects of maize straw biochar application on soil nitrogen (N) fractions, microbial biomass carbon (C), and wheat yields in a calcareous, sandy loam soil in the Loess Plateau region. Six maize straw biochar (BC) application rates were applied to the soil in July 2015, including control with no biochar (CK), BC1 (10 t ha−1), BC2 (20 t ha−1), BC3 (30 t ha−1), BC4 (40 t ha−1), and BC5 (50 t ha−1). Wheat was cultivated in the amended soil for 5 years using routine mineral N and P fertilization practices. Four to 5 years after biochar application, the soil contents of total N, microbial biomass C, and amino acid N significantly increased by 9.0–30.9%, 55.1–81.4%, and 64.5–68.2% (in 2019) and 6.5–10.9%, 68.6–139.7%, and 66.9–77.2% (in 2020), respectively, as compared to CK. Moreover, the content of unknown-acidolyzable nitrogen decreased by 45.0–63.1% (in 2019) and 83.5–89.6% (in 2020) compared with CK, respectively. Application of BC3 increased the total acidolyzable nitrogen, acidolyzable ammonium nitrogen, and amino-acid nitrogen contents in 0 to 30-cm soil layer by 6.3–7.8%, 23.0–25.2%, and 62.2–0.9% (in 2019) and 14.7–18.0%, 23.5–29.0%, and 41.9–107.6% (in 2020), respectively, as compared with CK. However, after 4 and 5 years, nonacid hydrolyzed N was the highest in BC5 (50 t ha−1) treatment, which increased by 27.0% and 44.8%, respectively, compared to the CK, while after 5 years, it was the lowest in BC3 (30 t ha−1) treatment, decreased by 35.4%. After 5 years, all biochar treatments significantly improved wheat yields compared to CK. The highest wheat yield was obtained in the BC3 treatment, which was 21.6% and 24.8% higher than the CK in years 4 and 5, respectively. In conclusion, the application of biochar as a soil conditioner can significantly affect the soil total and organic N fractions and microbial biomass after aging for 4–5 years and has a positive effect on improving soil nutrient supply capacity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen is one of the main factors limiting soil fertility and crop growth (Li et al. 2018). Soil organic nitrogen is one of the major forms of soil nitrogen (Dempster et al. 2012). Its chemical cycle, i.e., nitrogen fixation, nitrification, assimilation, ammonification, and denitrification are important factors affecting soil nitrogen availability and play an important role in maintaining soil nitrogen supply (Song et al. 2020). Soil microbial biomass, as one of the important sources of nutrients needed for plant growth, is an important driving factor for the soil nutrient transformation cycle (Wang et al. 2017). Most Chinese soils have low organic matter content and fertility status, so incorporation of crop residues, i.e., straw to the field is a popular soil fertilization practice in the early stages of rural China (Hu et al. 2020). China has a lot of straw resources, and straw is considered nutrient-enriched agricultural residues. In 2014, the annual production of straw was around 700 million tons, and during the years 1995–2005, about 62% of all straw was burnt in the field (Ren et al. 2019). Direct incorporation of straw into the soil in agriculture systems, on the other hand, would enhance the content of soil organic carbon, foster microbial decomposition, and emit GHG, i.e., N2O, CH4, CO2, and other GHG, resulting in soil nutrient depletion and significant global warming effects (Jing et al. 2020; Song et al. 2020).
Biochar is a highly aromatic carbon-containing solid substance produced by pyrolysis in the absence of oxygen or hypoxia, which has the characteristics of large specific surface area, adsorption, strong stability, and rich porosity (Haider et al. 2021a, b, c). In order to enhance the resource utilization of straw, crop straw can be processed into biochar (Hu et al. 2020). Straw biochar has been shown to enhance the content of soil organic matter while also maintaining soil quality, and straw-derived biochar has been shown to improve carbon sequestration in agricultural soils (El-Naggar et al. 2019; Jing et al. 2020). In recent years, the incorporation of biochar, as a new type of soil fertilizer improvement material, has become a trending research topic (Gul et al. 2015; Hu et al. 2020; Khan et al. 2021). Some studies suggest that the addition of biochar to agricultural soil can enhance the supply of nutrients in the soil for sustainable plant growth (Sarma et al. 2018; Haider et al. 2021b).
Nitrogen is needed for the metabolism of nucleic acid, enzymes, and nucleotides in plants, as well as for energy transfer (Manirakiza et al. 2019). The use of nitrogen is an essential process in the mineralization of organic carbon (Yang et al. 2019). Nitrogen can enhance the enzyme production and fertility status of soil, allowing carbon to be mineralized more effectively (Nieder et al. 2011). Nitrogen is the most limiting factor for microbial activity in semiarid and arid tropical zones of the world (Xu et al. 2003; Pokharel et al. 2020). Previous studies have indicated that adding biochar to agricultural soils may alter conditions that influence denitrification, nitrification, and other nitrogen transformation loss processes (Lehmann et al. 2011; Gul et al. 2015; Xu et al. 2016). Similarly, soil enzymes play an important role in the metabolic process that takes place in the soil, influencing the nutrient cycling, i.e., nitrogen and decomposition of soil carbon and nutrients (Oladele et al. 2019). Many factors influence the impact of biochar inclusion on soil enzyme production, including the form of biochar used, the relationship between enzymes and biochar, and the soil environment (Lehmann et al. 2011; Haider et al. 2021c).
Due to the prominent role of N nutrition in regulating crop production in semiarid and arid regions, the effect of biochar on soil nitrogen transformation processes are extremely important, as it must be replenished either as inorganic nitrogen fertilizer or cycled back to the soil in a complex organic form such as crop residues (Prommer et al. 2014). In the past few decades, research has focused on organic turnover mechanisms in the soil N cycle, such as organic matter depolymerization, which is currently thought to be the rate of limiting phase of dissolved organic N production (Hu et al. 2020). Biochar addition to soil may have a significant influence on the turnover of soil organic N fraction due to the properties stated above. Due to the numerous simultaneous soil N processes (Fig. 1), measuring and interpreting soil N transformation rates is complex.
Effects of biochar addition on soil enzyme production. Soil type, soil physical, chemical properties, and exogenous additives can affect soil enzyme activity. Adding an appropriate amount of biochar to the soil promotes enzymatic reaction by adsorbing oligomers in the soil, thereby increasing soil enzyme activity or enzyme production. However, excessive biochar addition inhibited the enzymatic reaction by protecting the binding sites of the enzymatic reaction, thereby reducing soil enzyme activity or enzyme production (modified from Prommer et al. 2014)
The biochar application strongly impacts the activities of soil enzymes (Hu et al. 2020; Song et al. 2020). Although the studies on the impact of biochar application on soil under conventional fertilizer application are available (Xu et al. 2003; Li et al. 2004; Jia et al. 2017); however, the effect of biochar application on various soil organic nitrogen fractions and crop yield in the dry farmland in a semiarid region like Loess Plateau is not clear. As a result, it is critical to investigate the effects of biochar amendments on nitrogen transformation processes, particularly dry farmland in a semiarid region like Loess Plateau soils where nitrogen fertilizer use is excessive, to designate appropriate times for nitrogen addition. Therefore, this study was conducted to evaluate the effect of biochar incorporation on soil organic nitrogen fractions, activities of soil enzymes, and soil carbon mineralization in dry farmland on the Loess Plateau. It was hypothesized that the application of biochar may significantly improve soil total nitrogen content and soil microbial biomass due to biochar’s alkaline nature and porous structure.
Materials and methods
Study site
The experiment was conducted at Lijiabu Town, Anding District, Dingxi City, Gansu Province, China (35°28′N, 104°44′E) (Fig. 2). It belongs to the hilly and gully region of the Loess Plateau and is also a typical dry farming area with an average altitude of 2000 m with average annual precipitation of 521 mm. The precipitation is mainly concentrated in the months of July–September. The average annual temperature of this region was about 6.5 °C, and the average sunshine duration was about 2476.6 h each year. The experimental soil belonged to Loessial soil (Chinese Soil Taxonomy Cooperative Research Group 1995), which equates to Calcaric Cambisols in WRB soil classification (FAO 1990). The soil used was typically cultivated soils in the Loess Plateau of Northern China, i.e., calcareous, sandy loam soil with low organic matter and fertility. The experimental soil had soil pH 8.4, average bulk density 1.2 g cm−3, wilting moisture 7.3%, saturated moisture 21.9%, organic matter content 12.0 g kg−1, total nitrogen 0.8 g kg−1, and total phosphorous (P2O5) 1.8 g kg−1.
Experimental design and materials
The experiment was set up in 2015 as a single factor random block design comprising of 6 biochar application levels, i.e., CK (0 t ha−1), BC1 (10 t ha−1), BC2 (20 t ha−1), BC3 (30 t ha−1), BC4 (40 t ha−1), and BC5 (50 t ha−1). Each treatment was repeated 3 times in different test blocks, with a total of 18 plots, with an area of 16.8 m2 (2.8 m × 6 m). The amount of biochar was calculated by natural air-dried weight. In March 2015, biochar was evenly spread into each plot according to the experimental design, and then it was incorporated into the plowing layer of soil with a rotary tiller (about 20 cm). From 2015 to 2020, the bread wheat variety “Dingxi 40” was planted in March and harvested in July. The wheat was seeded using a seeding rate of 188 kg ha−1 in a 20-cm spaced row set with a sowing depth of 7 cm. Before sowing in each year, urea 228 kg ha−1 (having 46% N) and superphosphate 750 kg ha−1 (having 14% P2O5), were applied accordingly to the conventional fertilizer rate, and the wheat was plowed according to the conventional farming method before sowing and after harvest (about 20 cm). At the end of July every year, the mechanical conventional method was used to harvest wheat, and the wheat in each plot is naturally air-dried and threshed, and the yield is recorded and measured. From 2015 to 2018, the experiment did not measure soil organic nitrogen fractions and microbial biomass and other related indicators.
The applied biochar was purchased from Liaoning Jinhefu Agricultural Science and Technology Cooperation Limited, which was prepared by pyrolysis of maize straw under 500 °C under anaerobic conditions, and 35% of the biomass could be converted into biochar. The basis properties of biochar were as follows; pH 9.2, cation exchange capacity 25.2 c mol kg−1, specific surface area 11.3 m2 g−1, soluble organ carbon content 432.4 mg kg−1, natural air-dried water content 5.1%, carbon content 53.3%, nitrogen content 1.0%, phosphorous content 0.3%, potassium content 0.5%, calcium content 0.8%, magnesium content 0.5%, and ash content 35.6%.
Collection and analysis of soil samples
After the harvesting of wheat in August 2020, soil samples were collected from 0 to 5 cm, 5 to 10 cm, and 10 to 30 cm depth by the 5-point method. After mixing, the samples were divided into two parts by quartile method, one of which was naturally dried through 0.25 mm and 0.15 mm and stored in a sealed bag, and other fresh samples were sieved through 2 mm and stored in an aseptic bag at 4 °C.
The air-dried soil samples (0.149 mm sieve) were weighed to determine the soil moisture contents. The soil sample was put into the bottom of the drying digestion tube, and a small amount of nonionic water (0.5–1.0 mL) was added to moisten the sample. The accelerant (2 g) and concentrated sulfuric acid (5 mL) were added and shaken well. The digestion tube was placed on the infrared digestion furnace for digestion. When the digestion liquid and soil particles were all turned gray and slightly green, they were continued to digest for 1 h. The cooled digestion tube was distilled directly into the Kjeldahl nitrogen analyzer, and the total nitrogen (TN) was determined by the Kjeldahl digestion method (Bremmer and Mulvaney 1982).
Soil microbial biochar (MBC) and microbial biomass nitrogen (MBN) were determined using fumigation-extraction methods based on the difference between C or N extracted with 0.5 mol·L−1 K2SO4 through chloroform-fumigation and unfumigated soil samples, using KEC (0.45) and KEN (0.54) factors, respectively (Varma and Oelmüller 2007).
Soil organic nitrogen fractions were determined by the Bremner method (Bremner 1965). For the preparation of soil acid hydrolysate, the sample soil of about 10.00 g was allowed to be sieved by passing through a 100-mesh sieve. The soil was put into a triangular flask with a grinding joint, n-octanol (2 drops) and 6 M HCI (20 mL) were added to the flask. The bottle was shaken to mix the soil with acid. The bottle was placed into a far-infrared digestion furnace with a temperature control function, installed in a condensing tube with glass grinding joint at the bottle mouth, and was connected to condensed water. After hydrolysis, the sample was filtered while hot with medium speed blue ribbon filter paper, and the filtrate was collected in a 100 mL beaker, and the remaining residue was washed with deionized water to make the volume of filtrate 60 mL. The beaker containing the filtrate was incubated in an ice bath; 5 M NaOH was added drop by drop into the solution and stirred to reach pH 5. The pH was neutralized with 0.5 M NaOH to the pH of 6.5 ± 0.1.
For the determination of total nitrogen acidolysis (TAN), acidolysis solution (5 mL) was sucked with a pipette with a wide end and was put into the digestion tube, nitrogen-fixing mixed catalyst (0.5 g) and concentrated sulfuric acid (2 mL) were added, and the mixture was heated at 380 °C until the digestion solution was clear. After cooling, the digestion tube was connected with the Kjeldahl nitrogen apparatus to estimate the TAN.
For the determination of acid hydrolyzed ammonia nitrogen (AMN), neutralized acidolysis solution (10 mL) was taken and put into the digestion tube, 3.5% MgO suspension (2.5 mL) was added, and AMN was determined with the Kjeldahl nitrogen determinator.
For the determination of acid-hydrolyzed ammonia nitrogen, neutralized acid hydrolysate (10 mL) was taken into the digestion tube; phosphoric acid borax buffer (pH 11.2; 10 mL) was added. The acid-hydrolyzed ammonia nitrogen was determined with the Kjeldahl nitrogen determinator. Amino-sugar nitrogen was calculated by subtracting acid-hydrolyzed ammonia nitrogen from the determination result.
For the estimation of amino acid nitrogen (AAN), neutralized acid hydrolysate (5 mL) was taken into a 50-mL small beaker, and 0.5 M NaOH solution (1 mL) was added. The sample was heated in 100 °C boiling water until the solution was reduced to 2 mL. After cooling at room temperature, citric acid (1 g) and hydrated ninhydrin (0.2 g) were added. The beaker was incubated at 100 °C in a water bath for 10 min. The mixture solution (10 mL) was taken, phosphoric acid borax buffer (20 mL), and 5 M NaOH (2 mL); the solution mixture was distilled for 4 min and was then titrated.
The unknown nitrogen (UAN) was calculated using the following formula:
The nonacid nitrogen was calculated by subtracting the total nitrogen acidolysis from the total nitrogen (TN).
Statistical analysis
The observed parameters were sorted by Excel 2016, the figures were developed out by Sigma-Plot 12.5, and the data were analyzed statistically by SPSS 19.0 software. Duncan’s new multiple range test was used for mean separation at p ≤ 0.05 level. The correlation attributes were calculated by the Pearson method.
Results
Total soil nitrogen
Biochar treatments had a significant effect on soil nitrogen content, which was significantly higher in the 0 to 5-cm soil layer as compared to the 10 to 30-cm soil layer (Table 1; Fig. 3). The total nitrogen contents of BC4 and BC5 treatments in the 0 to 5-cm soil layer in 2019 were significantly improved by 10.7% and 11.4%, respectively as compared to control treatment (p < 0.05, Fig. 3a). The total nitrogen content of BC4 and BC5 treatments increased by 9.8% and 10.9%, respectively, as compared to control in 2020 (p < 0.05, Fig. 3b). In the 5–10 cm layer, the total nitrogen content of BC3, BC4, and BC5 treatments in 2019 increased by 26.4%, 28.8%, and 30.9%, respectively, higher than control (p < 0.05, Fig. 3a), and the total nitrogen content of BC3, BC4, and BC5 treatments increased by 6.55%, 8.75%, and 10.25%, respectively, higher than control in 2020 (p < 0.05, Fig. 3b). In the 10 to 30-cm soil layer, the total nitrogen content of BC2, BC3, BC4, and BC5 treatments in 2019 were increased by 7.7%, 7.9%, 8.3%, and 9.0%, respectively, higher than control (p < 0.05, Fig. 3a), the total nitrogen content of BC2, BC3, BC4, and BC5 treatments were increased by 3.6%, 6.6%, 8.8%, and 10.3%, respectively, higher than control in 2020 (p < 0.05, Fig. 3b). However, there was no significant difference in total nitrogen content between the 2 years under BC1 treatment (p < 0.05, Fig. 3a; p < 0.05, Fig. 3b).
Effects of biochar addition on soil total nitrogen in different soil layers during a 2019 and b 2020. The results are the averages and standard deviations of three replicates. Bars sharing the same case letters, for interaction and main effects for a parameter, do not differ significantly at p ≤ 0.05. CK = without biochar; BC1 = 10 t ha−1; BC2 = 20 t ha−1; BC3 = 30 t ha−1; BC4 = 40 t ha−1; and BC5 = 50 t ha−1
Soil organic nitrogen fractions
Total acidolyzable nitrogen
The effects of biochar on the grouping of total acidolyzable nitrogen are given in Figs. 4 and 5. In 2019 and 2020, total acidolyzable nitrogen in the 0 to 30-cm soil layer increased firstly and then decreased with an increase of biochar addition and showed a decreasing trend within the deepening of the soil layer (Fig. 4a). The total acidolyzable nitrogen in the 0 to 30-cm soil layer ranged from 0.7 to 0.8 g kg−1 in 2019 and from 0.6 to 0.8 g kg−1 in 2020, respectively, accounting for a 69.8 to 88.2% increase in 2019 and a 66.1% to 86.9% increase in 2020, respectively, of total acidolyzable nitrogen in soils. The addition of biochar was greater than or equal to BC2, the total acidolyzable nitrogen content was significantly increased (p < 0.05). In 2019 and 2020, BC3 treatment increased the total acidolyzable nitrogen content in the 0 to 30-cm soil layer by 6.3–7.8% and 14.7% and 18.0% compared with control (p < 0.05), followed by BC4 and BC3 treatment in 2019, BC3 and BC4 treatment in 2020, and BC5 increased the last.
Effects of biochar addition on soil total acidolyzable N (a), amino acid N (b), and acidolyzable ammonia N (c) in different soil layers during years 2019 and 2020. The results are the averages and standard deviations of three replicates. Bars sharing the same case letters, for interaction and main effects for a parameter, do not differ significantly at p ≤ 0.05. CK = without biochar; BC1 = 10 t ha−1; BC2 = 20 t ha−1; BC3 = 30 t ha−1; BC4 = 40 t ha−1; and BC5 = 50 t ha−1
Effects of biochar addition on soil amino-sugar N (a), unknown-acidolyzable N (b), and non-acidolyzable N (c), in different soil layers during years 2019 and 2020. The results are the averages and standard deviations of three replicates. Bars sharing the same case letters, for interaction and main effects for a parameter, do not differ significantly at p ≤ 0.05. CK = without biochar; BC1 = 10 t ha−1; BC2 = 20 t ha−1; BC3 = 30 t ha−1; BC4 = 40 t ha−1; and BC5 = 50 t ha−1
Amino acid nitrogen
The amino acid nitrogen contents in soil increased with the increase of biochar supplemental level, and the highest content was observed in BC5 as compared to other treatments (Fig. 4). In 2019 and 2020, the variation range of amino acid nitrogen contents in the 0- to 30-cm soil layer was 0.2 to 0.5 g kg−1 and 0.2 to 0.5 g kg−, respectively, accounting for 28.6–43.9% and 27.5–45.1% of total soil nitrogen, respectively (Tables 1 and 2). Compared with control treatment, in the 0 to 5-cm soil layer, when biochar supplemental level was greater than BC1, amino acid nitrogen contents in soil significantly increased by 14.8–68.2%, respectively, in 2019 and 12.4–77.2%, respectively, in 2020 (p < 0.05); in the 5 to 30-cm soil layer, when biochar supplemental level was significantly greater than or equal to BC2, amino acid nitrogen contents in soil significantly increased by 15.1–64.5%, respectively, in 2019 and 17.6–66.9%, respectively, in 2020 (p < 0.05). In addition, the amino acid nitrogen contents in the 0 to 30-cm soil layer were increased by 4.4–6.7% in 2020 as compared with that in 2019.
Acidolyzable ammonia nitrogen
The acidolyzable ammonia nitrogen contents in soil were firstly increased and then decreased with the increase of biochar supplemental level (Fig. 4; 2019c; 2020c). In 2019 and 2020, the variation range of acidolyzable ammonia nitrogen contents in the 0 to 30-cm soil layer was 0.2 to 0.3 g kg−1 and 0.2 to 0.3 g kg−1, respectively, accounting for 17.9 to 27.5% and 19.0 to 28.7% of total soil nitrogen (Tables 1 and 2). Compared with control, BC3 treatment enhanced the acidolyzable ammonia nitrogen contents in soil by 23.0% and 23.2% in 2019 and 23.5% and 26.4% in 2020 in the 0 to 5-cm and 10 to 30-cm soil layers, respectively (p < 0.05), followed by BC2 treatment, which increased the acidolyzable ammonia nitrogen contents by 16.9% and 15.9% in 2019 and 16.3% and 15.5% in 2020 in the 0 to 5-cm and 10 to 30-cm soil layers, respectively (p < 0.05). In the 5 to 10-cm soil layer, compared with control, the acidolyzable ammonia nitrogen contents in the BC3 treatment increased by 25.2% in 2019 and 29.0% in 2020, respectively. However, under BC5 treatment, soil acidolyzable ammonia nitrogen contents were significantly decreased by 13.7 to 17.5%, respectively, in 2019 and 7.5 to 10.6%, respectively, in 2020. There was no significant difference in the soil acidolyzable ammonia nitrogen contents between BC1 and BC4 treatments.
Amino-sugar nitrogen
The application of biochar caused a significant increase in the amino-sugar nitrogen content (Fig. 5; 2019a; 2020a). In 2019 and 2020, the variation of amino-sugar nitrogen content in the 0 to 30-cm soil layer was 0.02–0.07 g kg−1 and 0.03–0.09 g kg−1, respectively, accounting for 2.5 to 7.8% in 2019 and 2.8 to 8.9% in 2020 of the total nitrogen contents in soil (Tables 1 and 2). BC3 treatment had the highest content of amino-sugar nitrogen in the 0 to 30-cm soil layer, which increased by 62.2 to 97.3% in 2019 and 91.9 to 107.6% in 2020 (p < 0.05), followed by BC4 treatment, that increased by 44.3–78.7%, respectively, in 2019 and 72.8–77.7%, respectively, in 2020 (p < 0.05). The amino-sugar nitrogen contents in BC2 treatment increased by 34.6–60.6%, respectively, in 2019 and 56.9–67.2%, respectively, in 2020, while in the BC1 treatment, only the 0 to 5-cm soil layer amino-sugar nitrogen content significantly increased by 45.3% in 2019 and 41.5% in 2020 (p < 0.05). The amino-sugar nitrogen contents under BC5 treatment, in the 0 to 30-cm soil layer increased by 29.2–70.6%, respectively, in 2019 and 43.6–57.7%, respectively, in 2020. However, the difference was nonsignificant compared with that of control in the 5–10 cm layer in 2019.
Unknown-acidolyzable nitrogen
Unknown-acidolyzable nitrogen contents showed a decreasing trend with the increase of biochar supplemental level (Fig. 5). In 2019 and 2020, the variation range of unknown-acidolyzable nitrogen contents in the 0 to 30-cm soil layer was 0.08–0.22 g kg−1 and 0.02–0.16 g kg−1, respectively, accounting for 8.0–24.5% and 1.6–18.5% of total nitrogen in the soil (Tables 1 and 2). Compared with control, unknown-acidolyzable nitrogen contents in BC3, BC4, and BC5 treatments was significantly decreased, among which BC5 treatment had the largest decreases of 45.0–63.1% in 2019 and 83.5–80.7% in 2020 (p < 0.05), followed by BC4 treatment by 35.2–57.6% in 2019 and 62.6–80.7% in 2020; in BC3 treatment, the reduction rate was 43.4–54.8% in 2019 and 42.0–48.1% in 2020. Similarly, in BC2 treatment, the unknown-acidolyzable nitrogen contents of 5–30 cm soil were significantly reduced by 26.9–40.5%. The effect of BC1 treatment on unknown-acidolyzable nitrogen contents in soil from the year 2019 to 2020 remained nonsignificant.
Non-acidolyzable nitrogen
In 2019 and 2020, the variation range of non-acidolyzable nitrogen contents in the 0 to 30-cm soil layer was 0.15–0.24 g kg−1 and 0.13–0.33 g kg−1, respectively, accounting for 17.3–25.9% and 15.5–31.1% of the total nitrogen content in soil (Tables 1 and 2). With the increase of the biochar addition, the non-acidolyzable nitrogen contents in the 0 to 30-cm soil layer firstly decreased and then increased and showed a downward trend with the deepening of the soil layer (Fig. 4; 2019c; 2020c). In the 0 to 10-cm soil layer compared with the control treatment, the non-acidolyzable nitrogen contents in the 5 to 10-cm soil layer of BC5 treatment increased by 44.8% in 2019, and there was a nonsignificant difference in the non-acidolyzable nitrogen contents in the other biochar treatments (p < 0.05). Correspondingly, in the 10 to 30-cm soil layer, compared with the control treatment, the non-acidolyzable nitrogen contents in BC2 and BC5 treatment increased by 21.4% and 26.9%, respectively, in 2019, and the non-acidolyzable nitrogen contents in BC2 and BC3 treatments decreased by 30.4% and 35.4%, respectively, in 2020 (p < 0.05).
Proportion of soil organic nitrogen fractions to soil total nitrogen
In 2019 and 2020, the proportion of organic nitrogen fractions in soil treated by biochar to soil total nitrogen was in descending order as follows; amino acid nitrogen > non-acidolyzable nitrogen > acidolyzable ammonium nitrogen > unknown-acidolyzable nitrogen > amino-sugar nitrogen (Table 2). In the 0 to 30-cm soil layer, the ratio of soil amino acid nitrogen to soil total nitrogen was increased gradually with the increase of biochar supplemental level. Compared with control, the ratio of soil acidolyzable ammonium nitrogen content to soil total nitrogen in BC3, BC4, and BC5 treatments increased significantly, among which BC5 treatment increased by 14.1–16.6% in 2019 and 14.1–16.6%, respectively, in 2020 (p < 0.05). The proportion of acidolyzable ammonium nitrogen content to soil total nitrogen showed a trend of increase and then decrease. Compared with control, BC3 treatment showed the highest increase, increasing by 13.5–16.0%, respectively, in 2019 and 15.5–21.0%, respectively, in 2020 (p < 0.05). BC5 treatment showed the highest decrease, decreasing by 22.3–24.3%, respectively, in 2019 and 12.5–16.6%, respectively in 2020 (p < 0.05). The proportion of unknown-acidolyzable nitrogen content in soil total nitrogen showed a decreasing trend. In 2019, the proportion of unknown-acidolyzable nitrogen content in soil total nitrogen of other biochar treatments decreased significantly except BC1 treatment, which showed a nonsignificant difference compared with the control. In 2020, the proportion of unknown-acidolyzable nitrogen content to soil total nitrogen in BC3, BC4, and BC5 treatments significantly decreased, and BC5 treatments showed the highest decrease by 49.6–67.1% in 2019 and 84.9–90.7% in 2020, respectively. The proportion of amino-sugar nitrogen to total nitrogen in soil showed a trend of first increasing and then decreasing, with the highest increase in BC3 treatment, 50.3–81.5% in 2019 and 79.8–93.1% in 2020, respectively, and the highest increase in the 0 to 5-cm soil layer was observed. The ratio from non-acidolyzable nitrogen to total nitrogen in soil layers increased by 16.3% in 2019 in BC5 treatment, decreased by 20.9% and 32.9% in BC2 treatment, and decreased by 38.2% and 18.6% in BC3 and BC4 treatments, respectively.
Soil microbial biomass carbon and nitrogen
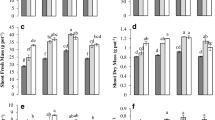
Compared with control, the addition of biochar significantly increased the soil microbial biomass carbon in the 0 to 30-cm soil layer in 2019 (Fig. 6a and b). The maximum increase of microbial biomass carbon in BC5 treatment was 55.1–81.4% (p < 0.05). However, in the 0 to 30-cm soil layer, the soil microbial biomass carbon content decreased in 2020 compared with that in 2019. In the 0 to 5-cm soil layer, the soil microbial biomass carbon content decreased in 2020 compared with that in 2019. In the 0 to 5-cm soil layer, the soil microbial biomass content of BC1 treatment did not differ significantly compared with control. In the 5 to 30-cm soil layer, the soil microbial biomass content of BC4 treatment had a nonsignificant difference compared with control. In addition to the above conditions, other biochar treatments could increase the soil microbial biomass carbon content in 0 to 30-cm soil layer, and the increase of microbial biomass carbon content in BC5 treatment was 68.6–139.7%. Compared with control in 2019, soil microbial biomass nitrogen content in biochar treatment improved significantly (Fig. 6). BC3 treatment had the highest increase in soil microbial biomass nitrogen contents, increasing by 66.3–78.9%, respectively, while BC4 treatment had a small increase in soil microbial biomass nitrogen content increasing by 27.0–42.1%, respectively, in 2019–2020. In 2020, the 0 to 5-cm soil layer showed a trend of the first decrease and then increase, and the 5 to 10-cm and 10 to 30-cm soil layers showed a trend of the first decrease, then increase and then again decrease trends, and the minimum values were all presented in BC2 treatment. BC2 treatment in the 10 to 30-cm soil layer significantly reduced 27.7%, compared with control, and a nonsignificant difference was found in other treatments compared with control (Fig. 6). The soil microbial biomass nitrogen content in the 0 to 30 cm soil layer decreased compared with that in 2019. In 2019 and 2020, the variation range of BC/BN in the 0 to 30-cm soil layer was 11.0–12.4% in 2019 and 2.9–3.6%, respectively, in 2020 as compared to control (Table 3). Compared with control, the addition of biochar in 2020 increased the BC/BN in the 0 to 30-cm soil layer (Table 3).
Effects of biochar addition on soil microbial biomass carbon (a) and soil microbial nitrogen (b) in different soil layers during years 2019 and 2020. The results are the averages and standard deviations of three replicates. Bars sharing the same case letters, for interaction and main effects for a parameter, do not differ significantly at p ≤ 0.05. CK = without biochar; BC1 = 10 t ha−1; BC2 = 20 t ha−1; BC3 = 30 t ha−1; BC4 = 40 t ha−1; BC5 = 50 t ha−1. MBC, microbial biomass carbon; MBN, microbial biomass nitrogen
Wheat yield
The addition of biochar caused a significant increase in wheat yield to varying degrees (Table 1; Fig. 7). In 2019 and 2020, the yield of wheat under the treatment of biomass charcoal was higher than that of CK. However, in 2019, only under the BC3 treatment, the wheat yield significantly increased by 21.6% compared with CK; and in 2020, the biomass charcoal treatment significantly increased the wheat yield compared with CK. The BC1, BC2, BC3, BC4, and BC5 treatments caused a 5.2%, 17.9%, 24.8%, 14.6%, and 10.3% increase in wheat grain yield, respectively, compared with control.
Effects of biochar addition on wheat yield during years 2019 and 2020. The results are the averages and standard deviations of three replicates. Bars sharing the same case letters, for interaction and main effects for a parameter, do not differ significantly at p ≤ 0.05. CK = without biochar; BC1 = 10 t ha−1; BC2 = 20 t ha−1; BC3 = 30 t ha−1; BC4 = 40 t ha−1; BC5 = 50 t ha−1
Correlation of soil organic nitrogen fractions with soil total nitrogen and microbial biomass carbon and nitrogen
In 2019, soil MBC content had a significantly positive correlation with soil AAN, AIN, and ASN content (p < 0.01), and a significant negative correlation with soil UAN content (p < 0.01; Table 4). However, correlations between soil MBC content and soil AMN and ASA in 2020 were nonsignificant. In 2019, soil MBN content was positively correlated with AAN, AIN, and ASN contents (p < 0.01), and with AMN content (p < 0.05), and were negatively correlated with the UAN content (p < 0.01). In 2020, there was a positive correlation between MBN content and AIN content (p < 0.01). There was a significant positive correlation between TN content and AAN and AIN content in 2 years (p < 0.01), and a significant negative correlation between TN content and UAN content in 2 years (p < 0.01).
Discussion
After 5 years, maize straw biochar amendment significantly affected soil total N, organic N fractions, microbial biomass C, and wheat yields. Soil total N increased with increasing biochar application rate, with the highest content in the BC5 treatment (Fig. 3). Biochar has a large specific surface area (11.3 m2 g−1), which can enhance the soil nitrogen uptake (Long et al. 2019) and reduce the loss caused by nitrogen leaching (Li et al. 2017), thus significantly increasing the total nitrogen content in the soil. The effect of biochar on microbial biomass carbon and nitrogen is not consistent due to differences in soil and biochar type in previous studies (Walelign and Mingkui 2015; Oladele et al. 2019). In the current study, the content of soil MBC increased with the increase of biochar content; on the other hand, the application of biochar increased the soil pH (Haider et al. 2021a), enhanced the soil water holding capacity (Pokharel et al. 2020) and porosity (Haider et al. 2021b), reduced soil bulk density (Rumpel 2011), and provided optimum living conditions for soil microorganisms survival (Gul et al. 2015), thus increasing the soil microbial biomass. Due to the presence of the higher carbon content and rich nutrient elements of biochar, the application of biochar can directly provide optimum nutrients for microbial growth (Chan and Xu 2009), and the strong adsorption of nutrients, i.e., nitrogen and phosphorous enhances the ability of soil to supply nutrients (Lehmann et al. 2011). The content of MBN decreased at first and then increased and then again decreased with the increase in the concentration of biochar, but the difference among treatments was not significant, and the content of BC1 (10 t ha−1) was the lowest in each soil layer. This might be due to the high pH value of biochar used in this experiment. With the increase of biochar concentration in soil, the soil pH value increases, which leads to a decrease in the fungal biomass in the soil. Consistently, Yuan et al. (2019) found that the soil MBN content was the highest after the addition of (30 t ha−1) wheat straw biochar for 1 year and decreased gradually with the increase of application rate, which may be due to the difference of soil acidity, biochar properties, pyrolysis temperature for biochar processing, feedstock used for biochar, climatic condition variations, and may be due to the different sampling years after adding biochar (Yang et al. 2019; Haider et al. 2021b). The ratio of BC/BN is an important index to measure the availability of soil microbial carbon and nitrogen and the change of microbial composition. The ratio of BC/BN showed a trend of increasing first and then decreasing and then increasing with the increase of biochar concentration in soil. Under the condition of conventional fertilizer, the addition of excessive biochar increased the C/N ratio of soil, resulting in the decrease of the proportion of active nitrogen that could be directly utilized by microorganisms in the soil (Yang et al. 2019), and the sequestration of nitrogen (Oladele et al. 2019), which was more like a carbon pool than a nitrogen source (Zhang et al. 2016) to soil microorganisms.
Soil acid hydrolyzed organic nitrogen is an active part of the soil nitrogen pool, which is easily distributed by human activities (Xu et al. 2016). The transformation rate of nonacid hydrolyzed nitrogen in the soil is slow, so it is a stable and difficult mineralization component in the soil nitrogen pool. In the current study, the addition of biochar significantly increased the content of total nitrogen in the soil acid hydrolysis but had no significant effect on the content of nonacid nitrogen. The overall performance of soil organic nitrogen was as follows amino acid nitrogen > non-acidolyzable nitrogen > acidolyzable ammonium nitrogen > unknown-acidolyzable nitrogen > amino-acidolyzable nitrogen, which was similar to many research results (Zhang et al. 2016). The results showed that with the increase in the biochar concentration, the changing trend of acid hydrolyzed total nitrogen and nonacid hydrolyzed nitrogen was opposite, and the nonacidified nitrogen decreased at first and then increased. Some studies (such as Lu et al. 2009; Li et al. 2013) reported that when nitrogen fertilizer was applied in soil, most of them were transformed into amino acid nitrogen and acid-hydrolyzed unknown nitrogen. With the increase of soil total nitrogen content, acid-hydrolyzed organic nitrogen begins to transform to nonacid hydrolyzed nitrogen (Fig. 8). The soil total nitrogen content increases with the increase of biochar concentration, which is consistent with the results of the above researchers. Acid ammonium nitrogen and acid amino-acid nitrogen are important sources of plant absorption and utilization of mineralization nitrogen, as well as the source and pool of soil mineralization organic nitrogen (Zhang et al. 2016), which is closely related to soil nitrogen supply capacity (Xiang et al. 2013). Amino acid nitrogen may come from the deamination of amino sugar, amide, purine, and pyrimidine, the decomposition of hydroxyl and other amino acids, and the release of fixed ammonium in the soil (Hu et al. 2020).
Effect of nitrogen application on soil organic nitrogen dynamics. After the application of nitrogen fertilizer in the soil, most of them would be transformed into soil amino acid nitrogen (AAN) and acid-hydrolyzed unknown nitrogen (UAN), and only a small part would be transformed into nonacid hydrolyzed nitrogen (AIN). However, with the increase of soil total nitrogen (TN) content, soil total acid-hydrolyzed organic nitrogen (TAN) would be transformed into nonacid hydrolyzed nitrogen (AIN)
The proportion of acid ammonium nitrogen and acid amino acid nitrogen in organic nitrogen fractions was higher, which was the same as that of Dang et al. (2011) in the study of typical soil organic nitrogen fractions in the Loess Plateau. Correlation analysis showed that amino acid nitrogen was positively correlated with soil total nitrogen and MBC, and acid hydrolyzed ammonium nitrogen was positively correlated with soil total nitrogen, which indicated that the application of biochar promoted the degradation of macromolecular complex organic matter by soil microorganisms on the basis of increasing soil total nitrogen and MBC content and increased inorganic nitrogen source and low molecular organic nitrogen in the soil (Li et al. 2017). However, the content of both decreased with the deepening of the soil layer, which was mainly due to the application of chemical fertilizers according to the conventional amount of fertilizers before sowing, which led to the higher nitrogen content in the surface layer of soil than in the lower layer. The mineralization rate of unknown nitrogen in the soil is slow, and it is easy to accumulate (Li et al. 2013; Fawzy et al. 2020). It is mainly composed of non-α-amino acid nitrogen, aliphatic amine, and aromatic amine (Xu et al. 2003; Nieder et al. 2011).
With the increase of biochar concentration, the content of unknown nitrogen in each soil layer showed a reducing trend. The correlation analysis showed that its content was negatively correlated with soil total nitrogen, amino acid nitrogen, and MBC content, which indicated that the addition of biochar promoted the decomposition and transformation of acid hydrolyzed unknown nitrogen in the soil. The proportion of amino-acid nitrogen in soil total nitrogen and acid hydrolyzed organic nitrogen fractions was relatively low (Nieder et al. 2011; Song et al. 2020), which was mainly due to the residue of the microbial cell wall (Jing et al. 2020). The content mainly reflected the accumulation degree of dead microorganisms in the soil (Hu et al. 2020). The results showed that the content of amino-acid nitrogen increased at first and then decreased with the increase of biochar concentration and deepening of the soil layer, and the content of biochar (30 t ha−1) was the highest, which was similar to the existing results (Yuan et al. 2019). The addition of optimum an amount of biochar improved the soil environment and provided a good environment for microbial survival (Haider et al. 2021a). Similarly, with the increasing concentration of biochar, the water-holding capacity of soil increased and the growth and reproduction of microorganisms were inhibited. In crux, the contents of total soil nitrogen, acid-hydrolyzed ammonium nitrogen, and amino-acid nitrogen were the highest under BC3 treatment, while those of unhydrolyzed nitrogen was the lowest. Under BC5 treatment, the contents of amino-acid nitrogen and acid-hydrolyzed unknown nitrogen were the highest and lowest, respectively, which indicated that the active part of soil organic nitrogen was under BC3 and BC5 treatments. Therefore, in the dry farming area of the Loess Plateau, the application of biochar is of great significance to improve the soil nitrogen supply capacity and crop yield.
Conclusions
The current study demonstrated that the soil total N, amino acid N, microbial biomass C, and wheat yield increased, while unknown nonacid hydrolyzable N decreased after 4–5 years of addition of maize straw biochar (10–50 t ha−1). However, variations were apparent among different levels of biochar application. The BC3 treatment (30 t ha−1) had the highest wheat yield among different treatments, which was mainly attributed to the beneficial effect of this treatment on soil N fractions, soil microbial biomass N, acid-hydrolyzed ammonium nitrogen, and amino-acid nitrogen content. Overall, the application of biochar at an optimum level can help improve soil nutrient supply capacity, soil microbial activities, and productivity of field crops in semiarid and arid regions.
References
Bremmer JM, Mulvaney CS (1982) Nitrogen-total. In: Page RH, Miller RH (eds) Methods of soil analysis. American Society of Agronomy, Madison, pp 595–624
Bremner JM (1965) Organic forms of nitrogen//Black C A. Methods of soil analysis. American Society of Agronomy Incorporation, Madison, pp 1148–1178
Chan KY, Xu Z (2009) Biochar nutrient properties and their enhancement. In: Lehmann J, Joseph S (eds) Biochar for Environmental Management: Science and Technology. Earthscan Publishers Ltd., pp 67–84
Chinese Soil Taxonomy Cooperative Research Group (1995) Chinese soil taxonomy (Revised Proposal). Institute of Soil Science/Chinese Agricultural Science and Technology Press, Academic Sinica/Beijing
Dang YA, Wang GD, Shi-Qing LI (2011) The changing characteristics of profile distribution of soil organic nitrogen component of the typical soil types on the loess plateau. Sci Agric Sin 44:5021–5030
Dempster DN, Gleeson DB, Solaiman ZM, Jones DL, Murphy DV (2012) Decreased soil microbial biomass and nitrogen mineralisation with eucalyptus biochar addition to a coarse textured soil. Plant Soil 354:311–324
El-Naggar A, Lee SS, Rinklebed J, Farooq M, Sarmah A, Zimmerman AR, Ahmad M, Shaheen SM, Ok YS (2019) Biochar application to low fertility soils: a review of current status, and future prospects. Geoderma 337:536–554
FAO (1990) Soil map of the world: revised legend. World soil resources report 60. Food and Agriculture Organization of the United Nations, Rome
Fawzy S, Osman AI, Doran J, Rooney DW (2020) Strategies for mitigation of climate change: a review. Environ Chem Lett 18:2069–2094
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H (2015) Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agric Ecosyst Environ 206:46–59
Haider FU, Coulter JA, Liqun C, Hussain S, Cheema SA, Wu J, Zhang R (2021a) An overview on biochar production, its implications, and mechanisms of biochar-induced amelioration of soil and plant characteristics. Pedosphere. https://doi.org/10.1016/S1002-0160(20)60094-7
Haider FU, Coulter JA, Cheema SA, Farooq M, Wu J, Zhang R, Shuaijie G, Liqun C (2021b) Co-application of biochar and microorganisms improves soybean performance and remediate cadmium-contaminated soil. Ecotoxicol Environ Saf 214:112112
Haider FU, Liqun C, Coulter JA, Cheema SA, Wu J, Zhang R, Wenjun M, Farooq M (2021c) Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf 11:111887
Hu L, Li S, Li K, Huang H, Wan W, Huang Q, Li Q, Li Y, Deng H, He T (2020) Effects of two types of straw biochar on the mineralization of soil organic carbon in farmland. Sustainability 12(24):10586. https://doi.org/10.3390/su122410586
Jia Q, Liao S, Rongyan BU, Zhang M, Ren T, Xiaokun LI, Cong R, Jianwei LU (2017) Effects of nitrogen application rate on fractionation of soil organic nitrogen relative to crop rotation mode. Acta Pedol Sin 54:1547–1558
Jing Y, Zhang Y, Han I, Wang P, Mei Q, Huang Y (2020) Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci Rep 10:8837
Khan I, Chen T, Farooq M, Wan-Ning D, Xu S, Li-xue W (2021) The residual impact of straw mulch and biochar amendments on soil physiochemical properties and yield of maize (Zea mays L.) under rainfed system. Agron J d 113:1102–1120
Lehmann J, Rillig M, Thies J, Masiello C, Hockaday W, Crowley D (2011) Biochar effects on soil biota - a review. Soil Biol Biochem 43:1812–1836
Li SQ, Li SX, Shao MA, Guo DY (2004) Effects of long-term application of fertilizers on soil organic nitrogen components and microbial biomass nitrogen in semiarid farmland ecological system. Sci Agric Sin 37:859–864
Li SS, Yang JC, Jiang HM, Zhang JF, Li LL, Zhang SQ, Pan P, Guo JM, Liu L (2013) Effects of organic and inorganic fertilizer on nitrogen pool and distribution of residual n fractions in flu- vo-aquic soil under the winter wheat system. J Agro-Environ Sci 32:1185–1193
Li Y, Yu YL, Zhang X, Yang QC, Zeng YQ, Han XR, Yang JF (2017) Effects of continuous application of biochar-based fertilizer and biochar on organic nitrogen fractions in brown soil. Chinese J Ecol 36:2903–2909
Li H, Dai M, Dai S, Dong X (2018) Current status and environment impact of direct straw return in china’s cropland - a review. Ecotoxicol Environ Saf 159:293–300
Long Z, Wang J, Hou Z (2019) Effects of cotton straw biochar returning and n application rate on soil organic nitrogen fractions in cotton field. J Shihezi Univ (Nat Sci) 37:154–161
Lu H, Li S, Jin F, Shao M (2009) Contributions of organic nitrogen forms to mineralized nitrogen during incubation experiments of the soils on the loess plateau. Commun Soil Sci Plant Anal 40:3399–3419
Manirakiza E, Ziadi N, St-Luce M, Hamel C, Antoun H, Karam A (2019) Nitrogen mineralization and microbial biomass carbon and nitrogen in response to co-application of biochar and paper mill biosolids. Appl Soil Ecol 142:90–98
Nieder R, Benbi DK, Scherer HW (2011) Fixation and defixation of ammonium in soils: a review. Biol Fertil Soils 47:1–14
Oladele S, Adeyemo A, Adegaiye A, Awodun M (2019) Effects of biochar amendment and nitrogen fertilization on soil microbial biomass pools in an alfisol under rain-fed rice cultivation. Biochar 1:163–176
Pokharel P, Ma Z, Chang SX (2020) Biochar increases soil microbial biomass with changes in extra- and intracellular enzyme activities: a global meta-analysis. Biochar 2:65–79
Prommer J, Wanek W, Hofhansl F, Trojan D, Offre P, Urich T, … Hood-Nowotny RC (2014) Biochar decelerates soil organic nitrogen cycling but stimulates soil nitrification in a temperate arable field trial. PLoS ONE 9(1):e86388. https://doi.org/10.1371/journal.pone.0086388
Ren J, Yu P, Xu X (2019) Straw utilization in China—status and recommendations. Sustainability 11(6):1762-. https://doi.org/10.3390/su11061762
Rumpel C (2011) Biochar for environmental management: science and technology. Nat Sci Soc 19:457–458
Sarma B, Farooq M, Gogoi N, Borkotoki B, Kataki R, Garg A (2018) Soil organic carbon dynamics in wheat - green gram crop rotation amended with vermicompost and biochar in combination with inorganic fertilizers: a comparative study. J Cleaner Prod 201:471–480
Song D, Chen L, Zhang S, Zheng Q, Ullah S, Zhou W, Wang X (2020) Combined biochar and nitrogen fertilizer change soil enzyme and microbial activities in a 2-year field trial. Eur J Soil Biol 99:103212
Varma A, Oelmüller R (2007) Advanced techniques in soil microbiology. Springer, Berlin Heidelberg
Walelign D, Mingkui Z (2015) Effect of biochar application on microbial biomass and enzymatic activities in degraded red soil. Afri J Agric Res 10:755–766
Wang D, Fonte SJ, Parikh SJ, Six J, Scow KM (2017) Biochar additions can enhance soil structure and the physical stabilization of c in aggregates. Geoderma 303:110–117
Xiang B, Miller S, Kort E, Santoni G, Daube B, Commane R, Angevine W, Ryerson T, Trainer M, Andrews A, Nehrkorn T, Tian H, Wofsy S (2013) Nitrous oxide (N2O) emissions from California based on 2010 Calnex airborne measurements. J Geophy Res (Atmospheres) 118:2809–2820
Xu YC, Shen QR, Ran W (2003) Content and distribution of forms of organic n in soil and particle size fractions after long-term fertilization. Chemosphere 50:739–745
Xu N, Tan G, Wang H, Gai X (2016) Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur J Soil Biol 74:1–8
Yang S, Sun X, Ding J, Jiang Z, Xu J (2019) Effects of biochar addition on the nee and soil organic carbon content of paddy fields under water-saving irrigation. Environ Sci Pollut Res Int 26:8303–8311
Yuan YH, Rui SY, Zhou JH, Liu GJ, Zhang WF, Li L, Fan HB (2019) Effects of biochar and calcium peroxide on soil enzyme activities and soil microbial community structure in upland red soil. Soil fertilizer Sciences in China, 93–101
Zhang MH, Lu JW, Zhao XL (2016) Effect of different organic materials on nitrogen mineralization in two purple soils. Huan jing ke xue 37:2291–2297
Acknowledgements
We are thankful to the reviewers for helping us to improve the quality of the manuscript.
Funding
This research was financially supported by the National Natural Science Foundation of China (Grant Numbers 41661049).
Author information
Authors and Affiliations
Contributions
P.Z. contributed to data collection and write up of the manuscript; F.U.H. contributed to writing, reviewing, and editing; S.H., M.F., and C.X. participated in coordination and helped to draft the manuscript; C.L. contributed to supervision and funding acquisition. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Amjad Kallel
Rights and permissions
About this article
Cite this article
Zhandong, P., Haider, F.U., Hussain, S. et al. Biochar amendment enhanced soil nitrogen fractions and wheat yield after four to five years of aging in Loess Plateau, China. Arab J Geosci 15, 523 (2022). https://doi.org/10.1007/s12517-022-09661-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-09661-8