Abstract
The addition of various quantities of raw materials could be used in order to improve the physical and pyroscopic performances of silica-alumina refractories manufacturing. Here we study the possibility of local kaolinitic clay (SB) valorization in low alumina fireclay refractory materials manufacturing. Alumina-rich commercial kaolin (AS) (33.4% alumina) was also used as an additive to (SB) clay (24% alumina), to improve the physical and pyroscopic performances of the manufactured samples. The thermal behavior of (SB) clay was studied to determine necessary parameters for the production of good chamotte. In order to elaborate the refractory samples, two mixtures were optimized; mixture M1 (80 wt.% of chamotte (SB) and 20 wt.% of (SB) clay as a binder in crude fine form) and mixture M2 (80 wt.% of chamotte (SB) and 20 wt.% of (AS) kaolin as a binder in crude fine form) and sintered at 1350 °C for 2 h after compaction and molding. The obtained samples were characterized by their bulk density, open porosity, shrinkage, cold crushing strength, microstructure by scanning electron microscope (SEM) micrographs, mineralogical composition, and their refractoriness under load. Our data suggest that the addition of the alumina-rich clay to the main mixture enhances the refractoriness from 1198 to 1213 °C (T0.5), the mechanical behavior of the manufactured pellets from 36 to 44 MPa, and the mullite amount from 25 to 29%, and decreases the open porosity from 19.6 to 18.6 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The geology of Tunisia is the result of a continuous and successive progression. Indeed, the Tunisian territory contains many useful geological resources and many deposits of raw materials considered economically viable and attractive for development. Among these raw materials, clays have been already explored in the ceramics and cement industry. Several works have been focused on the valorization of Tunisian clays in the ceramics field (Jeridi et al. 2008; Hachani 2011; Moussi et al. 2011; Bennour et al. 2015), and have suggested promising and satisfactory results. Among the well-used ceramic materials are refractories that are widely used in industrial fields mainly in furnaces construction such as electrical furnaces, blast furnaces, steel ladle, municipal waste incinerators, glass melting furnaces, and cement rotary kilns. Refractory materials, also, provide the protection of installations and reduce the thermal losses (Poirier 2011).
Silica-alumina refractories are mostly used in industrial processes such as petrochemicals and consist of 30% of the global market. Their manufacturing process requires sufficient dimensional accuracy and volume stability by prefiring a part of the clay between 1200 and 1500 °C (Routschka 2004) to produce chamotte. This latter is considered a basic component of those materials. Furthermore, chamotte transforms refractories into stable elements at high temperature and limits shrinkage and cracking during drying and sintering processes (Djangang et al. 2008; Minlheiro et al. 2005). Many research studies have already been conducted on silica-alumina refractories (e.g., Amrane 2003; Kolli et al. 2007; Kolli 2008; Djangang et al. 2008; Mécif 2010; Amrane et al. 2011; Seynou et al. 2013; Bouzidi 2012; Sadik et al. 2013; Sadik et al. 2014 ).
In the recent years, some works carried out on refractory materials have been concentrated on many important concepts. The research works of Rahman et al. (2015) and Sadik et al. (2016) have been focused on the exploration of local low-cost materials such as kaolinitic clays and dolomite to elaborate refractory materials with sufficient properties without carrying out high-cost thermal or chemical treatments or using expensive and rarely available materials such as olivine, spinel, bauxite, cordierite, and zirconium. Another concept that involves the protection of the environment through the valorization of organic waste in insulating refractories manufacturing was highlighted in some research works such as Hassan and Aigbodion (2014) and Serra et al. (2015). Also, studying degradation of refractories performance under the conditions of use (Chen et al. 2015) and refractory material recycling has been considered among the most important concepts in recent years.
The refractory technology is not very advanced in Tunisia. Consequently, the minorities of local refractory factories import expensive raw materials (bauxite and kaolin) from China and Ukraine or import commercialized refractory products. The import rate heightens the dependence of the country. It is worth investigating some local kaolinitic clays in fireclay materials manufacturing to reduce raw materials importation and to valorize rejects and low-cost substances. For this aim, Tunisian kaolinitic clay (SB) from the Sidi Bader quarry has been chosen to be evaluated in terms of its suitability to fireclay refractories elaboration because of its refractory potential. The Sidi Bader clays have been studied in many recent works in ceramic (Moussi 2012; Bennour et al. 2015), in the adsorption of textile dyes (Chargui et al. 2018), in geo-medicine (Khiari 2017) and in mineralogical study (Felhi et al. 2008). Commercial kaolin (AS) with higher alumina content was used in this work, on the one hand, as an additive to (SB) clay in order to compare the pyroscopic resistance with the mechanical behavior of refractory products, and on the other hand, to study the effect of the chemical and mineralogical compositions of the raw materials on the physical properties of the elaborated pellets especially the refractoriness under load, the mechanical resistance, and the open porosity. The addition of kaolin (AS) was based on many studies adopting the same approach (e.g., Aladesuyi et al. 2017; Amrane et al. 2011; Sadik et al. 2013; Sadik et al. 2014).
Materials and methods
Geological setting
Despite the scarcity of kaolins in Tunisia, some kaolin deposits exist in different regions: the halloysite of Aïn Khemouda in the central part of Tunisia (Jamoussi 2001; Delphine 2004), the kaolinitic clays of the Numidian Flysch in the far North of Tunisia (Ilavsky 1966; Rouvier 1967, 1994; Jamoussi 2001), and the volcano-sedimentary deposits of Tamra Basin (Ilavsky 1966; Rouvier 1967; Dermech 1990). The most significant reserves of kaolin in Tunisia belong to the Numidian Flysch.
The (SB) clay, studied in this work, is from Sidi Bader quarry that is situated about 4 km away from Tabarka town to the east (Fig.1). It’s located in Touila Mountains. This deposit belongs to the Numidian Flysch which presents the main outcrop of the extreme north Tunisian, formed by sandstones and argillites. The Numidian Flysch was formed in a complex of large channels at turbidity filled with high density, observable along several tens of kilometers (Yaich et al. 2000). The origin of the flysch sediments has been frequently debated. Based on the presence of current wrinkles in the Numidian formations (Wildi 1983; Parize et al. 1986; Talbi 1998), it is estimated that these sediments are from northern sources and this hypothesis has been approved by the recent works of (Fildes et al. 2009).
The geographic location of Sidi Bader quarry (Mahmoudi et al. 2016) modified
The (SB) samples of Oligocene-Lower Miocene age used in this study are collected in an alternance of white to beige clays and sandstone intercalation of the quarry (Fig. 2).
Methods
The chemical analysis of the raw materials is performed by X-ray fluorescence using S8 Tiger apparatus, the results are expressed as the percentage of concentration of all oxides of each element, and the loss on ignition is measured with calcination at 1000 °C.
The X-ray diffraction data are carried out using a PANalytical X'Pert Pro Diffractometer (Cu-Kα radiation, λ = 1.5418 Å, 2ϴ range 5–70°). The relative amount of phases was estimated using the application EVA of DIFFRAC plus software (Bruker) (Serna et al. 2014).
The differential thermal analysis (DTA) and thermogravimetry analysis (TGA) of the raw materials are run using Linseis apparatus, and dilatometry is realized using DIL 801L apparatus.
Fired specimens are tested for bulk density BD (g/cm3) which is massively divided by bulk volume and open porosity OP (%) which is an open pore volume as a percentage of bulk volume and shrinkage S (%). Bulk density and open porosity are measured by using the hydrostatic method according to the standard and calculated by using the following equations:
Shrinkage (%) is calculated using the dimensions of the materials before and after heat treatment using the following equation:
where Ls is the height of the raw specimens and Lc is the height of the fired specimens.
The compressive strength is determined by uniaxial compression until the failure of specimens using a TONI Technik apparatus; the charge of compression is 50 kN with 1 kN/s as a compression rate. The refractoriness under load test of the refractory manufactured pellets is realized according to the EN ISO 1893; cylindrical pellets (Ø 50 cm× H 50 cm) cut from the refractory bricks made with the optimized mixtures M1 and M2 are subjected to a load of 2 kg/cm2 of the section of the tested pellets with temperature variation. The apparatus is equipped with a recorder which tracks the dimensional variations of the specimens as the function of the temperature and gives a curve (Amrane 2012).
Preparation of the refractory pellets
The first experimental tests are carried out to (i) evaluate the thermal and mechanical behavior of the clays and (ii) to optimize a good chamotte based on (SB) clay. As a first step, raw (SB) clay and (AS) commercial kaolin are crushed into fine particles (<400 μm), and uniaxially shaped using a hydraulic press into cylinder specimens (Ø 3 cm × H 3 cm). They are sintered from 1350 to 1550 °C for 1 h; the heating rate is 5 °C/min. A second batch of cylinder specimens based on (SB) clay is sintered at 1350 and 1500 °C varying calcination cycle 2, 4, and 6 h at 2 °C/min as a firing rate. The best (SB) chamotte grog is obtained after sintering at 1500°C for 6 h at 2 °C/min as firing rate of cubic specimens (7×7×7 cm) is based on (SB) clay. The sintered chamotte is grounded and sieved into different particle size fractions: course (1–5mm), middle (100 μm–1mm), and fine (<100 μm) (Table 1).
The specimens elaboration is realized by shaping two optimized mixtures (M1) and (M2) where chamotte grog (SB) is used as a main component: the first mixture (M1) is composed of 80 wt.% of chamotte grog (SB) and 20 wt.% of (SB) clay as a binder in crude and fine form; the second mixture (M2) is formed with 80 wt.% of chamotte (SB) and 20 wt.% of (AS) kaolin as a binder in a crude fine form.
These mixtures are moisturized by adding 5 wt.% of water, shaped into cylinder specimens (Ø 3 cm × H 3 cm) by using hydraulic press, dried for 24 h at 105 °C, and then, fired at 1350 °C for 2 h.
Results
Clay characterization
Table 2 presents the XRF and XRD results of raw materials. The SB clay shows a relatively high SiO2 content (61.9%). The AS clay is relatively richer in Al2O3 (33.4 %) and the loss of ignition is 12%. The latter material presents higher kaolinite content. Figure 3 depicts the emplacements of raw materials according to their silica and alumina amounts, on the binary silica-alumina diagram showing the fate of the chemical composition of raw materials after firing at high temperature (1550°C), whereas the ternary diagram (SiO2-Al2O3-Oxydes) in Fig. 4 shows that SB and AS clay are closer to the SiO2-Al2O3 side than the rest of minerals (Kirabira 2003). According to Routschka (2004) and Fayyad et al. (2012), the usual refractory clays have TiO2 content ranging from 1 to 4%, Fe2O3 not exceeding 2.5%, and (Na2O+K2O+CaO+MgO) not exceeding 6%. The nature and the quality of those impurities will control the melting point and the quantity of liquid phase after firing. According to the chemical composition results, SB clay and AS kaolin are suitable to be used in low alumina refractory bricks manufacturing (ISO 10081) due to their low amounts of iron oxide, TiO2 and K2O. Therefore, the relatively important amount of alumina in AS clay will improve the mechanical and pyroscopic behavior of the SB clay by increasing mullite amount (Bouzidi 2012) relying on the fact that the relatively high mechanical properties of refractory products are essentially due to their mullite-composed structure (Sadik et al. 2013).
Studied raw materials locations in the binary silica-alumina diagram (modified) (Routschka 2004)
Ternary diagram of SiO2-Al2O3-Oxides, showing the locations of SB, AS clays and the European commercially marketed kaolins (Kirabira 2003)
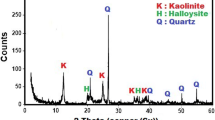
The mineralogical composition of raw materials presented by XRD patterns (Fig. 5) and semi-quantitative analysis (Table 2) shows that the identified mineral phases of SB clay are kaolinite (55%), illite (9%), quartz (34%), and anatase (3%) whereas the identified mineral phases of AS kaolin are kaolinite (85%), illite (8%), quartz (7%), and anatase (4%). The presence of quartz, illite, and anatase in the raw materials explains the presence of SiO2, K2O, and TiO2, respectively, in agreement with the chemical composition.
The DTA/TGA analysis of SB and AS clays shows a relatively close behavior. Figure 6 presents the (DTA/TGA) curves of SB clay. The following data are observed: (i) endothermic peak between 60 and 100 °C accompanied by the release of absorbed and interlayer water, (ii) endothermic peak between 500 and 550 °C corresponding to the dehydroxylation of kaolinite, and (iii) exothermic peak between 900 and 1000 °C corresponding to the structural reorganization of kaolinite. The losses on ignition of SB clay and AS kaolin are almost 8% and 11%, respectively.
The dilatometric curve of SB clay (Fig. 7) shows expansion from 20 to 500 °C followed by alpha and beta quartz transformation at 570 °C (Jouenne 1990). The expansion of the SB clay is almost 0.5% and the shrinkage is about 3%.
Chamotte optimization
As a first step, SB clay and AS commercial kaolin are calcinated in a temperature ranging from 1350 to 1500 °C for 1 h, with 5 °C/min as firing rate. To optimize a good chamotte, SB clay was, then, heated at high temperature (1500 °C) for 2, 4, and 6 h with 2°C/min as firing rate. For SB clay, the experimental observations of the first firing cycle have shown that calcination in the first temperature range (1350–550 °C) caused cracks and the expansion of the pellets at 1400 °C (Fig. 8). The result of characterizations (Table. 4) shows the increase of the open porosity to 29.6% and the decrease of the cold crushing strength from 38.2 to 21.3 MPa. When we vary the duration of the calcinations cycle (2, 4, and 6 h), the open porosity and cold crushing resistance gradually decrease to 8% and 14.71 MPa, respectively. The bulk density maximum is at 1350 °C (2.11 g/cm3) but decreases gradually when firing time and heating temperature increase, the same for the shrinkage that becomes negative (expansion) from 1400 °C (Table 3).
Discussions
Physical properties
The degradation of the properties of SB clay during the first and the second firing cycles is probably related to (i) the appearance of cristobalite which hinders the densification of the components according to (Zawrah and Hamzawy 2002 and El-Kheshen and Zawrah 2003) and (ii) the appearance of the amorphous phase. This is approved by the XRD analysis which shows the increase of the mullite and cristobalite contents at 1400 °C explaining the cracking and the expansion of the pellets. The amount of the amorphous phase increases and reaches its maximum 80 wt.% after heating at 1500 °C for 6 h explaining possibly the decrease in porosity at this stage. In general, the appearance of the amorphous phase improves the densification as the apparent density increases with firing temperature because of highly viscous silica formation (Sadik et al. 2014). The increase in sintering temperature causes the increase in bulk density, shrinkage, and cold crushing strength out of mullite synthesis and the decrease in open porosity. However, the surface defect appearing at calcinations temperature exceeding 1350 °C for SB clay could be related to the deterioration of crystalline phases and the development of vitreous matrix. Moreover, the shape of some cracks suggests a problem of molding pressure.
For the AS commercial kaolin, heating test proves that shrinkage values are relatively important, due to the relatively high amount of kaolinite (85%), reaching a maximum of 23.7% at 1350 °C. At higher temperature, lower linear deformation is observed (11.5% at 1550 °C). The open porosity slightly increases with temperature (from 3.1% at 1350 °C to 3.7% at 1550 °C). The studied specimens show a similar mechanical behavior at temperature below 1400 °C (37 MPa). On the other hand, bulk density obtained at the same range is around 2.18 g/cm3 when it is higher at lower temperatures. This is related to the development of the vitreous amorphous phase (Sadik et al. 2014) as reported by the XRD analysis (Table 4).
These results affirm the increase of mullite content from 42% at 1350 °C to 43% at 1550 °C; the cristobalite content goes down sharply from 16% at 1350 °C to 1% at 1550 °C, while amorphous phase rises from 40 to 56% which is less than SB clay because of the less silica amount in the AS clay. All the results assert that the AS clay is fully densified and less porous than SB clay; moreover, it presents a sufficient thermal and mechanical behavior; accordingly, it is suitable to be added to the SB clay to improve its thermal and mechanical behavior in order to elaborate the refractory final specimens. Based on previous results, firing SB clay at 1500 °C for 6 h with 5 °C/min as firing rate is selected as a suitable condition to elaborate stable chamotte to ensure the stability of the refractory bricks and approximate the industrial experimental procedure.
The pre-calcinated chamotte at 1500 °C for 6 h is crushed (75% main particle size 1–5 mm, Table 1). The refractory final specimens (Fig. 9) are manufactured by using the optimized two mixtures M1 and M2 and sintered at 1350 °C for 2 h.
Refractory pellets properties
Table 4 summarizes the technological properties of the SB and AS based on cylindrical refractory pellets formulated by the mixtures M1 and M2. The obtained results are almost similar for both mixtures, except for the cold crushing strength which increases to 44 MPa for M2 compared to 36 MPa for M1. This is caused by the addition of 20% of the AS, clearly richer in silica and alumina amounts when compared to SB. This should improve the mullite content (± 5%) in accordance with the mineralogical analysis (± 4%) which shows that the mullite content in M1 is 25% as much as it is 28% for M2. The bulk density of the manufactured refractory pellets is almost the same (2 g/cm3). The addition of SB chamotte helps in reducing the shrinkage to almost 3%. The open porosity is relatively high for M1 and M2 with 19% and 20%, respectively, because of the dominance of the coarse phase. Compared to other research works carried out on refractory silica-alumina bricks such as Seynou et al. (2013) and Routschka (2004) shown in (Table 4), the refractory elaborated pellets properties are sufficient and the obtained results are almost within the standards range.
Thermo-mechanical properties
The commercial kaolin (AS) ameliorates some properties, essentially the mechanical and thermal ones. The cold crushing strength soars from 36 to 44 MPa. The bulk density is almost 2 g/cm3. The use of chamotte SB as the main component, in both mixtures, helps decrease shrinkage to 2.8%. Besides, the refractoriness under load, which is the resistance of refractory material to deformation when heated at high temperature (Fayyad et al. 2012), is tested at 0.2 N/mm2 to assess their softening at high temperature. Both M1 and M2 refractory specimens are evaluated and the results are illustrated in Table 3.
The temperature ISO (T0.5) is the beginning of the softening of the refractory specimens. It corresponds to 1198 °C for M1 and 1213 °C for M2. The temperature T5 is the beginning of failure when the refractory specimen is not able to resist under this temperature. It corresponds to 1364 °C for M1 and 1384°C for M2. The refractoriness under load depends, mainly, on the properties of the fired bricks and, in particular, on the porosity: when porosity increases, refractoriness decreases. Referring to Shaw (1972) and Galdina (1983), the mechanical properties of fire-clay refractory bricks depend on the chemical and mineralogical composition of the raw materials and porosity of the produced bricks. Most fluxing materials such as alkalis, earth’s alkaline, iron oxide, and manganese oxide, which are usually present in small amounts in fire clay, tend to decrease the resistance of the refractory bricks to deformation under load. The temperature of deformation of fireclay refractories increases with increasing alumina. Porosity, greatly, influences refractoriness under load values which explains that for denser bricks, the deformation point occurs at relatively higher temperature. Therefore, these obtained results confirm that the possibility of using a local kaolinitic clay ameliorated with the addition of a low percentage (20 to 40%) of a substance rich on alumina content instead of using expensive alumina-rich imported clays might be economically significant to elaborate silica-alumina refractories with satisfactory quality and could be able to meet the Tunisian market needs of refractory materials.
Conclusions
This work deals with the valorization of the Sidi Bader (area of Tabarka, North West Tunisia) kaolinitic clay in refractory silica alumina bricks. The chemical and mineralogical composition of the investigated raw materials shows alumina content between 20 and 35% and silica content less than 65%, while the mineralogical composition consists mainly of kaolinite associated with illite, quartz, and anatase. Commercial alumina-rich clay (AS) is added to the Sidi Bader clay. The characterization and optimization results of the chamotte grog provide a less dense and more porous chamotte, but mineralogically stable by sintering the Sidi Bader clay for 6 h at 1500 °C. The characterization of the refractory specimens manufactured using two mixtures M1 (80 wt.% of chamotte grog (SB) and 20 wt.% of (SB) clay as a binder in crude fine form) and M2 (80 wt.% of chamotte (SB) and 20 wt.% of (AS) kaolin as a binder in crude fine form) displays that the results are almost within the international standards. The AS kaolin addition to the main mixture ensures its mechanical and thermal behavior. However, those bricks are likely to be used in pizza and bread ovens construction. The obtained results are highly encouraging, but further investigation is necessary to refine this potential valorization. Adding more than 20% of alumina-rich clay will possibly yield better results.
References
Aladesuyi O, Pal M, Das SK, Ajanaku KO (2017) Phase and microstructural evolution during sintering of mixture of 75:25. Nigerian kaolin and calcined alumina powder compacts, JMES, 2017 8(8):2682–2838
Amrane B (2003) Élaboration et caractérisation d’un matériau réfractaire thermorésistant pour supports de cuisson rapide des produits céramiques. In: Mémoire de mastère. Université de Boumerdes, Faculté des Sciences de l’Ingénieur, p 117
Amrane B (2012) Thèse de doctorat en métallurgie, Modelisation du comportement thermomecanique des ceramiques par la mecanique de l’endommagement : cas des refractaires silico-alumineux façon, Ecole Nationale Polytechnique d’Alger. Algérie:187
Amrane B, Ouedraogo E, Mamen B, Djaknoun Mesrati N (2011) Experimental study of thermo-mechanical behaviour of alumina-silicat refractory materialsbased on mixture of Algerian kaolinitic clays. Ceram Int 37:3217–3227
Bennour A, Mahmoudi S, Srasra E, Boussen S, Htira N (2015) Composition, firing behavior and ceramic properties of the Sejnène clays (Northwest Tunisia). Appl Clay Sci 115:30–38
Bouzidi N (2012) Thèse de doctorat en Génie des procédés, Influence des impuretés des kaolins sur les propriétés des produits de cuisson. Université de Bjaia, Algériee, p 135
Chargui H, Hajjaji W, Wouters J, Yans J, Jamoussi F (2018) Direct orange34 dye fixation by modified Kaolin. Clay Miner 53:271–287
Chen L, Mlfiet A, TomJones P, Blanpin B, Guo M (2015) Degradation mechanisms of alumina–silica runner refractories by carbon steel during ingot casting process. Ceram Int 42:10209–10214
Delphine B (2004) Néogenèses silico-alumineuses en contexte cryptokarstique de l'halloysite de Beez (Namur, Belgique) et de Aïn Khamouda (Kasserine, Tunisie). PhD Thesis, University Paris- Sud XI, France. 231
Dermech M (1990) Le complexe de l'oued Bélif-Sidi Driss (Tunisie septentrionale) Hydrothermalisme et métallogénie, PhD Thesis, University Tunis El manar, Tunisia. 336
Djangang, C.N., Elimbi, A., Melo, U.C., Lecomte, G.L., Nkoumbou, C., Soro, N., Bonnet,J., Blanchart, P., Njopwouo, D., 2008. Sintering of clay-chamotte ceramic composites for refractory bricks, Ceram Int, 34, 1207–1213.
El-Kheshen A, Zawrah M (2003) Sinterability, microstructure and properties of glass/ceramic composites. Ceram Int 29:251–257
EN ISO (1893) 2007. Produits réfractaires -- Détermination de l'affaissement sous charge -- Méthode différentielle avec élévation de la température
Fayyad SM, Al Marahleh G, Abu-Ein S (2012) Improvement of the Refractoriness under Load of Fire-Clay Refractory Bricks Adv. Theor Appl Mech 5, 2012(4):161–172
Felhi M, Tlili A, Gaied ME, Montacer M (2008) Mineralogical study of kaolinitic clays from Sidi El Bader in the far north of Tunisia. Appl Clay Sci 39:208–217
Fildes C, Stow DAV, Riahi S, Soussi M, Patel U, Milton JA, Marsh S (2009) European Provenance of the Numidian Flysch in northern Tunisia. Terra Nova 22(2):94–102
Galdina N (1983) Improvement in the quality of refectory materials. Glas Ceram 7-8:439–442
Hachani M (2011) Thèse de doctorat. In: Valorisation céramique de quelques argiles du Crétacé inférieur: formation Bouhedma (Altlas centro-méridional) et Duiret (Plateforme saharienne). Université de Carthage, Faculté des Sciences de Bizerte, p 177
Hassan SB, Aigbodion VS (2014) Effect coal ash on some refractory properties of alumino-silicate (Kankara) clay for furnace lining. Sciences direct:1–8
Ilavsky J (1966) Les argiles de Tunisie. Service Géologique, Office Nationale des Mines, Tunisie, p 150
Jamoussi F (2001) Caractérisation minéralogique, géochimique, géotechnique et utilisation industrielle des argiles de Tunisie. Doc. Thesis, University Tunis, Tunisie.437
Jeridi K, Hachani M, Hajjaji W, Moussi B, Medhioub M, Lopez-Galindo A, Zargouni F, Labrincha J, Jamoussi F (2008) Technological behaviour of some Tunisian clays prepared by dry ceramic processing. Clay Miner 43(3):339–350
Jouenne CA (1990) Traite de Céramiques et Matériaux Minéraux, Editions Septima Paris, 1990
Khiari I (2017) Etudes de quelques argiles utilisées dans les domaines cosmétiques et pharmaceutiques en méditerrané occidentale. Thèse de doctorat en sciences géologique, Université de Sfax, Tunisie .208
Kirabira JB (2003) Properties of Ugandan minerals and fireclay refractories. Doctoral thesis, Stocholm, Swedenpp, pp 1–50
Kolli M 2008 Elaboration et caractérisation thermomécanique de réfractaires à base de kaolin DD3. Thèse de doctorat en optique et mécanique de précision, Université Ferhat Abbas-Setif UFAS (Algerie). 175
Kolli M, Hamidouche M, Fantozzi G, Chevalier J (2007) Elaboration and characterization of a refractory based on Algerian kaolin. Ceram Int 33:1435–1443
Mahmoudi S, Bennour A, Meguebli A, srasra, E., Zargouni, F. (2016) Characterization and traditional ceramic application of clays from the Douiret region in South Tunisia. Appl Clay Sci 127–128(2016):78–87
Mécif A (2010) Elaboration et Etude Des Réfractaires à Base de Mullite et du Zircon. Thèse de doctorat en physique, Université de Mentouri – Constantine, Algérie. 123
Minlheiro FAC, Freire MN, Silva AG, Holanda PJNF (2005) Densification behaviour of red firing Brazilian kaolinitic clay. Ceram Int 31:757–763
Moussi B (2012) Mode de genèse et valorisation de quelques argiles de la région de Nefza-Sejnane (Tunisie Septentrionale). Thèse de doctorat en sciences géologique, Université de Carthage, Tunisie. 157
Moussi B, Medhioub M, Hatira N, Yans J, Hajjaji W, Rocha F, Labrincha JA, Jamoussi F (2011) Identification and use of white clayey deposits from the area of Tamra (northern Tunisia) as ceramic raw materials. Clay Miner 46:165–175
Parize O, Beaudoin B, Burollet PF, Cojan G, Fries G, Pinault M (1986) La provenance du matériel gréseux numidien est septentrionale (Sicile et Tunisie). CR Acad Sci Paris 18:1671–1674
Poirier, J., 2011. Les céramiques réfractaires de l’élaboration aux propriétés d’emploi. Verres céramiques & composites, vol. 1, n°2 28-42
Rahman H, Tariqul Islam Md, Ibn Minhaj T, Azad MAK, Mehedi Hassan MD, Haque R, Md AA (2015) Study of thermal conductivity and mechanical property of insulating firebrick produced by local clay and petroleum coal dust as raw materials. Science Direct Procedia Engineering 105(2015):121–128
Routschka G (ed) (2004) Pocket manual—refractory materials: basics, structures and properties, 2nd edn. Essen, Vulkan-Verlag
Rouvier H (1967) Géologie de l'extrême Nord tunisien: tectoniques et paléogéographies superposées à l'extrémité orientale de la chaîne nord magrébine. Annale des Mines et de la Géologie. Office Nationale des Mines, Tunisie, p 29
Rouvier H (1994) Notice explicative de la carte géologique de la Tunisie à 1/50.000.Nefza, feuilleN° 10.OfficeNationale des Mines,Tunisie
Sadik C, Albizane A, El Amrani E (2013) Production of porous firebrick from mixtures of clay and recycled refractory waste with expanded perlite addition, 2002. J Mater Environ Sci 4:123–130
Sadik C, Albizane A, El Amrani E (2014) Recent advances in silica-alumina refractory: a review. Journal of Asian Ceramic Societies 2:83–96
Sadik C, Moudden O, El Bouari A, El Amrani I (2016) Review on the elaboration and characterization of ceramics refractories based on magnesite and dolomite. Journal of Asian Ceramic Societies 4(2016):219–233
Serna F, Lagneau J, Carpentier JM (2014) La diffraction des rayons X: une technique puissante pour résoudre certains problèmes industriels et technologiques. Chimie nouvelle n° 116
Serra MF, Conconie MS, Gaune MR, Suarez G, Aglietti EF, Randtorff NM (2015) Mullite (3Al2O3·2SiO2) ceramics obtained by reaction sintering of rice husk ash and alumina, phase evolution, sintering and microstructure. Journal of Asian ceramic societies:7
Seynou M, Flamen P, Swadogo M, Tirlocq J, Ouedraogo R (2013) Refractory bricks based on Tikaré (Burkina Faso) Kaolinitic clay material. Journal de la société Ouest-Africaine de chimie. 18 éme année, n°035.ISSN 0796-6687
Shaw K (1972) Refractories and their uses Applied Science Publishers Ltd, London
Talbi F (1998) Petrologie, géochimie, études des phases fluides et gîtologie liées au magmatisme néogène de la Tunisie septentrionale. Thèse de doctorat. Université Tunis II. 368
Wildi W (1983) La chaîne tello-rifaine (Algérie, Maroc, Tunisie): structure, stratigraphie et évolution du Trias au Miocène. Rev Géol Dyn Géogr Phys 24:201–297
Yaich C, Hooyberghs HJF, Durlet C, Renard M (2000) Corrélation stratigraphique entre les unités oligo-miocènes de Tunisie centrale et le Numidien. C.R. Acad. Sci. Paris 331:499–506
Zawrah M, Hamzawy E (2002) Effect of the cristobalite formation on sinterability microstructure and the properties of glass/ceramic composites. Ceramics international 28:123–130
Acknowledgements
Special thanks to all the staff and collaborators of the Ministry of Higher Education and Scientific Research of Tunisia, the University of Namur (UNamur), the Belgian Ceramic Research Center (Mons, Belgium), the Water Researches and Technologies Center Borj Cedria-Tunisia. Special thanks also to Pr Saidi Talha and Pr Bouneb Yahya who did the linguistic revision of this paper.
Funding
This work was supported by the Tunisian Belgium Wallonie-Bruxelles International WBI research project “Valorisation des argiles tunisiennes.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Zeynal Abiddin Erguler
Rights and permissions
About this article
Cite this article
Grine, O., Moussi, B., Hajjaji, W. et al. Low-cost northern Tunisian kaolinitic clay-based refractory materials and effect of a rich alumina clay addition. Arab J Geosci 14, 1595 (2021). https://doi.org/10.1007/s12517-021-08099-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-021-08099-8