Abstract
Groundwater is the primary source for drinking purposes in all over the world. The present study is carried out to evaluate the groundwater suitability for drinking purposes. For this purposes, thirty groundwater samples were collected from active boreholes in the investigated region and analyzed for concentrations of various physico-chemical parameters. Chadha diagram was used to better understand the hydro-geochemistry, while fuzzy comprehensive assessment method (FCAM) was introduced to delineate the overall groundwater quality for drinking purposes. Groundwater is slightly alkaline in nature in this study region, and the dominant hydrochemical facie is HCO3−-Na+ which is a reflection of the predominant rock-water interaction in the region. Results of FCAM demonstrated that 73% of groundwater samples were most suitable for drinking purposes and remaining 27% were poor in quality which were unsuitable for drinking purposes in the investigated region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is the most important resource for various uses around the world (Chen et al. 2019; Li et al. 2019), and developing countries typically depend on it for their daily needs such as drinking, irrigation, and industrial purposes. Especially, India and China are mainly facing water crisis (Singh et al. 2019; Subba Rao et al. 2020; Chen et al. 2018; Narsimha and Sudarshan 2017a), and predominantly, the quality is the main issue in both the developing countries. Therefore, a number of statistical methods have been implemented to understand the pollution sources of groundwater in various regions. More recently, Subba Rao et al. (2020) assessed the quality criteria for groundwater use by applying various statistical methods and found that the rock-water interactions, silicate weathering, ion exchange, and chemical fertilizers influenced the groundwater quality in the Wanapathy region, South India. In another study, Chandrasekar et al. (2019) noticed that groundwater quality is largely contaminated by natural/geological factors and anthropogenic process. Mostly from agricultural fertilizers, municipal wastes, industrial wastes, mismanagement of solid and liquid wastes are essential factors which mainly deteriorates the groundwater quality (Adimalla 2020; Chandrasekar et al. 2019; Liu et al. 2019; Mukate et al. 2019; Narsimha and Sudarshan 2017b; Verma et al. 2018; Wu et al. 2020; Taloor et al. 2020). However, a number of novel approaches and models introduced to understand the overall quality of groundwater for drinking purposes. Some of the recent research fields include the following:
-
Mukate et al. (2019) developed a new integrated water quality index (IWQI) model to assess the groundwater quality for drinking suitability of water.
-
Verma et al. (2018) compared two significant approaches of spatial distribution and water quality index (WQI) techniques to evaluate the drinking water quality. This study gives profound insights into Gangetic plain region of India.
-
Adimalla and Qian (2019) carried out an assessment of groundwater quality for drinking by using WQI and human health risk assessment in the agricultural region of Nanganur, South India. The results are useful in the understanding of the overall quality of groundwater for drinking uses and also its impact on residence health.
-
Adimalla (2020) conducted an investigation on controlling factors and mechanism of groundwater quality variation in the semiarid region of South India by applying various statistical techniques. This study also implemented that multiple techniques which were most useful tools to achieve a better understanding of groundwater chemistry and contamination factors.
To date, most studies have evaluated the groundwater quality in several regions in India (Singh et al. 2018, 2019; Adimalla and Qian 2019; Elumalai et al. 2017a, b; Mthembu et al. 2020; Sakram and Adimalla 2018; Subba Rao et al. 2017; Subba Rao et al. 2020). These studies majorly emphasized on quality of groundwater for various purposes such as drinking and irrigation. However, to improve the comprehensive understanding of the overall quality of groundwater for drinking purposes, in this study we then used the profound fuzzy comprehensive assessment method (FCAM). Therefore, this paper took Mothkur region as the research area to study the overall quality of groundwater to provide a scientific basis for understanding the water quality in South India. The main aim of this study is to investigate the overall groundwater quality for drinking purposes by applying FCAM model. Moreover, this study also provides useful information about groundwater quality for local and international researchers.
Study region
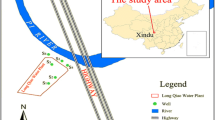
The Mothkur is located in the northern part of Nalgonda district, Telangana State, India. Geographically, the investigated region lies between latitude 17.4147° and 17.4542°N, and longitude 79.1130° and 79.1561°E, with covering an area of 210 km2. The sampling map was drawn with the help of Survey of India (SOI) Topo sheet No. 56O3 (Fig. 1). The study area reported an average rainfall of 562 mm in South-West monsoon, and also, 74.8% of the annual rainfall occurs during the monsoon season. The South-West monsoon starts in the month of June and ends in September. Especially, the significant recharge of groundwater ensues in this monsoon period. The entire study region is dominated by hard rock terrain which occupy comprise granites, gneisses (Archaean crystalline rocks). These rocks primarily having secondary porosity which forms the repository for groundwater. Typically, the groundwater occurs under the water table conditions in weathered zone and confined and semi-confined in fractured zone. The majority of the aquifer zones encountered within the depth range from 10 to 20 m bgl (CGWB 2013).
Materials and methods
Sample collection and analysis
The systematic sampling of groundwater was carried out in the month of November 2016 to comprehend the quality of groundwater for drinking purposes. A Garmin (Garmin eTrex 30) GPS (Global Positioning System) was used for recording the groundwater sampling locations in the study region. A total of thirty groundwater samples were collected from bore wells/hand pumps. The physico-chemical parameters such as pH (hydrogen ion concentration), electrical conductivity (EC), and total dissolved solids (TDS) were tested/measured in the field at the time of sampling by using portable water quality kit (pH/EC/TDS meter Hanna HI9811-5). The bicarbonate (HCO3−) was measured by titrating with standard HCl, while chloride (Cl−) was estimated by titrating with standard AgNO3. Total hardness (TH as CaCO3) and calcium (Ca2+) were determined titrations method, using standard EDTA. The magnesium (Mg2+) was calculated, taking the difference between TH and Ca2+. Potassium (K+) and sodium (Na+) were estimated by using Flame Photometer (Model 130, Systronics Flame Photometer). Sulfate (SO42−) and nitrate (NO3−) were analyzed by using UV visible spectrophotometer (APHA 2012). Fluoride (F−) was analyzed using a fluoride ion-selective electrode with a Thermo-Orion four star benchtop pH/ISE meter (APHA 2012).
The accuracy of the physico-chemical parameters was evaluated using blank samples, parallel samples, and internal standards water quality procedures of American Public Health Associated (APHA 2012). The accuracy of each groundwater sample was computed the following formula (Eq. 1):
where charge balance error percentage (%CBE), total concentrations of cations (Ca2+, K+, Na+, and Mg2+), and the total concentrations of anions (SO42−, NO3−, F−, HCO3−, and Cl−) and all cations and anions are expressed in milliequivalent per liter (meq/L). The computed %CBE was observed to be within the acceptable limit of ± 5% (Domenico and Schwartz 1990).
Fuzzy comprehensive assessment method
The fuzzy comprehensive assessment method (FCAM) is applied to characterize the groundwater quality for drinking purposes and the following steps are used to compute the FCAM (He and Wu 2019; Wang et al. 2017; Dahiya et al. 2007): The groundwater quality grade (Gk) of each sample is typically determined by the assessing matrix (Bk×m) exemplified as
where “k” indicates the number of samples, “n” expresses the number of parameters, “m” denotes the number of water quality grades, “xkj” represents the weighted subordinate degree of sample “k” to grade “m,” and “Gk” specifies the water quality grade of sample “k.”
The weight vector “A(k)” is calculated by using the following equation:
where \( ``{a}_i^k" \) represents the weight of the parameter “i” of the “kth” sample\( \left({\sum}_{i=1}^n{a}_i^k=1\right) \), “c” is the concentration of a parameter of a give sample, “Sij” denotes the standardized concentration of parameter “i” of grade “j,” and \( ``{a}_{i\times m}^k" \) represents the subordinate degree matrix which generally determined by subordinate function (SF) (Wang et al. 2020; Gong and Jin 2009). The subordinate degree matrix is computed as follows:
where “rij” indicates the subordinate degree of parameter “i” to the grade “j” and is computed using the following SF equations (when i = 1):
when i = 2, 3, - - - - - - - -, m − 1,
And when i = m,
Results and discussions
Hydrogeochemical facies
The Chadha diagram reveals hydrochemical regime with respect to the presence of ions viz., Ca2+, Mg2+, Na+, K+, CO32−, HCO3−, Cl−, and SO42− (Chadha 1999). The relative concentration of the cations and anions represented in milliequivalent percentage (% meq/L). In this Chadha diagram, the difference in between alkaline earths (Ca2+ + Mg2+) and alkali metals (Na+ + K+) which are expressed as percentage reacting values is plotted on “X” axis, while the difference in between the weak acidic anions (CO32− + HCO3−) and strong acidic anions (Cl− + SO42−) is plotted on the “Y” axis.
The Chadha diagram is plotted and portrayed in Fig. 2. According to this diagram, there are 26% of groundwater samples whose anions and cations were plotted in sub-field (3) which indicates that weak acidic anions exceed strong acidic anions. Moreover, groundwater in the investigated region is significantly dominated by the alkali metals (Na+ + K+) exceed alkaline earths (Ca2+ + Mg2+) and weak acidic anions (CO32− + HCO3−) exceed strong acidic anions (Cl− + SO42−). Furthermore, this sub-field water is mostly dominated by Na+-HCO3− type (Chadha 1999). However, only three groundwater samples are shown in the sub-field of alkali metals exceed alkaline earths and strong acidic anions exceed weak acidic anions. Typically, this kind of water creates salinity problems in both agricultural/irrigation and drinking practices.
Chadha diagram (Chadha 1999) showing the hydrochemical facies of groundwater in the study area. (1) Alkaline earths exceed alkali metals; (2) alkali metals exceed alkaline earths; (3) weak acidic anions exceed strong acidic anions, (4) strong acidic anions exceed weak acidic anions; (5) alkaline earths and weak acidic anions exceed both alkali metals and strong acidic anions, respectively; HCO3−-Ca2+·Mg2+, HCO3−-Ca2+ or HCO3−-Mg2+ types; (6) alkaline earths exceed alkali metals and strong acidic anions exceed weak acidic anions; Cl−·SO42−-Ca2+·Mg2+, Cl−-Ca2+·Mg2+ or SO42−-Ca2+·Mg2+ types; (7) alkali metals exceed alkaline earths and strong acidic anions exceed weak acidic anions; SO42−·Cl−-Na+, Cl−-Na+, or SO42−-Na+ types; and (8) alkali metals exceed alkaline earths and weak acidic anions exceed strong acidic anions; HCO3−-Na+ type
Groundwater use for drinking purposes
The hydrogen ion concentration (pH) of the groundwater ranges from 7.0 to 8.4 with a mean of 7.5 in the study region (Table 1). The pH designates that slightly alkaline in nature. However, all collected groundwater samples are within the prescribed limit of 6.5 to 8.5 (BIS 2012). Electrical conductivity (EC) range varied considerably from 676 to 4016 μS/cm, with an average value of 1513.17 μS/cm, and correspondingly, TDS varied from 446 to 2650 mg/L (mean 1000.63 mg/L). About 70% of groundwater samples were classified as fresh (TDS < 1000 mg/L), and the remaining samples were categorized as brackish water (1000 ≤ TDS ≤ 10,000 mg/L). Typically, water hardness may be increased due to the excessive presence of Ca2+, Mg2+, SO42−, Cl−, and HCO3− (Elumalai et al. 2017a, b; Mthembu et al. 2020; Subba Rao et al. 2020). In this study region groundwater, the total hardness (TH) as CaCO3− varied from 280 to 1185 mg/L with an average value of 462 mg/L (Table 1). The analysis data specifies that a vast majority (87%) of groundwater samples have TH values below 600 mg/L; therefore, such groundwater is most suitable for drinking purposes.
The cation and anion dominance of the study region groundwater samples are Na+ > Ca2+ > Mg2+ > K+ and HCO3− > Cl− > SO42− > NO3− > F−, respectively. The range of Na+ in the collected groundwater samples ranged from 129 to 545 mg/L and was found the 43% of samples beyond the WHO permissible limit of 200 mg/L. Mg2+ concentrations varied from 15 to 176 mg/L, and about 29 samples were found to be well within the maximum permissible threshold value of 100 mg/L as described by the BIS (2012). Calcium was found to vary from 24 to 242 mg/L with a mean value of 76.30 mg/L (Table 1), and a total of twenty-nine groundwater samples were found to contain Ca2+ at concentrations within the BIS maximum permissible limit of 200 mg/L. Potassium has no specific limit (BIS 2012). However, the concentration of K+ was found to differ from 1.4 to 6 mg/L (Table 1).
The HCO3− content of groundwater samples ranges from 299 to 628 mg/L, with a mean of 410.73 mg/L (Table 1). However, HCO3− has no specific limit. The chloride content of groundwater samples varies from 10 to 785 mg/L with an average value of 222.50 mg/L (Table 1), and about 37% of samples exceed the desirable limit of 250 mg/L. However, all collected groundwater samples are within the maximum permissible limit of 1000 mg/L (BIS 2012). In the study region, SO42− is in the range of 18 to 240 mg/L with an average value of 73.50 mg/L (Table 1) and are found to be within the maximum permissible limit of 400 mg/L as prescribed by the Indian Standard specifications for drinking water (BIS 2012). NO3− ranges for 13–82 mg/L with mean value 38.70 mg/L (Table 1), and about 37% of groundwater sampling locations that are not recommended for drinking purposes due to the concentration of nitrate are very higher than the prescribed limit of 45 mg/L (BIS 2012). The fluoride is often found in the hard rock terrain, and it is an essential element in the development of bones. The BIS (2012) stipulated that fluoride concentration in drinking water should not be exceeded the 1.2 mg/L. However, in the present study region groundwater, the fluoride concentration ranges from 0.63 to 1.85 mg/L with an average value of 1.13 mg/L, and about 27% of total collected groundwater samples exceed the prescribed limit of 1.2 mg/L (Table 1).
Overall groundwater quality based on FCAM
Physicochemical parameters such as pH, TDS, TH, Na+, Ca2+, Mg2+, Cl−, SO42−, F−, and NO3− were selected to evaluate the overall groundwater quality by using the fuzzy comprehensive assessment method (FCAM) model (He and Wu 2019; Kiurski-Milošević et al. 2015). In this study, national groundwater quality standards “Bureau of Indian Standards” guidelines were used. However, groundwater quality based on FCAM is classified into five grades such as grade-I (excellent water quality), grade-II (good water quality), grade-III (fair water quality), grade-IV (poor water quality), and grade-V (very poor water quality) (He and Wu 2019; Wang et al. 2017; Gong and Jin 2009).
The computed FCAM values in the study region groundwater are listed in Table 2 and Fig. 3. The distribution of groundwater quality is shown in Fig. 4. As can be seen from Table 2, twenty-two groundwater samples are classified as good quality water which indicates that these groundwater samples are more suitable for drinking purposes in the study region. Moreover, eight groundwater samples are classified as poor quality water and these groundwater are not recommending for drinking purposes, but it can be used for irrigation purposes in the study region. The poor quality groundwater samples were associated with main pollutes such as chloride, nitrate, fluoride, and sulfate. The nitrate and fluoride contamination is prevalent in the study region groundwater. Therefore, intensive treatment is promptly required before groundwater can be utilized for drinking purposes in the study region.
Conclusions
The results indicate that Na+ is the dominant cation and HCO3− is the dominant anion in the study region. The majority of the groundwater that is dominated by HCO3−-Na+ facie indicates the rock-water interaction is a dominant process in the study region. Groundwater is of the alkaline condition. The cation and anion dominance of the study region groundwater samples are Na+ > Ca2+ > Mg2+ > K+, and HCO3− > Cl− > SO42− > NO3− > F−, respectively. The Na+ and Cl− concentration of 43% and 37% of groundwater samples are found to be more than the acceptable limit of BIS in the study region. The concentration of NO3− in 63% of groundwater samples is below its desirable limit of 45 mg/L, and the remaining samples are unsuitable for drinking purposes. About 73% of groundwater of the study region shows F− content is below the safe limit of 1.2 mg/L, and the rest of the groundwater samples are above its safe limit of 1.2 mg/L. As per the classification of fuzzy comprehensive assessment method (FCAM), about 22 and 8 groundwater samples in the study area come under good and poor water quality type for drinking purposes, respectively.
References
Adimalla N (2020) Controlling factors and mechanism of groundwater quality variation in semiarid region of South India: an approach of water quality index (WQI) and health risk assessment (HRA). Environ Geochem Health 42(6):1725–1752. https://doi.org/10.1007/s10653-019-00374-8
Adimalla N, Qian H (2019) Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol Environ Saf 176:153–161. https://doi.org/10.1016/j.ecoenv.2019.03.066
APHA (2012) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
BIS (2012) Indian standard specifications for drinking water, IS:10500, Bureau of Indian Standards, New Delhi
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7(5):431–439
Chandrasekar T, Sabarathinam C, Nadesan D, Rajendiran T, Banajarani Panda BR, Keesari T, Mohan Viswanathan P, Utharapathi V, Alagappan R (2019) Geochemical (process based) characterization of groundwater along the KT boundary of South India. Geochemistry 79(1):62–77
Chen J, Wu H, Qian H, Li X (2018) Challenges and prospects of sustainable groundwater management in an agricultural plain along the Silk Road Economic Belt, north-west China. Int J Water Resour Dev 34(3):354–368
Chen J, Huang Q, Lin Y, Fang Y, Qian H, Liu R, Ma H (2019) Hydrogeochemical Characteristics and Quality Assessment of Groundwater in an Irrigated Region, Northwest China. Water 11(1)
Dahiya S, Singh B, Gaur S, Garg VK, Kushwaha HS (2007) Analysis of groundwater quality using fuzzy synthetic evaluation. J Hazard Mater 147(3):938–946. https://doi.org/10.1016/j.jhazmat.2007.01.119
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology. Wiley, New York
Elumalai V, Brindha K, Elango L (2017a) Regional and temporal variation in minor ions in groundwater of a part of a large river delta, southern India. Environ Monit Assess 189:305. https://doi.org/10.1007/s10661-017-6006-3
Elumalai V, Brindha K, Lakshmanan E (2017b) Human exposure risk assessment due to heavy metals in groundwater by pollution index and multivariate statistical methods: a case study from South Africa. Water 9:234
Gong L, Jin C (2009) Fuzzy comprehensive evaluation for carrying capacity of regional water resources. Water Resour Manag 23:2505–2513. https://doi.org/10.1007/s11269-008-9393-y
He S, Wu J (2019) Hydrogeochemical characteristics, groundwater quality, and health risks from hexavalent chromium and nitrate in groundwater of huanhe formation in Wuqi County, Northwest China. Expo Health 11:125–137. https://doi.org/10.1007/s12403-018-0289-7
Kiurski-Milošević JŽ, Vojinović-Miloradov MB, Ralević NM (2015) Fuzzy model for determination and assessment of groundwater quality in the city of Zrenjanin. Serbia HemijskaIndustrija 69(1):17–28. https://doi.org/10.2298/hemind131215016k
Li P, Tian R, Liu R (2019) Solute geochemistry and multivariate analysis of water quality in the Guohua Phosphorite Mine, Guizhou Province, China. Expo Health 11(2):81–94
Liu J, Jin D, Wang T, Gao M, Yang J, Wang Q (2019) Hydrogeochemical processes and quality assessment of shallow groundwater in Chenqi coalfield, Inner Mongolia, China. Environ Earth Sci 78(12):347
Mthembu PP, Elumalai V, Brindha K, Li P (2020) Hydrogeochemical processes and trace metal contamination in groundwater: impact on human health in the Maputaland Coastal Aquifer, South Africa. Expo Health 12:403–426. https://doi.org/10.1007/s12403-020-00369-2
Mukate S, Wagh V, Panaskar D, Jacobs JA, Sawant A (2019) Development of new integrated water quality index (IWQI) model to evaluate the drinking suitability of water. Ecol Indic 101:348–354
Narsimha A, Sudarshan V (2017a) Assessment of fluoride contamination in groundwater from Basara, Adilabad District, Telangana State, India. Appl Water Sci 7(6):2717–2725. https://doi.org/10.1007/s13201-016-0489-x
Narsimha A, Sudarshan V (2017b) Contamination of fluoride in groundwater and its effect on human health: a case study in hard rock aquifers of Siddipet, Telangana State, India. Appl Water Sci 7(5):2501–2512. https://doi.org/10.1007/s13201-016-0441-0
Sakram G, Adimalla N (2018) Hydrogeochemical characterization and assessment of water suitability for drinking and irrigation in crystalline rocks of Mothkur region, Telangana State, South India. Appl Water Sci 8(5):143. https://doi.org/10.1007/s13201-018-0787-6
Singh S, Ghosh NC, Gurjar S, Krishan G, Kumar S, Berwal P (2018) Index-based assessment of suitability of water quality for irrigation purpose under Indian conditions. Environ Monit Assess 190:29. https://doi.org/10.1007/s10661-017-6407-3
Singh S, Ghosh NC, Krishan G, Kumar S, Gurjar S, Sharma MK (2019) Development of indices for surface and ground water quality assessment and characterization for Indian conditions. Environ Monit Assess 191:182. https://doi.org/10.1007/s10661-019-7276-8
Subba Rao N, Marghade D, Dinakar A, Chandana I, Sunitha B, Ravindra B, Balaji T (2017) Geochemical characteristics and controlling factors of chemical composition of groundwater in a part of Guntur district, Andhra Pradesh, India. Environ Earth Sci 76(21):747
Subba Rao N, Sunitha B, Adimalla N, Chaudhary M (2020) Quality criteria for groundwater use from a rural part of Wanaparthy District, Telangana State, India, through ionic spatial distribution (ISD), entropy water quality index (EWQI) and principal component analysis (PCA). Environ Geochem Health 42(2):579–599. https://doi.org/10.1007/s10653-019-00393-5
Taloor AK, Pir RA, Adimalla N, Ali S, Manhas DS, Roy S, Singh AK (2020) Spring water quality and discharge assessment in the Basantar watershed of Jammu Himalaya using geographic information system (GIS) and water quality Index (WQI). Groundw Sustain Dev 10:100364. https://doi.org/10.1016/j.gsd.2020.100364
Verma DK, Bhunia GS, Shit PK, Tiwari AK (2018) Assessment of groundwater quality of the Central Gangetic Plain Area of India using geospatial and WQI techniques. J Geol Soc India 92(6):743–752
Wang H, Cai Y, Tan Q, Zeng Y (2017) Evaluation of groundwater remediation technologies based on fuzzy multi-criteria decision analysis approaches. Water 9:443. https://doi.org/10.3390/w9060443
Wang J, Yan H, Xin K, Tao T (2020) Risk assessment methodology for iron stability under water quality factors based on fuzzy comprehensive evaluation. Environ Sci Eur 32:81. https://doi.org/10.1186/s12302-020-00356-z
WHO (2011) Guidelines for drinking water quality. World Health Organization, Geneva
Wu C, Fang C, Wu X, Zhu G, Zhang Y (2020) Hydrogeochemical characterization and quality assessment of groundwater using self-organizing maps in the Hangjinqi gasfield area, Ordos Basin, NW China. Geosci Front. https://doi.org/10.1016/j.gsf.2020.09.012
Acknowledgments
Thanks are due to Editor-in-Chief and anonymous reviewers for their constructive comments helped us in bringing the manuscript to its present form.
Funding
The author received financial support from the Department of Science and Technology (DST)-Science and Engineering Research Board (SERB) Government of India, New Delhi, and the Research Funds for Start-Up Research Grant (Young Scientists) project (SR/FTP/ES-13/2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Recent advanced techniques in water resources management
Rights and permissions
About this article
Cite this article
Adimalla, N. Groundwater quality delineation based on fuzzy comprehensive assessment method (FCAM): a case study. Arab J Geosci 13, 1256 (2020). https://doi.org/10.1007/s12517-020-06265-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-020-06265-y