Abstract
Based on the long-term (12 years) data on soil respiration (SR) measurements in representative conifer forests of the Krasnoyarsk region (Central Siberia), we demonstrated specific characteristics of SR. The study sites located closely to Arctic Circle were related to different latitudes, including 56° N (SAE site, Krasnoyarsk), 60° N (ZOTTO site, Zotino), and 64° N (Tura site, Evenkia). Study sites were selected in forest ecosystems (> 41 years old) and represented a wide range of the climate, stand, permafrost availability, and soil types. However, the mean seasonal values of SR (mean ± SD) varied in a relatively narrow range from 2.11 ± 0.69 in the forest-steppe zone (SAE) to 2.82 ± 1.77 μmol CO2 m−2 s−1 in northern larch taiga (Tura). Nevertheless, a twofold difference in the duration of growing season among northern and southern locations results in a variation of total seasonal CO2 emissions (mean ± SD) from 216 ± 136 g C m−2 in larch stands of northern taiga to 369 ± 115 g C m−2 in larch stands of southern forest-steppe zone. Evaluation of the multiannual dynamics of SR over the past 1.5 decades (1995–2010) showed the escalation of SR in the permafrost larch stands (Tura) in 2005–2010. That has been coupled with the enhanced activity of the soil heterotrophic microbiota in 2007–2010. Our study highlights the importance of further monitoring of SR in permafrost Siberian ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Greenhouse gas (GHG) emissions, mostly carbon (C) dioxide (CO2), have been extensively studied over the past 3 decades all over the world (Luo and Zhou 2006; IPCC 2014). SR is assessed to be the second-largest CO2 terrestrial flux after photosynthesis (e.g., Raich and Potter 1995). SR can compete with annual fossil fuel loading of atmospheric CO2 (Dixon et al. 1994; Boden et al. 2009; IPCC 2014). Ongoing climate change in high latitudes, aggravated by the ecosystem disturbances, such as wildfires, permafrost degradation, landslides, and insect outbreaks, has the potential to completely modify SR (e.g., Casper 2010; Osawa et al. 2010; IPCC 2014; Abbott and Jones 2015). Hashimoto et al. (2015), on the base of the semi-empirical model, parameterized with over 1000 data points estimated mean annual global SR trend as increased at the rate of 0.09 Pg C per year. Zhao et al. (2017) conducted global biome-specific research on SR (on the base of comprehensive global SR databases) and showed an increasing trend in average annual global SR. Jian et al. (2018), in review research, reported on ongoing worldwide increasing of SR caused by rising temperatures during 1961–2014, including boreal forest ecosystems. In the modeling study using the Community Land Model (CLM) version 4.5, Parazoo et al. (2018) showed the possibility of a shift of permafrost-affected ecosystems from C sink to source. SR rates, documented in many high-latitude habitats, vary widely, and the range of this variation might reach 100 times due to the latitudinal, bioclimatic, and soil environmental differences (e.g., Bond-Lambert and Thompson 2010a). Thus, SR studies across high-latitude sites have shown a high variability of SR, which made up 0.8–6.3 μmol CO2 m−2 s−1 in Alaska forest ecosystems (Gordon et al. 1987; Grogan and Chapin III 1999; O’Neill et al. 2003; Kim et al. 2012), 1.8–14.7 μmol CO2 m−2 s−1 in Finland forest ecosystems (Komulainen et al. 1999; Maljanen et al. 2001; Kumpu et al. 2018), and 1.8–6.8 μmol CO2 m−2 s−1 in forest ecosystems of Sweden (Gardenas 2000; Högberg et al. 2001; Bhupinderpal-Singh et al. 2003; Eliasson et al. 2005). Since climate change causes the acceleration of the permafrost ecosystems’ feedback, the SR modeling, as a climate function, becomes more complicated as far as many variables influence the SR value, e.g., climate parameters, landscape forms, stand types, and soil biological and physical-chemical processes.
The large area occupied by the Siberian forest locates within the highly vulnerable cryolithic zone with the vast amount of C stored in the frozen soil and permafrost (Casper 2010; Osawa et al. 2010; Masyagina et al. 2009, 2015, 2019). However, due to the remoteness and low accessibility, studies on the GHG dynamics rarely extend into the northern taiga forest ecosystems of the Yenisei River basin (Schulze et al. 1999; Yanagihara et al. 2000; Morishita et al. 2010; Masyagina et al. 2019). Krasnoyarsk region—the second biggest region of the Russian Federation—occupies a large area of 2.34 million km2 from the Sayan Mountains to the Taymyr Peninsula in the south-north direction. The dominant tree species are larch (> 70% of the forested area), and pine (about 6% of the forested area). The successful expansion of these light taiga species at this territory is explained by their fire-tolerance (Schulze et al. 2012). The larch-dominated area coincides with the zone of permafrost distribution (Abaimov et al. 2004). Southern regions, lacking the permafrost or having discontinuous permafrost patches (island permafrost), are covered by pine, dark conifers, and mixed (conifer and broadleaf tree species) forests (Pleshikov 2002; Osawa et al. 2010). Therefore, the Krasnoyarsk region may serve as an excellent polygon to contribute to the understanding of how Siberian forest ecosystems have responded to climate change over the past few decades.
Here, we compared SR rates of three groups of sites located at the different latitudes of the Krasnoyarsk region (Russia). Chosen study areas sited at 56° N (Krasnoyarsk, Siberian afforestation experiment (SAE) sites, southern taiga), 60° N (ZOTTO observatory, middle taiga at the southern part of Turukhansk district), and 64° N (Tura sites, northern taiga permafrost forests near Tura settlement, Evenkia). In this study, based on the 12-year monitoring of three study sites in central Siberia, we seek the unique characteristics of SR from permafrost and non-permafrost regions with different trees cover. According to our first hypothesis, higher SR rates are supposed to be in a warmer climate of the south in the Krasnoyarsk region (larch and pine forests at the SAE sites). We expect lower SR rates in the north (pine forests in ZOTTO and larch forests in Tura). In general, rhizosphere temperature and soil water conditions strongly control SR (Jiang et al. 2005; Luo and Zhou 2006; Yanagihara et al. 2000; Morishita et al. 2010; Qu et al. 2018; Makhnykina et al. 2020). From our common idea and previous research, SR of the same species of larch forests in the northeastern part of China has been limited by the soil moisture condition because of limited precipitation (Jiang et al. 2005). Same to China, a limited amount of precipitation also characterizes Siberia that might hinder SR in Siberia in the same way. According to the previous and current studies conducted in the permafrost region in the central Siberia, the main environmental factors affecting SR, besides the hydrothermal regime of soils, are pH and ground vegetation stock in postpyrogenic larch associations (Masyagina et al. 2015; Köster et al. 2018). Since SR is mainly a temperature-dependent process (Yvan-Durocher et al. 2012), the second hypothesis assumes that SR will depend on environmental conditions (temperature and water content as the main variables) and their seasonal fluctuations in three contrasting examined areas. Therefore, maximum SR values are expected in the middle of the growing period due to both higher soil microbial and root activity and increased C input to the soil at this period. However, severe climate conditions in Tura and ZOTTO may decrease SR, and vice versa, more favorable environmental conditions in Krasnoyarsk (SAE) may promote SR. To find out the site-specific drivers of SR, we examined soil C and N contents, C/N, soil microbial activity, soil hydrothermal factors, tree root biomass, and ground vegetation cover-related parameters. Therefore, our study has three tasks: (1) to assess the spatial and seasonal dynamics of SR rates in the key forest types of the Krasnoyarsk region, (2) to show the factors relating to the SR and estimate the effect of tree species on SR in contrasting sites (northern or southern locations), and (3) to evaluate for the first time the changes in SR in the high-latitude Tura site over the past 15 years (1995–2010). This study is expanding the understanding of SR levels and its variation at a central part of the large Siberian region, and emphasizing the urgency of monitoring SR in the permafrost area of high latitude Siberian ecosystems.
Materials and methods
Study sites location
The research sites were settled in the Krasnoyarsk region, Russia, namely in three contrast by the environmental conditions sites: SAE near Krasnoyarsk city, ZOTTO sites near Zotino village, and Tura sites near Tura settlement (Fig. 1, Table 1), and related to the various latitudes. We measured SR rates and other characteristics of the southern (SP: SAE site O2) and northern (NP: ZOTTO sites Z1-Z2) pine stands and the southern (SL: SAE site O1) and northern (NL: Tura sites T1-T14) larch stands (Fig. 1, Table 1).
CM, Cambisols; CR, Cryosols; HS, Histosols; PH, Phaeozems; PZ, Podzols (IUSS Working Group WRB 2015); ND, no data available; *Kuzmichev et al. (2005) as for 2002; **t ha−1; T3 and T13 were described according to Prokushkin et al. (2018)
SAE sites (O1–O2 sites, Fig. 1, Table 1) established in the forest-steppe and southern taiga zones (near Krasnoyarsk) belong to the Sukachev Institute of Forest SB RAS, Federal Research Center “Krasnoyarsk Science Center SB RAS.” In 1971, Prof. N.V. Orlovskii organized here the monoculture plantations of six main forest-forming species: Siberian spruce, Scots pine, Siberian stone pine, Siberian larch, silver birch, and aspen to estimate the individual tree species effects on the soil processes (Menyailo et al. 2002; Matvienko et al. 2014). The region is characterized by continental climatic conditions with annual precipitation of 500 mm per year. The mean summer air temperature is about 20 °C (noon). Soil temperature at a depth of 20 cm is varied from − 4 to − 14 °C in winter and from 10 to 12 °C in summer. The soil is Phaeozems (Table 1), according to IUSS Working Group WRB (2015). In 1971–1972, seedlings of six tree species (2 to 3 years old) were planted in 2400 m2 monoculture plots at a density of 40000 stems ha−1, following homogenization of the 0–20 cm soil (Shugalei 2005). Data on forest inventory and soil biochemical and meteorological properties were published by Menyailo et al. (2002), Menyailo and Hungate (2005), Menyailo (2008), Shugalei (2005), and Vedrova (2005).
ZOTTO (Z1–Z2, Fig. 1, Table 1) sites were located in the middle taiga at the southern part of Turukhansk district of Krasnoyarsk region near Sredneyeniseiskiy Field Station of the Sukachev Institute of Forest SB RAS, Federal Research Center “Krasnoyarsk Science Center SB RAS” (ZOTTO - Zotino tall tower observatory) (Makhnykina et al. 2016). The most influencing factor at the natural ecosystems of the studied territory is the level of underground waters, which regulated water regime at the different topography (bogs, forests, etc.), and determined water availability. The research plots were placed on gently undulating, alluvial sands (classified as Podzols) with no underlying permafrost (Table 1). Climate is continental. This moderately cold area has sufficient water availability. The mean annual air temperature is − 3.7 °C, and annual precipitation is 590 mm (Pleshikov 2002). We established two research plots, which represented different stand types: lichen pine stand (Z1) and green mosses pine stand (Z2) of the same age (Table 1). In the tree cover, pine (Pinus sylvestris L.) prevailed. Ground vegetation in the lichen pine stand (Z1) was characterized by lichens Cladonia stellaris (Opiz) Pouzar et Vezda and Cl. arbuscular (Wallr) Flot. Ground cover dominants in the pine stand with green mosses were Pleurozium schreberi (Brid.) Mitt. and Dicranum polysetum Michx. Dominants of grass-dwarf shrub layer were evergreen dwarf-shrubs Vaccinium vitis-ideae (L.) Avror., Empetrum nigrum L., Arctostaphylos uva-ursi L., and evergreen fern Lycopodium complanatum L. (Trefilova et al. 2009).
Tura study sites comprised several long-term plots located in northern taiga forests near the Evenkian Field Station (Tura, Krasnoyarsk region, Fig. 1, Table 1) of the Sukachev Institute of Forest SB RAS, Federal Research Center “Krasnoyarsk Science Center SB RAS.” Natural larch stands, typical for Tura study area, were characterized by a wide age range of 41–276 years old and located on the different landscape types with permafrost, of various (northern and southern) exposition, and variable microclimatic environment. The climate was sharply continental (mean annual air temperature − 9.5 °С) with wide temperature fluctuation and precipitation distribution. Cold winters (mean air temperature in January is − 36.7°С) and moderately warm summers (mean air temperature in July is 16.3 °С) with annual precipitation of 250–548 mm are usual for the studied region. The growing period lasted 69–80 days (Prokushkin et al. 2008).
Field measurements
At every studied area, we estimated SR rates (μmol CO2 m−2 s−1) with the same methodological approaches (IR-gas analysis) but employing different types of equipment. The measured SR values included soil microbial respiration, root respiration associated with mycorrhiza, and CO2 diffusion as a source of chemical-physical processes (Jong et al. 1974). The soil temperature (TS5) of the mineral soil at a depth of 5 cm was assessed simultaneously with SR measurements. This soil depth was chosen to measure TS5 because it is an essential depth for the plant root systems. Another reason why we used the soil depth of 5 cm, and not litter layer (O horizon), despite that it is known for higher microbial activity and larger root’s contribution, was because the litter layer had a high intra-site heterogeneity of its thickness. Therefore, TS5 was a more stable parameter characterizing the rate of SR, which could be used for the comparative research of locations with a different geographical background.
At the SAE sites (Fig. 1), the SR was estimated using a LI-8100A automated soil flux system (LI-COR Biosciences Inc., Lincoln, Nebraska, USA) with a chamber (D = 20 cm, 4.82 l), which was placed onto the polyvinyl chloride (PVC) collar (4 collars in SL and 4 collars in SP). We decided on the depth of collar insertion according to the detailed pre-examination (Wang et al. 2005). The system calculated the rate of CO2 flux (μmol CO2 m–2 s–1) based on the change in CO2 concentration in the chamber when the latter was closed for 1 min. Measurements were made on a twice-a-month basis from May to October 2011. TS5 was recorded with a DT400 sensor (Comark Instruments, UK) twice a month, simultaneously with SR measurements. Volumetric soil water content (θv, %) in each collar was measured once a month, using a Theta Probe ML2x sensor with an HH2 portable data logger (Delta T Devices Ltd., UK). This device calculated θv based on the difference in electric resistance to constant current between the soil and water. According to Shugalei (2005), the bulk density of the mineral soil layer varied from 1.05 to 1.09 kg l−1. The gravimetric soil water content at a depth of 5 cm (θg) was assessed using the Eq. (1) of linear regression (R2 = 0.98) received empirically for the SAE site.
where θg is gravimetric soil water content (%), θv is volumetric soil water content (%).
At the ZOTTO sites (Fig. 1), we measured SR rates using the LI-8100A (LI-COR Biosciences Inc., Lincoln, Nebraska, USA) from June to October 2013. On each site, five PVC collars (D = 20 cm) were settled randomly and left for the whole season. The depth of installation of the collar into the mineral soil depended on the height of the moss layer and the thickness of soil organic horizon—it was 2–3 cm for the lichen pine stand, and about 13–15 cm for the green-moss pine stand. With insufficient collar installation depth, CO2 fluxes were taken from the organic horizon, which violates the reliability of calculations. The distance between the collars did not exceed 2 m, which made it possible to more reliably estimate the spatial variation of CO2 flux from the soil surface. SR measurements were assessed in the daytime once in 3 days. At the same time of measuring SR, TS5 was registered using a Soil Temperature Probe Type E (Omega, USA). θv (%) was measured at a depth of 5 cm using a Theta Probe Model ML2 (Delta T Devices Ltd., UK) with the same routine as TS5 and SR (Makhnykina et al. 2016). The soil bulk density of the mineral layer was estimated to be 1.82 kg l−1 (Santruckova et al. 2003). The θg was calculated using equation (2).
where θg is gravimetric soil water content (%), θv is volumetric soil water content (%), BD is soil bulk density, and WD is water density (0.997 kg m−3).
At the Tura sites (Fig. 1), SR was studied from June to September of 1995–2010 (Table 1). At every site, we set a 20-m transect, where we placed from 6 to 20 PVC collars (D = 10 cm) for subsequent settlement of chamber of the IR gas analyzer Li-Cor 6200 (LI-COR Biosciences Inc., Lincoln, Nebraska, USA). Collars were put on the soil organic layer for 1–2 cm to prevent gas leaking, which could disturb the results as shown by Wang et al. (2005). The SR measurements were started after 24 h to achieve equilibrium of the soil gas rate. Simultaneously with SR measurements, TS5 was registered using an electronic thermometer Checktemp 1 (CheckTemp, Hanna, USA).
Ground vegetation cover height (VegH) and litter depth (LitD) were measured along with the SR measurements and further used to calculate the forest floor vegetation and organic layer stocks.
Soil sampling
At the Tura sites, the mineral soil water content was determined by the gravimetric method applied on the mineral soil samples collected at the same depth (5 cm) close to the collars used for the SR measurements. The θg (%) was calculated after drying of the obtained samples at 105 °C to a constant weight. The soil bulk density of the mineral horizon was estimated to be 0.93 kg l−1 (Köster et al. 2018).
Mineral soil samples were collected on each site in August (on SAE in 2011; on ZOTTO in 2013; on Tura site in 2007–2010) to assess how SR related to the other chemical and biological parameters of soil. We excavated mineral soil samples down to the top 10-cm soil of the active layer with 3–4 replications close to the SR collars, and then put them into the refrigerator and kept at 4 °C until transportation. After the transportation, samples were delivered to the laboratory and processed as described earlier (Menyailo et al. 2002; Masyagina et al. 2015; Makhnykina et al. 2016). Then collected soil material was sieved to obtain < 2-mm fraction. Root material remained after the soil sieving was used for root biomass evaluation. Root biomass was assessed at the Tura sites (larch roots) and the ZOTTO sites (pine roots). Soil and roots were dried at 60 °C for 48 h. Then, dried soil material was separated into subsamples for soil C and N contents analyses, and for the incubation experiments for the evaluation of soil microbial activity.
Laboratory analyses and incubations
Mineral soil C and N contents
As for the SAE sites, total soil C and N contents were determined by Dumas combustion and gas chromatography (Heraeus elemental analyzer; Menyailo et al. 2002). As for ZOTTO and Tura sites, total soil C and N contents were evaluated by the element analyzer Elementar Vario EL (Elementar, Germany) (Makhnykina et al. 2016; Masyagina et al. 2019).
Soil microbial activity
To obtain characteristics of soil microbial activity (soil basal respiration (BR, mg CO2-C kg−1 day−1) and substrate-induced respiration (SIR, mg CO2-C kg−1 day−1)), we conducted the soil incubation experiments. As for the SAE sites, obtained mineral soil subsamples were incubated in 200-ml glass containers at the soil absolute moisture of 58%. The rates of BR and SIR were measured using the LI-8100A system (Matvienko et al. 2014). As for Tura and ZOTTO sites, soil subsamples (5 g) were incubated in 250-ml glass flasks at the soil absolute moisture of 60%. For SIR estimation, we added 0.1 ml of glucose-mineral solution in a flask to obtain glucose concentration equal to 10 mg g−1 of soil. Then flasks were sealed with resin stoppers hermetically and incubated at 22 °C. Soil gases from the flasks were taken twice. First, just after the flasks had been sealed, and last — after 24 h (in case of BR) or after 3 h (in case of SIR). The CO2 concentration was analyzed with a gas chromatographer Agilent 6890N at a Collective Using Center of Sukachev Institute of Forest SB RAS (Krasnoyarsk, Russia) (Masyagina et al. 2015).
Data analyses
Studied parameters (SR, TS5, θg, C and N contents, C/N, BR, and SIR) were tested for normality before analysis. When data sets were non-normally distributed, we performed a log-transformation to normalize the data. We used two-way factorial analysis of variances (ANOVAs) to test the main and combined effects of the study site (location and latitude: SAE, ZOTTO, Tura) and time (month of the growing period: June, July, August, September) on variables: SR, TS5, θg, C and N contents, C/N ratio, BR, and SIR (Table 4). The Tukey HSD (p adjusted) was used for the Tukey multiple comparisons of means. Principal component analysis (PCA) was performed and visualized using R-package “factoextra”. Correlation analysis between the parameters (SR, TS5, θg, C and N contents, C/N, BR, SIR, roots biomass, VegH, and LitD) was determined with Pearson’s product-moment correlation coefficients (significant at p < 0.05 or p < 0.01, Online Resource 3–4 of Electronic Supplementary Materials). R-package “aplpack” with function “faces” was used to create the Chernoff faces diagram (Online Resource 1 of Electronic Supplementary Materials, Chernoff 1973). The analyses of the obtained data were performed using RStudio version 3.6.2 – © 2009–2020 RStudio, Inc.
Results
SR rates across the different habitats of the Krasnoyarsk region: spatial and seasonal analysis
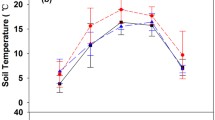
Study sites within the large region of the Russian Federation demonstrated a wide diversity of environmental conditions and ecosystem characteristics (Tables 2 and 3). Analysis of the main and combined effects of the study site (location) and time (month of a growing period) on the examined parameters across the dominant forest types of Krasnoyarsk region (SAE, ZOTTO, and Tura) has shown that sites had significant differences for all studied parameters, except for the SR (Table 4). SR rates did not differ significantly among the study sites (Fig. 2, Table 4). Three parameters had clear seasonal dynamics — TS5, θg, and SR (Fig. 2, Table 4).
SR rates (μmol CO2 m−2 s−1), TS5 (°C), and θg (%) in larch (NL–Tura, SL–SAE) and pine (NP–ZOTTO, SP–SAE) stands of different locations (northern or southern) over the growing period. The horizontal line within the box shows the median, box boundaries indicate 25th and 75th percentiles, and whiskers represent highest and lowest values; dots above or below whiskers show outliers. Medians followed by the different letters in the same curve are significantly different at p < 0.05 according to the Tukey multiple comparisons of means analysis. The upper case letters describe differences in SR between the different locations (NL, NP–northern, or SL, SP–southern) within the same month. The lower case letters describe the differences of SR, TS5, and θg values at the same location in different months
Despite the spatial variation of SR found in our study (0.4–12.5 μmol CO2 m−2 s−1, Fig. 2), there were no significant differences in the SR between the northern and southern locations (Fig. 2).
Despite the high SR heterogeneity in Tura sites (0.40–12.5 μmol m−2 s−1), we observed its distinct seasonal dynamics with maximum values in June–August (significantly different from September) and minimum—in September, which was accompanied by the fast drop in soil temperatures at this period (Fig. 2). At ZOTTO and SAE sites, the analysis of seasonal SR fluctuations showed a clear seasonal temperature-SR relation over the growing season (Fig. 2). Here, SR values appeared to be visually higher (but not significantly) in August (ZOTTO) and the middle of the growing season (July–August; SAE).
Environmental and biological factors affecting SR rates
As PCA showed, all three study sites took place in three different clusters (Fig. 3). The main variation was captured by two first components (РС1– Tura and РС2-ZOTTO); thus, they describe 94.1% of all dispersion (Fig. 3). Biological and environmental parameters (soil bulk density, annual snowfall, D, SIR, H, C, etc.) equally contributed to the PC1. Environmental parameters (annual Tair, season duration, Jan T, SAT > 5, summer TS5, June T, etc.) were responsible for PC2 (Online Resource 2 of Electronic Supplementary Materials). PC3 (SAE)—the minimal principal component—falls to the SR, SR per season, and BR, which are inter-correlated between each other (Fig. 3). The orientation of the SR in the PCA plot (Fig. 3) accounts for the combination of factors where each variable influences the SR. Figure 3 indicates that the mean summer θg, stand age, and C/N are the closest variables to the SR, so they can be determined as the most influential (namely, positively correlated) variables. PCA plot points to the negative correlations between SR and annual Tair, season duration, and air T in January. Inter-correlations in the PCA plot (Fig. 3) correspond to the tables of correlations between studied factors in Online Resource 3-4 of Electronic Supplementary Materials. Thus, the overall regional patterns of the SR were governed by the environmental variables (air temperature, θg), stand age, and ground vegetation-related parameters (VegH, LitD) (Online Resource 3 of Electronic Supplementary Materials). However, in the case of the distinct study site, we obtained the site-specific correlations between the parameters (Online Resource 4 of Electronic Supplementary Materials). Thus, at the SAE sites, SR better correlated with θg and soil microbial activity (BR, SIR) among all available studied parameters (Online Resource 4 of Electronic Supplementary Materials). At the ZOTTO sites, there was a significant correlation between SR and TS5. In the Tura study area, SR significantly depended on the stand age, air temperature, TS5, θg, soil C and N contents, root biomass, VegH, and LitD. Remarkably, soil θg has influenced SR in SAE and Tura in the opposite way. We found a negative correlation between SR and θg in the water-saturated soils of Tura sites; while, in the SAE sites, SR was positively related to θg.
Principal component analysis (PCA) of the studied environmental parameters (variables PCA), correlations, and different locations (individuals PCA). Individuals PCA: 1–Tura-NL, 2–ZOTTO-NP, 3–SAE-SL, 4–SAE-SP. Variables PCA: mean SR (SR), stand age (SA), mean annual air temperature (annual.Tair), mean summer TS5 (summer.TS5), mean summer θg at a depth of 5 cm (summer.WC), mean soil C content (C), mean soil N content (N), C/N (C.N), mean soil BR (BR), mean soil SIR (SIR), litter thickness (LitD), SR per season (SR.per.season), soil bulk density (soil.BD), average DBH of a tree (D), average H of a tree (H), annual snowfall (annual.snowfall), annual precipitation (annual.precipitation), growing season duration (season), sum of air temperatures higher than 5 °C (SAT.5), mean July air temperature (June.T), mean January air temperature (Jan.T). Contributions of parameters are presented in the Online resource 2 of Electronic Supplementary Materials
We found the soil microbial activity to be varied strongly within three sites (10.3–86.0 mg CO2-C kg−1 day−1 as for mean BR; 18.9–399.6 mg CO2-C kg−1 day−1 as for SIR, Table 4), but significantly correlated with SR only at one study site, i.e., SAE (Online Resource 4 of Electronic Supplementary Materials). We examined how soil microbial activity (BR, SIR) linked to the different studied parameters. It has been shown that BR and SIR were closely correlated to the soil C and N contents, C/N ratio, and microclimatic parameters (TS5, θg) (Fig. 4, Online Resource 3 of Electronic Supplementary Materials). Figure 4 shows that, at the ZOTTO sites, the soil microbial activity is very low. For example, BR and SIR values are limited to 20 and 50 mg CO2-C kg−1 day−1, respectively (Fig. 4). Whereas, at the Tura sites, these values are higher than the level of 100 (as for BR) and 300 (as for SIR) mg CO2-C kg−1 day−1, respectively.
At the SAE sites, soil C (2.4–7.4%) and N (0.16–0.46%) contents were highest among all study sites. Soil C content was the lowest at ZOTTO (0.20–1.47%) compared to Tura (1.76–7.12%) and SAE sites. The range of soil N content at the ZOTTO sites was narrower compared to Tura sites (0.01–0.04% vs. 0.08–0.32%) (Fig. 4i).
We observed the highest variability of the soil C/N ratio at the ZOTTO sites (C/N = 23–36, Fig. 4c, f). At the Tura sites, with all their heterogeneity, the C/N range was rather narrow (21–25), and, at SAE, soil C/N was the lowest, and its range was the narrowest (15–17).
The tightest relationship (r = 0.98, p < 0.01, Fig. 4i, Online Resource 3 of Electronic Supplementary Materials) was revealed between C and N contents in the mineral soil. There is a clear distribution of C–N paired values among the study sites in Fig. 4i where Tura values slightly cross SAE values and fully distinguish from ZOTTO values.
Since two study sites (SAE and ZOTTO) were presented by the mono-aged stands, we could assess the influence of stand age on SR only among the larch stands located in Tura. We found a strong correlation between the stand age and SR in Tura sites (Fig. 5, Online Resource 3-4 of Electronic Supplementary Materials).
Variation of the SR rates (μmol CO2 m−2 s−1) in forest stands of different age over the growing period (N = 871). Every point represents one measurement (an average of 4–20 statistical replications). The green line represents a linear regression for the Tura study site only. The corresponding equation designates the stand age-SR relationship in Tura sites
To study how the tree species (larch and pine) influence the SR, we compared the SR rates in all three research sites (Fig. 6). In our study, soils of northern locations (Tura and ZOTTO) differed by the characteristics from each other. Therefore, we could not extract the tree species’ effect on SR. However, in the southern locations (SAE sites: SL and SP), soils were the same. Therefore, at the southern sites, the tree species’ effect on SR can be extracted. Within SAE sites, there were no significant differences in SR among the tree species observed; SR in the larch stand was visually slightly higher than that in the pine stand. In northern locations, SR in larch (NL) and pine (NP) stands did not differ significantly as well (Fig. 6). The same species of northern and southern locations did not differ either.
SR (μmol CO2 m−2 s−1) rates depending on the dominant tree species (larch or pine) of different locations (NL–Tura, SL–SAE, NP– ZOTTO, SP–SAE), but regardless of the studied period in the month of the growing season. The horizontal line within the box indicates median; box boundaries indicate 25th and 75th percentiles, and whiskers indicate highest and lowest values; dots above or below whiskers indicate outliers. Medians followed by the different letters in the same curve are significantly different at p < 0.05 according to the Tukey multiple comparisons of means analysis.
SR changes in high latitudes over the past 15 years
To understand the long-term chronological changes of SR in high-latitude forest ecosystems, we addressed to Tura site (64° N), which is rather close to Arctic Circle. As far as we gathered big data on SR in the natural permafrost larch stands at the Tura site for a long-term period (1995–2010), we could evaluate changes of SR over the 15 years. Figure 7a shows the significant changes in SR during the study period. Thus, in 2000–2004, a significant decrease in SR was observed in intact larch stands in Tura sites, while over the past 5 years period (2005–2010), the increase of SR was found. Furthermore, we found an increase in BR over a 2007–2010 period (Fig. 7).
SR (μmol CO2 m−2 s−1) and BR (mg CO2-C kg−1 day−1) rates in chronology (1995–2010) in Tura site. The blue line represents GAM (generalized additive models) for SR and linear regression for BR. Grey shadows represent confidence intervals (standard error). The horizontal line within the box indicates median; box boundaries indicate 25th and 75th percentiles, and whiskers indicate highest and lowest values; dots above or below whiskers indicate outliers. The letter describes differences in SR presented at the year periods compared at p < 0.05 according to the Tukey multiple comparisons of means analysis.
Discussion
SR rates across the different habitats of the Krasnoyarsk region: spatial analysis
This study presented the long-term research of SR rates measured in the key forest types of the central Siberia in the Krasnoyarsk region, Russia (SAE, ZOTTO, and Tura) over the growing period (June–September). All these sites were in middle latitudes (56–64° N) but represented contrasting geographical locations characterized by a different climate, landscape, and environment (Table 2). We found that the site location has caused the difference in the majority of the studied parameters between study sites, except for the SR (Table 4). Our first prediction that SR would be higher in the southern location appeared wrong. There were no significant differences in SR observed among the study sites. Glagolev et al. (2018) also found that SR measured in the south taiga subzone (Plotnikovo, Tomsk, Russia) did not differ significantly from the values obtained in tropical forests. Kudeyarov and Kurganova (2005) estimated about 100 sites in regions throughout Russia (excluding permafrost zone), and they reported similar SR rates (0.46–6.67 μmol m−2 s−1) over the growing season in the northern and southern taiga of Russia. However, we obtained larger seasonal SR values per growing season at southern locations (i.e., 1049 and 1352 g CO2 m−2 in SAE), compared to northern sites (1150 g CO2 m−2 in ZOTTO and 793 g CO2 m−2 in Tura) due to the longer growing season in southern parts (SAE)—150 days vs. 74 days in Tura or 120 days in ZOTTO. Ongoing climate change has already caused warming processes in high latitudes coupling with permafrost degradation. Besides, the climate-induced extension of the duration of the growing season, mostly owing to earlier spring, causes earlier phenological start—bud break and physiological activity of trees, which results in inputs of roots exudates to the rhizosphere. This, in turn, stimulates soil microbiota and can escalate the SR in permafrost habitats.
High variability of the SR across these contrasting study sites was within the range of 0.4–12.5 μmol CO2 m−2 s−1 (Fig. 2). Figure 8 shows a similar pattern of such wide SR variation across the forest ecosystems worldwide (Online Resource 5 of Electronic Supplementary Materials). Variations of the environmental and biological factors (TS5, θg, C and N contents, C/N, BR, and SIR) were likely contributed to both temporal and spatial variability in SR within sites. It coincides well with the other studies, where SR heterogeneity has linked to microclimate parameters (Chen and Tian 2005; Bond-Lambert and Thompson 2010b; Kim et al. 2012; Yvan-Durocher et al. 2012; Kumpu et al. 2018). Moreover, the large variety in SR has been attributed to the litter quality (Hobbie 1996), root production, and root respiration (Kajimoto 2010), as well as to soil microbial biome structure and activity (Bond-Lambert et al. 2018). A stand age correlated with SR and contributed to its variation in Tura sites (Fig. 5, Online Resource 3-4 of Electronic Supplementary Materials). Abaimov et al. (2000) displayed that forest fire history through the change in the landscape characteristics and high heterogeneity of environmental conditions can cause large SR variability. Namely, the recovering of the active layer depth in the course of postpyrogenic succession has been associated with the changes in SR (Masyagina et al. 2015). Yanagihara et al. (2000) reported on the SR range of 0.6–12.6 μmol m−2 s−1 related to the depth of active layer (ordinary soil depth to the front of frozen soil) at the north- and south-facing slopes in > 220-year-old L. gmelinii stands in Tura. Specific cryogenic micro-topography (or microrelief, not flat ground surface), namely earth hummock, is often developed in the permafrost zone (Kajimoto 2010). It provides patterned environments on the forest floor, so-called troughs, and hummocks. Soil temperature on earth hummocks is usually 3–5 °C or even 5–8 °C higher than that in depression sites (troughs). Troughs are much moister than hummocks. These patterns in hydroclimatic characteristics result in differences in plant species composition developed in the earth hummock and troughs, which also contribute to SR spatial variability (Osawa et al. 2010).
SR (μmol CO2 m−2 s−1) measured across the forest ecosystems worldwide based upon 33 publications (N = 135). References linked to the legend are displayed in Online Resource 2 of Electronic Supplementary Materials
The lack of pronounced microrelief, i.e., flat ground surface, could narrow the SR variability. Sawamoto et al. (2000) observed a very narrow variety of the SR (4.16–4.20 μmol m−2 s−1) in a natural L. gmelinii forest on the relatively flat sites in Northeastern Siberia. Similarly, a lower variation of the SR observed in SAE and ZOTTO can be explained by the simple structure of forest stands, low biodiversity, flat landscape, and limited observations with only one type of stand age presented.
SR rates across the different habitats of the Krasnoyarsk region: seasonal analysis
The second hypothesis occurred to be right since we found distinct seasonal dynamics of important variables—TS5, θg, and SR (Table 4). Strong seasonal dynamics of the SR observed in Tura sites showed the maximum values in June–August (significantly different from September) and minimum—in September (Fig. 2). First, the seasonal dynamics of the SR correlated to the TS5 (Fig. 2, Online Resource 4 of Electronic Supplementary Materials). Therefore, the higher SR corresponds to the higher TS5. Second, the high June SR rates in Tura, despite the low TS5, might be linked to the fast and active decomposition of larch litter accumulated in the year before. In the next year, in June, the decomposition has quickly continued after the snow has melted, thus, thereby stimulates the soil microbial respiration that has resulted in high SR values over the growing period in Tura site. Third, such SR fluctuations may be also associated with a specific hydrothermal regime with limited precipitation (20–30 mm) in July at a rather low TS5 (6–14 °C) due to the cold shallow active layer. This similar to drought, conditions seem to inhibit microbial mineralization of the soil organic matter and autotrophic respiration (Masyagina et al. 2015).
At the ZOTTO and SAE sites, the examination of seasonal SR dynamics exhibited a visual seasonal temperature-dependent variation in SR over the growing period (Fig. 2). However, at the ZOTTO sites, SR tends to be maximal (but not significantly) in August, which corresponds to the most favorable microclimatic conditions (Makhnykina et al. 2020). At the SAE sites, SR was highest (but not significantly) in the middle of the growing season (July–August), possibly due to the high dependency SR from the seasonal changes in the soil θg (Online Resource 4 of Electronic Supplementary Materials, Matvienko et al. 2014). At all sites, we expected the higher SR in the middle of the growing period due to both higher microbial and root activity, as well as the higher plant photosynthesis and photosynthate allocation to the roots (Högberg et al. 2001), and an increase of carbon inputs to the soil at this period. However, at the Tura site, the vegetation activity, as well as SR, coupled with the available rich soil organic matter stocks in the middle of the growing season, maybe neglected by the harsh microclimatic factors. In the same way, midsummer limitation of the soil water content in ZOTTO can potentially decrease SR values and its variability.
Environmental and biological factors affecting SR rates
In our study, the regional patterns of SR over the growing period were showed to depend principally on the fluctuations of environmental factors (air temperature, θg) and ground vegetation-related parameters (VegH, LitD) (Online Resource 3-4 of Electronic Supplementary Materials). As Fig. 3 shows SR correlates to the combination of factors where each variable influences the SR. PCA indicates that the mean summer θg at a depth of 5 cm, stand age, C/N are the most influential (positively correlated) variables for SR. Negative correlations were found between SR and annual air temperature, season duration, and air temperature in January. Inter-correlations in the PCA plot (Fig. 3) supported the tables of correlations between the studied factors in Online Resource 3-4 of Electronic Supplementary Materials.
Performed PCA put all three study sites in three different clusters (Fig. 3), pointed thus to completely different sets of correlations in every study site (Online Resource 4 of Electronic Supplementary Materials). Biological and environmental parameters (soil bulk density, annual snowfall, D, SIR, H, C contents, etc.) are equally contributed to the PC1 (Tura sites), which coincides well with correlations found between SR and air temperature, TS5, θg, soil C and N contents, root biomass, VegH, and LitD (Online Resource 2, 4 of Electronic Supplementary Materials). Environmental parameters (annual air temperature, season duration, air temperature in January, SAT > 5, summer TS5, air temperature in June, etc.) were responsible for PC2 (ZOTTO sites); here was a significant correlation between SR and TS5 (Fig. 3, Online Resource 2, 4 of Electronic Supplementary Materials). Finally, PC3 (SAE)—the minimal principal component—was influenced by the SR, SR per season, and BR, which inter-correlated between each other (Fig. 3, Online Resource 4 of Electronic Supplementary Materials).
Surprisingly, there was no moisture limitation found in SR rates across all Tura sites through 12 years of our monitoring. θg of the study sites may be supplied from frozen soil in the permafrost zone, even though the total amount of precipitation has coincided with the steppe region after Whittaker’s scheme (Whittaker 1975; Osawa et al. 2010). Irrespective of our initial expectation, θg showed a very weak negative correlation with SR in the Tura larch stands under limited precipitation of less than 400 mm per year. Another thought to be an important driver for SR—TS5 was weakly correlated with SR. Morishita et al. (2010) found a negative correlation between SR and θg at the 105-year-old larch stand near Tura. According to Köster et al. (2018), the soil moisture, mosses biomass, and the total C content of the soil did not significantly affect SR in Tura. Numerous correlations obtained in our study confirm the spatial and environmental heterogeneity of the Tura site, which causes high variability in SR in Tura sites and restrains its prediction.
Generally, SR is assumed to be an integral value of the ecosystem processes, including pedogenesis, soil organic matter transformation, root and soil microbial activity, and it is widely using for evaluation of the forest ecosystems’ sustainability and functioning (Jong et al. 1974). Its integral nature causes complexity in modeling and predicting SR’ temporal and spatial dynamics since all widely varying CO2-generating processes and their response to various fluctuating environmental factors should be considered (Zobitz et al. 2008). Soil microbial activity is an important process contributing to the SR (Laganière et al. 2012; Wang et al. 2018), which remains highly uncertain (Zhou et al. 2009). In our study, soil microbial activity varied strongly within three sites (Table 4), but significantly related to the SR only at SAE (Online Resource 4 of Electronic Supplementary Materials). Wang et al. (2018) also found high spatial variation in the soil heterotrophic respiration in a latitudinal gradient across tropical and cold-temperate zones. As Online Resource 3 of Electronic Supplementary Materials and Fig. 4a–h, j, k display, soil microbial activity (BR, SIR) closely correlates to the soil C and N contents, C/N ratio, and microclimatic variables (TS5, θg). There was a similar pattern on how the soil microbial activity depends on the C/N ratio across the study sites. Soil microbial activity was very low at the ZOTTO sites, e.g., BR and SIR values were limited to 20 and 50 mg CO2-C kg−1 day−1, respectively. This could be due to the high C/N values (24–36) at the ZOTTO sites (Fig. 4c, f), which point to low soil fertility characteristics. Also, low BR in ZOTTO might be mediated through the high soil bulk density (Online Resource 3-4 of Electronic Supplementary Materials). The higher bulk density leads to soil compaction, which limits access to water and oxygen by soil microbiota (Wang et al. 2018). At the Tura sites, however, these values were higher than 100 (BR) and 300 (SIR) mg CO2-C kg−1 day−1 (Fig. 4c, f). In our study, the soil microbial activity is very variable, which seems to be related to the site and environmental heterogeneity of a Tura site. The range of the soil N contents at the ZOTTO site was narrower compared to Tura sites (Fig. 4h, k); therefore, it is relevant that the soil microbial activity is constrained at the ZOTTO site. At the SAE sites, soil C and N contents were highest among all study sites, whereas the soil microbial activity was in the same range as that is at the Tura site (Fig. 4g, h, j, k). Therefore, we supposed that soil microbiota in the northern larch stands (Tura) in temperature-limited conditions of soil N deficit shows activity, which is similar to the southern location (SAE).
The maximum range of the soil C/N ratio was observed at the ZOTTO sites (Fig. 4c, f) compared to Tura sites, despite their heterogeneity, and at the SAE sites, where the soil C/N was of the lowest value and its range was the narrowest. We observed the tightest relationship (r = 0.98, p < 0.01, Fig. 4i, Online resource 3 in Electronic Supplementary Materials) between C and N contents in the mineral soil. Figure 4 i presents a clear distribution of C–N paired values among the study sites where Tura values slightly cross SAE values and fully distinguish from ZOTTO values. In our study, these C–N contents trends well corresponded to the site-specific microbial activity. This finding supports the idea that, in mineral soil, C and N contents are the main indicators of soil microbial activity (Qu et al. 2018). Furthermore, the correlation between C, N, and SR can explain, why, despite the summer TS5 limitation to 10 °C, soil microbial activity in permafrost soils is comparable to the BR and SIR values in soils of the southern locations (SAE sites, Fig. 4g, h, j, k).
Vegetation-relative parameters (VegH, LitD) are important factors for SR since the ground vegetation cover and litter (Gao et al. 2018) are thermal insulating layers which are regulating the soil thermal regime. Here, VegH and LitD are mostly to discuss in Tura sites since the data on these parameters for ZOTTO and SAE sites are not fully available. In Tura sites, SR was found to be positively correlated with LitD and negatively correlated with VegH (Online resource 3-4 in Electronic Supplementary Materials). It is due to the LitD is contributed to the CO2 emission processes, which are a part of SR, since, in boreal forests, litter (or soil organic layer) represents the main rooting zone and is an important habitat for soil microbiota (Brassard et al. 2009; Gao et al. 2018; Gentsch et al. 2018). Soil organic layer was found to have a linear relationship with an increase in SR (Maier and Kress 2000; Sulzman et al. 2005). Makhnykina et al. (2020) observed the lowest CO2 emission flux (1.1 ± 0.1 μmol CO2 m−2 s−1) at the disturbed plot due to the absence of vegetation and genetic soil horizons, thus supporting our finding. Litter was found to be an important factor for winter SR in pine and larch forests in China (Gao et al. 2018). Kumpu et al. (2018) suggested that the thickness of the soil organic layer could be a strong source of SR variation. VegH parameter describes the height of ground vegetation. Ground vegetation has contributed significantly to the C cycle of boreal ecosystems (Kolari et al. 2006). Its CO2 accumulation activity thus can decrease the SR owing to the emitted CO2 refixation (Widén 2002). Variations in VegH are also associated with the site micro-topography, which is typical for the permafrost areas (Komulainen et al. 1999; Osawa et al. 2010).
As far as the tree species effects on the soil processes were well shown (Menyailo and Hungate 2005), we tried to check how the different tree species (larch or pine) influence the SR. For this, we compared SR rates at all three study sites (Fig. 6). Within SAE sites, there were no significant differences in SR among the tree species observed. In the northern locations, SR in larch (NL) and pine (NP) stands did not differ significantly as well (Fig. 6); although, there was a limitation of this study—different soil types in northern locations. Our findings were agreed to the results reported by Menyailo and Hungate (2005). Opposite to our results, Gao et al. (2018) showed that winter SR was higher in pine forests compared to larch forests in northeastern China. They explained higher SR in the pine forest by the higher soil microbial biomass and fungi abundance. Regarding the other parameters, we found that the soil BR rates in larch forest appeared to be significantly higher compared to the pine stand. Since we found similar SR among the sites, the high BR in larch stands (NL) should have been compensated by the low root respiration due to significantly low root biomass compared to the pine stands (NP).
Finally, we would attract the interest to Tables 2 and 3, which briefly show the wide variety of environmental parameters and soil biochemical processes within the Krasnoyarsk region, Russia. Interestingly, in the studied latitudinal gradient of the study sites, the lower mean annual air temperature corresponds to the higher (but non-significant) mean SR rate (Tables 2 and 3). The same relationship has been observed in the latitudinal changes in SR across Alaska, USA (Kim et al. 2012). They showed that the latitudinal gradient of annual mean air temperature might affect the distribution of soil CO2 efflux in a north-south direction over Alaska. Thus, different regional patterns of SR observed in the Krasnoyarsk region can be included in general GHG simulation models even though there was a big spatial and temporal heterogeneity. Observed high SR heterogeneity potentially would cause a high complexity in GHG monitoring and modeling over the large territory. To overcome this problem, the further expansion of new study sites for continuous observation of GHG emissions and related parameters should be done. Besides, the data findings gained from this research can be included in the existing SR databases and employed for building and validation of regional and state-scale models of С budgets, as well as used to monitor the annual fluctuations in the С cycle under ongoing climate change.
SR changes in high latitudes over the past 15 years
Past decades “global climate change” topic has been headed among the other ecological issues (Ciais et al. 2013; Millar et al. 2017; Bjorkman et al. 2018; Gaüzère et al. 2018; Parazoo et al. 2018). Especially, it points to the maximum impact of climate change on high-latitude ecosystems (Kattenberg et al. 1996; Boy et al. 2019; Ito et al. 2020), where a considerable amount of soil C is stored in permafrost (Tarnocai et al. 2009). Highly likely, positive feedback of the high latitudes to climate change would develop due to a gradual increase in air temperatures that would stimulate the release of CO2 from the soils because of enhanced decomposition of the soil organic matter. For that purpose, we evaluated changes in SR over 15 years (1995–2010) at the Tura site (64° N), which is rather close to the Arctic Circle. According to our findings (Fig. 7a, b), in intact Tura larch stands, SR has increased significantly in the past 5-year period (2005–2010) compared to the previous 10 years. An increasing trend of SR was also estimated for the boreal forest (Zhao et al. 2017). This significant SR escalation over the past 5 years can relate to the accelerated soil microbial respiration activity (i.e., heterotrophic respiration, Fig. 7c). Bond-Lambert et al. (2018) also point to this possibility. Laganière et al. (2012) report on higher SR linked to higher BR in the Canadian boreal forest, as well. Tremblay et al. (2018) reported the increasing soil heterotrophic respiration in boreal soils transplanted to warmer regions. Tang et al. (2020) investigated the spatial and temporal pattern of soil heterotrophic respiration for the 1980–2016 period on a global scale (using Global Soil Respiration Database) and showed a significant and increasing trend of BR in temperate and boreal zones. Our study has limitations on providing the long-term soil temperature (TS5) data for this region for all period of investigation to support the role of climate change for the SR in Tura. However, our findings—enhanced SR and soil heterotrophic respiration—confirm that such an increase in SR and BR might be due to the raise of soil carbon availability as a substrate for soil microbiota over the past decades. Such boosted carbon availability can be caused by the permafrost thawing (Jansson and Tas 2014; Parazoo et al. 2018; Boy et al. 2019) or due to the increased tree root exudates input as an acclimation of trees to the warmer environment (Norby and Luo 2004). However, in this study, we did not consider the specific soil processes as a C stabilization on minerals (Gentsch et al. 2018), which can constrain permafrost soil organic C degradability and should be accounted for that issue in future research.
Conclusions
Actual SR at the three different latitudinal gradations (SAE, ZOTTO, and Tura) showed very similar rates; however, the variation of SR was very high. At the Tura site, SR has slightly accelerated (but not significantly), which may be associated with the higher microbial activity (BR). Due to the abundance of SR data in Tura (12 research plots, long monitoring period from 1995 to 2010), we have evaluated the high SR spatial and seasonal heterogeneity (0.4–12.5 μmol m−2 s−1). The SR was correlated to plenty of factors and parameters, such as TS5, θg, C and N contents, C/N, BR, SIR, micro-topography, tree age, and the month of the growing season. However, despite the similar rates of SR observed in latitudinal locations, the duration of the growing season has differed from 74 days in Tura to 150 days in SAE. Due to this, southern locations showed larger seasonal SR values per growing season (i.e., 1049 and 1352 g CO2 m−2 in SAE) compared to the northern sites (1150 g CO2 m−2 in ZOTTO and 793 g CO2 m−2 in Tura).
The set of SR-affected parameters have differed in various study sites. Thus, at the SAE sites, SR strongly correlated to the θg and microbial activity (BR, SIR). At the ZOTTO sites, TS5 was the only factor related to the SR. At the Tura sites, SR was significantly dependent on the environmental parameters (air temperature, TS5, θg), soil C and N contents, tree age, root biomass, and ground vegetation-related parameters (VegH, LitD). Inter-site comparison of the SR showed no effect of dominant tree species on the SR rates.
Climate change in high latitudes, as a fact, is generally accepted. The effect of warming on the SR is an important component in predicting the general response of net C balance in boreal ecosystems at high latitudes under climate change. In the high-latitude permafrost site (Tura, 64° N), we observed the SR escalation over the past 5 years (2005–2010), coupled with the accelerated soil microbial activity (2007–2010); however, other factors, which did not account for that SR increase, could be involved too.
References
Abaimov AP, Zyryanova OA, Prokushkin SG, Koike T, Matsuura V (2000) Forest ecosystems of the cryolithic zone of Siberia; regional features, mechanisms of stability and pyrogenic changes. Eurasian J For Res 1:1–10
Abaimov AP, Prokushkin SG, Sukhovol’skii VG, Ovchinnikova TM (2004) Evaluation and prediction of postfire condition of Gmelin larch on permafrost soils in Middle Siberia. Lesovedenie 2:3–11 (In Russian)
Abbott BW, Jones JB (2015) Permafrost collapse alters soil carbon stocks, respiration, CH4, and N2O in upland tundra. Glob Chang Biol 21:4570–4587
Bhupinderpal-Singh NA, Lofvenius MO, Högberg MN, Mellander PE, Högberg P (2003) Tree root and soil heterotrophic respiration as revealed by girdling of boreal Scots pine forest: extending observations beyond the first year. Plant Cell Environ 26:1287–1296
Bjorkman AD, Myers-Smith IH, Elmendorf SC, Normand S, Rüger N, Beck PSA, Blach-Overgaard A, Blok D, Cornelissen JHC, Forbes BC, Georges D, Goetz SJ, Guay KC, Henry GHR, HilleRisLambers J, Hollister RD, Karger DN, Kattge J, Manning P, Prevéy JS, Rixen C, Schaepman-Strub G, Thomas HJD, Vellend M, Wilmking M, Wipf S, Carbognani M, Hermanutz L, Lévesque E, Molau U, Petraglia A, Soudzilovskaia NA, Spasojevic MJ, Tomaselli M, Vowles T, Alatalo JM, Alexander HD, Anadon-Rosell A, Angers-Blondin S, Beest M, Berner L, Björk RG, Buchwal A, Buras A, Christie K, Cooper EJ, Dullinger S, Elberling B, Eskelinen A, Frei ER, Grau O, Grogan P, Hallinger M, Harper KA, Heijmans MMPD, Hudson J, Hülber K, Iturrate-Garcia M, Iversen CM, Jaroszynska F, Johnstone JF, Jørgensen RH, Kaarlejärvi E, Klady R, Kuleza S, Kulonen A, Lamarque LJ, Lantz T, Little CJ, Speed JDM, Michelsen A, Milbau A, Nabe-Nielsen J, Nielsen SS, Ninot JM, Oberbauer SF, Olofsson J, Onipchenko VG, Rumpf SB, Semenchuk P, Shetti R, Collier LS, Street LE, Suding KN, Tape KD, Trant A, Treier UA, Tremblay JP, Tremblay M, Venn S, Weijers S, Zamin T, Boulanger-Lapointe N, Gould WA, Hik DS, Hofgaard A, Jónsdóttir IS, Jorgenson J, Klein J, Magnusson B, Tweedie C, Wookey PA, Bahn M, Blonder B, van Bodegom PM, Bond-Lamberty B, Campetella G, Cerabolini BEL, Chapin FS III, Cornwell WK, Craine J, Dainese M, de Vries FT, Díaz S, Enquist BJ, Green W, Milla R, Niinemets Ü, Onoda Y, Ordoñez JC, Ozinga WA, Penuelas J, Poorter H, Poschlod P, Reich PB, Sandel B, Schamp B, Sheremetev S, Weiher E (2018) Plant functional trait change across a warming tundra biome. Nature 562:57–62. https://doi.org/10.1038/s41586-018-0563-7
Boden TA, Marland G, Andres RJ (2009) Global, regional and national fossil-fuel CO2 emissions, TN: Carbon Dioxide Information Analysis Center, ORNL, Oak Ridge. http://cdiac.ornl.gov/trends/emis/overview 2006.html
Bond-Lambert B, Thompson A (2010a) A global database of soil respiration data. Biogeosciences 7:1915–1926
Bond-Lambert B, Thompson A (2010b) Temperature-associated increases in the global soil respiration record. Nature 464:582–597
Bond-Lambert B, Bailey VL, Chen M, Gough CM, Vargas R (2018) Globally rising soil heterotrophic respiration over recent decades. Nature 560:80–83
Boy M, Thomson ES, Acosta Navarro J-C, Arnalds O, Batchvarova E, Bäck J, Berninger F, Bilde M, Brasseur Z, Dagsson-Waldhauserova P, Castarède D, Dalirian M, de Leeuw G, Dragosics M, Duplissy E-M, Duplissy J, Ekman AML, Fang K, Gallet J-C, Glasius M, Gryning S-E, Grythe H, Hansson H-C, Hansson M, Isaksson E, Iversen T, Jonsdottir I, Kasurinen V, Kirkevåg A, Korhola A, Krejci R, Kristjansson JE, Lappalainen HK, Lauri A, Leppäranta M, Lihavainen H, Makkonen R, Massling A, Meinander O, Nilsson ED, Olafsson H, Pettersson JBC, Prisle NL, Riipinen I, Roldin P, Ruppel M, Salter M, Sand M, Seland Ø, Seppä H, Skov H, Soares J, Stohl A, Ström J, Svensson J, Swietlicki E, Tabakova K, Thorsteinsson T, Virkkula A, Weyhenmeyer GA, Wu Y, Zieger P, Kulmala M (2019) Interactions between the atmosphere, cryosphere, and ecosystems at northern high latitudes. Atmos Chem Phys 19:2015–2061. https://doi.org/10.5194/acp-19-2015-2019
Brassard BW, Chen HYH, Bergeron Y (2009) Influence of environmental variability on root dynamics in northern forests. Crit Rev Plant Sci 28:179–197
Casper JK (ed) (2010) Greenhouse gases: worldwide impacts. Facts on file, an imprint of InfoBase Publishing, New York
Chen H, Tian HQ (2005) Does a general temperature-dependent Q10 model of soil respiration exist at biome and global scale. J Integr Plant Biol 47:1288–1302
Chernoff H (1973) The use of faces to represent statistical association. JASA 68:361–368
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, De Fries R, Galloway J, Heimann M, Jones C, Le Quéré C, Myneni RB, Piao S, Thornton P, Carbon and Other Biogeochemical Cycles (2013) Climate Change 2013: The Physical Science Basis. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Dixon RK, Brown S, Houghton RA, Solomon AM, Trexler MC, Wisniewski J (1994) Carbon Pools and Flux of Global Forest Ecosystems. Science 263:185–190
Eliasson PE, McMurtrie RE, Pepper DA, Stromgren M, Linder S, Agren GI (2005) The response of heterotrophic CO2 flux to soil warming. Glob Chang Biol 11:167–181
Gao D, Peng B, Fan Z, Pei G, Bai E (2018) Different winter soil respiration between two mid-temperate plantation forests. For Ecol Manag 409:390–398. https://doi.org/10.1016/j.foreco.2017.11.029
Gardenas AI (2000) Soil respiration fluxes measured along a hydrological gradient in a Norway spruce stand in south Sweden (Skogaby). Plant Soil 221:273–280
Gaüzère P, Iversen LL, Barnagaud J-Y, Svenning J-C, Blonder B (2018) Empirical Predictability of Community Responses to Climate Change. Front Ecol Evol 6:186. https://doi.org/10.3389/fevo.2018.00186
Gentsch N, Wild B, Mikutta R, Čapek P, Diáková K, Schrumpf M, Turner S, Minnich C, Schaarschmidt F, Shibistova O, Schnecker J, Urich T, Gittel A, Šantrůčková H, Bárta J, Lashchinskiy N, Fuß R, Richter A, Guggenberger G (2018) Temperature response of permafrost soil carbon is attenuated by mineral protection. Glob Chang Biol 24:3401–3415
Glagolev MV, Ilyasov DV, Terentieva IE, Sabrekov AF, Mochenov SY, Maksutov SS (2018) Methane and carbon dioxide fluxes in the waterlogged forests of south and middle taiga of Western Siberia. IOP Conf Series: Earth and Environmental Science 138:012005. https://doi.org/10.1088/1755-1315/138/1/012005
Gordon AM, Schlentner RE, Van Cleve K (1987) Seasonal patterns of soil respiration and CO2 evolution following harvesting in the white spruce forests of interior Alaska. Can J For Res 17:304–310
Grogan P, Chapin FS III (1999) Arctic soil respiration: effects of climate and vegetation depend on season. Ecosystems 2:451–459
Hashimoto S, Carvalhais N, Ito A, Migliavacca M, Nishina K, Reichstein M (2015) Global spatiotemporal distribution of soil respiration modeled using a global database. Biogeosciences 12:4121–4132. https://doi.org/10.5194/bg-12-4121-2015
Hobbie SE (1996) Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol Monogr 66:503–522
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottosson-Lövenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
IPCC: Climate Change 2014: (2014) Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change In: Core Writing Team, Pachauri RK, Meyer LA (eds) IPCC, Geneva
Ito A, Reyer CPO, Gädeke A, Ciais P, Chang J, Chen M, François L, Forrest M, Hickler T, Ostberg S, Shi H, Thiery W, Tian H (2020) Pronounced and unavoidable impacts of low-end global warming on northern high-latitude land ecosystems. Environ Res Lett 15:044006
IUSS Working Group WRB. (2015) World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106 FAO, Rome
Jansson JK, Tas N (2014) The microbial ecology of permafrost. Nat Rev Microbiol 12:414–425
Jian J, Steele MK, Day SD, Thomas RQ (2018) Future global soil respiration rates will swell despite regional decreases in temperature sensitivity caused by rising temperature. Earth’s Future 6:1539–1554. https://doi.org/10.1029/2018EF000937
Jiang J, Shi F, Li B, Luo Y, Chen J, Chen J (2005) Separating rhizosphere respiration from total soil respiration in two larch plantations in northeastern China. Tree Physiol 25:1187–1195
Jong DE, Schappert HJV, MacDonald KB (1974) Carbon dioxide evolution from virgin and cultivated soil as affected by management practices and climate. Can J Soil Sci 54:299–307
Kajimoto T (2010) Root system development of larch trees growing on Siberian permafrost. In: Osawa A, Zyryanova OA, Matsuura Y, Kajimoto T, Wein RW (eds) Permafrost ecosystems: Siberian Larch Forests. Ecological Studies, Vol. 209, Springer Scicnce+Business Media B.V., pp 99–122
Kattenberg A, Giorgi F, Grassl H, Meehl GA, Mitchell JFB, Stouffer RJ, Tokioka T, Weaver AJ, Wigley TML (1996) Climate models-projections of future climate. In: Houghton JT, Meiro Filho LG, Callender BA, Henris N, Kattenberg A, Maskell K (eds) Climate change 1995: the science of climate change. 2nd assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 285–357
Kim Y, Kim S-D, Enomoto H, Kushida K, Kondo M, Uchida M (2012) Latitudinal distribution of soil CO2 efflux and temperature along the Dalton Highway, Alaska. Polar Science. https://doi.org/10.1016/j.polar.2012.11.002
Kolari P, Pumpanen J, Kulmala L, Ilvesniemi H, Nikinmaa E, Grönholm T, Hari P (2006) Forest floor vegetation plays an important role in photosynthetic production of boreal forests. For Ecol Manag 221:241–248
Komulainen V-M, Tuittila E-S, Vasander H, Laine J (1999) Restoration of drained peatlands in southern Finland: initial effects on vegetation change and CO2 balance. J Appl Ecol 36:634–648
Köster E, Köster K, Berninger F, Prokushkin A, Aaltonen H, Zhou X, Pumpanen J (2018) Changes in fluxes of carbon dioxide and methane caused by fire in Siberian boreal forest with continuous permafrost. J Environ Manag 228:405–415. https://doi.org/10.1016/j.jenvman.2018.09.051
Kudeyarov VN, Kurganova IN (2005) Respiration of Russian soils: database analysis, long-term monitoring, and general estimates. Eurasian Soil Sci 38:983–992
Kumpu A, Mäkelä A, Pumpanen J, Saarinen J, Berninger F (2018) Soil CO2 efflux in uneven-aged and even-aged Norway spruce stands in southern Finland. iForest 11:705–712. https://doi.org/10.3832/ifor2658-011
Kuzmichev VV, Pshenichnikova LS, Tretyakova VA (2005) Productivity of six tree species plantations for three decades in the Siberian afforestation experiment. In: Binkley D, Menyailo O (eds) Tree species effects on soils: implications for global change. Springer, Netherlands, pp 269–279
Laganière J, Paré D, Bergeron Y, Chen HYH (2012) The effect of boreal forest composition on soil respiration is mediated through variations in soil temperature and C quality. Soil Biol Biochem 53:18–27. https://doi.org/10.1016/j.soilbio.2012.04.024
Luo Y, Zhou X (2006) Soil respiration and the environment. Elsevier, Burlington
Maier CA, Kress LW (2000) Soil CO2 evolution and root respiration in 11 year-old loblolly pine (Pinus taeda) plantations as affected by moisture and nutrient availability. Can J For Res 30:347–359
Makhnykina AV, Prokushkin AS, Vaganov EA, Verkhovets SV, Rubtsov AV (2016) Dynamics of the CO2 fluxes from the soil surface in pine forests in Central Siberia. J Siberian Federal Univ Biol 3(9):338–357
Makhnykina AV, Prokushkin AS, Menyailo OV, Verkhovets SV, Tychkov II, Urban AV, Rubtsov AV, Koshurnikova NN, Vaganov EA (2020) The impact of climatic factors on CО2 emissions from soils of Middle-Taiga forests in Central Siberia: emission as a function of soil temperature and moisture. Russ J Ecol 51:46–56. https://doi.org/10.1134/s1067413620010063
Maljanen M, Hytönen J, Martikainen PJ (2001) Fluxes of N2O, CH4 and CO2 on afforested boreal agricultural soils. Plant Soil 231:113–121
Masyagina OV, Prokushkin AS, Prokushkin SG (2009) Long-term soil respiration observations in burnt area (of 1994) and in adjacent larch stand in central Evenkia (Russia). In: C/H2O/Energy balance and climate over boreal regions with special emphasis on eastern Eurasia: Proceedings of 4th International WS. Yakutsk, Russia, Nagoya, pp 99–100
Masyagina OV, Evgrafova SY, Titov SV, Prokushkin AS (2015) Dynamics of soil respiration at different stages of pyrogenic restoration succession with different-aged burns in Evenkia as an example. Russ J Ecol 46(1):27–35
Masyagina OV, Evgrafova SY, Bugaenko TN, Kholodilova VV, Krivobokov LV, Korets MA, Wagner D (2019) Permafrost landslides promote soil CO2 emission and hinder C accumulation. Sci Total Environ 657:351–364. https://doi.org/10.1016/j.scitotenv.2018.11.468
Matvienko AI, Makarov MI, Menyailo OV (2014) Biological sources of soil CO2 under Larix sibirica and Pinus sylvestris. Russ J Ecol 3:174–180
Menyailo OV (2008) The effect of afforestation on mineralization of soil organic matter. Russ J Ecol 39(1):21–25
Menyailo OV, Hungate BA (2005) Tree species effects on potential production and consumption of carbon dioxide, methane, and nitrous oxide: the Siberian afforestation experiment. In: Binkley D, Menyailo O (eds) Tree species effects on soils: implications for global change. Springer, Netherlands, pp 293–305
Menyailo OV, Hungate BA, Zech W (2002) Tree species mediated soil chemical changes in a Siberian artificial afforestation experiment. Plant Soil 242:171–182
Millar R, Fuglestvedt J, Friedlingstein P et al (2017) Emission budgets and pathways consistent with limiting warming to 1.5 °C. Nat Geosci 10:741–747. https://doi.org/10.1038/ngeo3031
Morishita T, Masyagina OV, Koike T, Matsuura Y (2010) Soil respiration in larch forests. In: Osawa A, Zyryanova O.A, Matsuura Y, Kajimoto T, Wein RW (eds) Permafrost ecosystems: Siberian Larch Forests. Ecological Studies. Vol. 209, Springer Scicnce+Business Media B.V., pp 165–182
Norby RJ, Luo Y (2004) Evaluating ecosystem responses to rising atmospheric CO2 and global warming in a multi-factor world. New Phytol 162:281–293. https://doi.org/10.1111/j.1469-8137.2004.01047.x
O’Neill KP, Kasischke ES, Richter DD (2003) Seasonal and decadal patterns of soil carbon uptake and emission along an age sequence of burned black spruce stands in interior Alaska. J Geophys Res–Atmos 108
Osawa A, Zyryanova OA, Matsuura Y, Kajimoto T, Wein RW (2010) Permafrost ecosystems: Siberian larch forests. Ecol Stud 209. https://doi.org/10.1007/978-1-4020-9693-8_1
Parazoo NC, Koven CD, Lawrence DM, Romanovsky V, Miller CE (2018) Detecting the permafrost carbon feedback: talik formation and increased cold-season respiration as precursors to sink-to-source transitions. Cryosphere 12:123–144. https://doi.org/10.5194/tc-12-123-2018
Pleshikov FI (ed) (2002) Forest ecosystems of the Yenisei River meridian. Nauka SB RAS, Novosibirsk (in Russian)
Prokushkin AS, Tokareva IV, Prokushkin SG, Abaimov AP, Guggenberger H (2008) Fluxes of dissolved organic matter in larch forests in the cryolithozone of central Siberia. Russ J Ecol 39:151–159
Prokushkin AS, Hagedorn F, Pokrovsky OS, Viers J, Kirdyanov AV, Masyagina OV, Prokushkina MP, McDowell WH (2018) Permafrost regime affects the nutritional status and productivity of larches in Central Siberia. Forests 9:1–18
Qu LY, Kitaoka S, Koike T (2018) Factors controlling soil microbial respiration during the growing season in a mature larch plantation in Northern Japan. J Soils Sediments 18:661–668. https://doi.org/10.1007/s11368-017-1799-9
Raich JW, Potter CS (1995) Global patterns of carbon dioxide emissions from soils. Glob Biogeochem Cycles 9:23–36
Santruckova H, Bird M, Kalaschnikov YN, Grund M, Elhottova D, Simek M, Grigoriev SA, Gleixner G, Arneth A, Schulze D, Lloyd J (2003) Microbial characteristics of soils on a latitudinal transect in Siberia. Glob Chang Biol 9(7):1106–1117. https://doi.org/10.1046/j.1365-2486.2003.00596.x
Sawamoto T, Hatano R, Yajima T, Takahashi K, Isaev AP (2000) Soil respiration in Siberian taiga ecosystems with different histories of forest fire. Soil Sci Plant Nutr 46:31–42
Schulze E-D, Lloyd J, Kelliher FM et al (1999) Productivity of forests in the Eurosiberian boreal region and their potential to act as a carbon sink – a synthesis. Glob Chang Biol 5:703–722
Schulze E-D, Wirth C, Mollicone D, von Lüpke N, Ziegler W, Achard F, Mund M, Prokushkin A, Scherbina S (2012) Factors promoting larch dominance in central Siberia: fire versus growth performance and implications for carbon dynamics at the boundary of evergreen and deciduous conifers. Biogeosciences 9:1405–1421. https://doi.org/10.5194/bg-9-1405-2012
Shugalei LS (2005) The Siberian afforestation experiment: history, methodology, and problems. In: Binkley D, Menyailo O (eds) Tree species effects on soils: implications for global change. Springer, Netherlands, pp 257–268
Sulzman EW, Brant JB, Bowden RD, Lajtha K (2005) Contribution of aboveground litter, belowground litter, and rhizosphere respiration to total soil CO2 efflux in an old growth coniferous forest. Biogeochemistry 73:231–256
Tang X, Fan S, Du M, Zhang W, Gao S, Liu S, Chen G, Yu Z, Yang W (2020) Spatial and temporal patterns of global soil heterotrophic respiration in terrestrial ecosystems. Earth Syst Sci Data 12:1037–1051. https://doi.org/10.5194/essd-12-1037-2020
Tarnocai C, Canadell JG, Schuur EAG, Kuhry P, Mazhitova G, Zimov S (2009) Soil organic carbon pools in the northern circumpolar permafrost region. Glob Biogeochem Cycles 23:GB2023. https://doi.org/10.1029/2008gb003327
Trefilova OV, Vedrova EF, Oskorbin PA (2009) The stock and structure of large woody debris in the pine forests of the Yenisei plain. Lesovedenie 4:16–23 (in Russian)
Tremblay SL, D’Orangeville L, Lambert M-C, Houle D (2018) Transplanting boreal soils to a warmer region increases soil heterotrophic respiration as well as its temperature sensitivity. Soil Biol Biochem 116:203–212. https://doi.org/10.1016/j.soilbio.2017.10.018
Vedrova EF (2005) Biochemistry of carbon and nitrogen in the Siberian afforestation experiment. In: Binkley D, Menyailo O (eds) Tree species effects on soils: implications for global change. Springer, Netherlands, pp 281–292
Wang WJ, Zu YG, Wang H, Hirano T, Sasa K, Koike T (2005) Effects of collar inserting and parameters selection on the soil respiration measurement by LI-6400 in a larch forest. J Forest Res 10:57–60
Wang Q, He N, Xu L, Zhou X (2018) Microbial properties regulate spatial variation in the differences in heterotrophic respiration and its temperature sensitivity between primary and secondary forests from tropical to cold-temperate zones. Agric For Meteorol 262:81–88. https://doi.org/10.1016/j.agrformet.2018.07.007
Whittaker RH (1975) Communities and ecosystems, 2nd Revise edn. MacMillan Publishing Co, New York
Widén B (2002) Seasonal variation in forest-floor CO2 exchange in a Swedish coniferous forest. Agric For Meteorol 111:283–297
Yanagihara Y, Koike T, Matsuura Y, Mori S, Shibata H, Satoh F, Masuyagina OV, Zyryanova OA, Prokushkin AS, Prokushkin SG, Abaimov AP (2000) Soil respiration rate on the contrasting north- and south-facing slopes of a larch forest in central Siberia. Eurasian J For Res 1:19–29
Yvan-Durocher G, Caffrey JM, Cescatti A, Dossena M, del Giorgio P, Gasol JM, Montoya JM, Pumpane J, Staehr PA, Trimmer M, Woodward G, Allen AP (2012) Reconciling the temperature dependence of respiration across timescales and ecosystem types. Nature 487:472–476. https://doi.org/10.1038/nature11205
Zhao Z, Peng C, Yang Q, Meng F-R, Song X, Chen S, Epule TE, Li P, Zhu Q (2017) Model prediction of biome-specific global soil respiration from 1960 to 2012. Earth’s Future 5:715–729. https://doi.org/10.1002/2016EF000480
Zhou T, Phi P, Hui D, Luo Y (2009) Global pattern of temperature sensitivity of soil heterotrophic respiration (Q10) and its implications for carbon-climate feedback. J Geophys Res Biogeosci 114:G02016
Zobitz JM, Moore DJP, Sacks WJ, Monson RK, Bowling DR, Schimel DS (2008) Integration of process-based soil respiration models with whole-ecosystem CO2 measurements. Ecosystems 11:250–269. https://doi.org/10.1007/s10021-007-9120-1
Acknowledgments
The research was performed using the subject of a basic project No. 0356-2019-0009. We thank all anonymous reviewers for the constructive comments that have substantially improved this manuscript.
Funding
The research was funded by the Russian Foundation of Basic Research (projects No. 19-29-05122, 18-41-242003, 18-34-00736, 18-05-60203), RFBR-NSFC (project No. 19-54-53026), and Japan Society for the Promotion of Science “KAKENHI” (Grant Number 19H02987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Haroun Chenchouni
Electronic supplementary material
ESM 1
(DOCX 304 kb)
Rights and permissions
About this article
Cite this article
Masyagina, O.V., Menyailo, O.V., Prokushkin, A.S. et al. Soil respiration in larch and pine ecosystems of the Krasnoyarsk region (Russian Federation): a latitudinal comparative study. Arab J Geosci 13, 954 (2020). https://doi.org/10.1007/s12517-020-05939-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-020-05939-x