Abstract
The abandoned mining wastes still represent one of the significant environmental hazards. Kef Ettout tailings is one example that was exposed to severe ambient conditions and must be assessed to determine its potential risks. The initial mine wastes, the tailings, and agricultural soils were investigated. The results showed that the winds and runoff water distributed the potentially toxic metals and the alkaline pH of tailings and soils, the carbonate, and TOC content controlled the metal bioavailability. About 22% of Pb and 70 and 98% of Zn and Cd, respectively, were leached from tailings. Despite the initial wastes were richer in Zn (1.5 times) than in Pb, the tailings kept much more Pb (1.6 times) than Zn. In agricultural soils, the mean concentrations of Pb, Zn, and Cd were 69, 141, and 1.8 mg kg−1, respectively. The enrichment factor and geoaccumulation index showed that more than 75% of soils were considered strongly contaminated. Speciation results indicated that about 97% of initial wastes metals were bounded to residual fractions. However, in tailings, 9–30, 4–10, and < 6% of Pb, Zn, and Cd, respectively, were in stable forms. Redistribution index (Utf) and relative binding intensity (IR) of metal tailing had confirmed that the tailings continued to provide more Zn quantity than Pb and Cd. In soils, the highest percentages of Pb and Zn were closely associated with organic matter, the Cd was significantly bounded to the exchangeable fraction, and the mean decreasing factor mobility order was Cd (66) < Pb (73) < Zn (78). Therefore, this tailings type must be rehabilitated to limit its risks, particularly of Zn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The tailings without rehabilitation were still a worldwide problem since they pose a serious threat to environment and human health as they continued to be often potentially pollutants (Liakopoulos et al. 2010; Nganje et al. 2011; Bian et al. 2012; Wang et al. 2017; Khamseh et al. 2017). The nature of pollutants and their concentrations depended on the nature, the composition and the mineralogy of the ore bodies and their gangues, and the ambient conditions such as climate, hydrology, geochemistry, and particle sizes. These toxic contaminants could be leached from the waste rock piles by natural drainage with surface water run-on and by the wind that often cause a threatening of the ecosystem (Kossoff 2014; Ouchir et al. 2016).
Potentially toxic metals present in soil may come from agricultural activities (Huang and Jin 2008), urbanization, and industrialization (Zhong et al. 2012). These nonbiodegradable metals persist for long periods in terrestrial environments (Kabata-Pendias and Pendias 2001). Also, fine particles of tailings are exposed to physical agents like wind that can disperse them into the surrounding area and contaminate the land and agricultural soils (Abdallah et al. 2012; Yadav and Jamal 2015; Lama et al. 2017). The dispersion of the contaminants occurs also by rainfall and after by the runoff towards the plain where we have the fertile soils.
Several Pb-Zn mines were active in the north of Tunisia. Kef Ettout is among the first mines of Pb-Zn of Tunisian atlas. This ore exploitation had started in 1883. After the mine closure (1975), the direct waste rejections were stopped. However, the improperly abandoned wastes contained potentially toxic metals. The climate of northern mining district is characterized by severe climatic conditions since the heavy rains and average temperatures between winter and summer are very important. The important wind speed, particularly in winter, let the Aeolian transport of contaminants is an important agent of pollutant dispersion. Thus, these mine tailings exposed to severe ambient conditions are highly susceptible to alteration and erosion and are important sources of metal pollution that will likely cause potential and serious risks to the population, soils, and water resources.
To assess contamination of polluted tailings of Kef Ettout and the surrounding agricultural soils, the total metal concentrations of sampled sediments from tailings and soils were analyzed. Quantifying the total metal content in the sampled sediments allowed us to have an idea about the abundance and the limits of dispersion of potential pollutants in the studied site. However, they were insufficient to have an exact idea about the bioavailable metal quantities and consequently about the toxicity levels of these mobile metals for the environmental and human health (Rao et al. 2008; Camden-Smith and Tutu 2014). Thus, the total concentrations should be complemented by dosage of metal quantities bonded to exchangeable and carbonate fraction (F1), reducible fraction (F2), oxidizable (sulfides/organics) fraction (F3), and metal quantity involved into crystal of the minerals (F4) (Rodriguez et al. 2009; Buccolieri et al. 2010; Trifi et al. 2018). It is also necessary to determine the parameters that controlled the balance between the immobile (without environmental impact) and mobile potentially toxic metal quantities in tailings and agricultural soils surrounding the pollution sources (Salomons and Stigliani 1995). Many ambient parameters ruled the metal speciation in the tailings and soils. The most factors that could influence the metal mobility are the pH, the nature and the concentration of organic matter, and the texture of sediments (Bolan et al. 2014; Lama et al. 2017). The clay minerals and their mineralogy and the oxides of Fe and Mn controlled also the long-term metal behavior (Staunton 2002).
The goal is particularly to have representative characterization of the mine tailings and surrounding agricultural soil in order to identify potential environmental problems for possible soil remediation or. The results allow estimations of potential pollution and an extent of affected zones and may provide a scientific basis to see if it is possible to predict a remediation or protection strategies of these natural resources, cultivated crops, and human health in these agricultural areas. To take stock of this site, the proposed studies were (1) the characterization of contamination level, the spatial dispersion of the potentially toxic metals of mine wastes, and the surrounding agricultural soils, (2) the assessment of the contamination of the polluted tailings, and (3) the calculation of the pollution level of studied environment, the mobility factor (MF), redistribution index (Utf), and relative binding intensity (IR) of metals to assess the evolution of the order and the stability degree of various metals in the tailings and therefore the degree and the order of their danger to the environment.
Material and methods
Study area
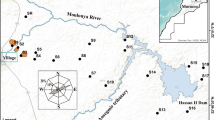
Geologically, Kef Ettout site is part of a Maaden catchment that has a high-contrast topographic character. Many valleys notching the relief are observed. The down basin is characterized by low slopes and the up basin shows stronger slopes (from 0° to 49.7°). The transition between the high and low areas is fast and accompanied with steep slopes. This basin includes several exploited mines such as Sidi Ahmed, Ain Erroumi, and Kef Ettout (Fig. 1). In Kef Ettout area, the relief is contrasted where we have on one hand the forests area (Fig. 1) with steep slopes and difficult reliefs to be sampled and on the other hand the agricultural soil with gentle slopes. In the pen plain soils, the domes and crests are usually observed. The macroscopic observations indicate that the soil texture and color (dark gray) on the studied soils are relatively homogeneous and the land use is variable (Fig. 1).
Geological map of the of Wadi El Maadene catchment area and location of study area (Rouvier 1987; Batik 1980)
Khanguet Kef mine is one of the oldest mines of Tunisia and considered essentially as a high producer of lead (Pb) and zinc (Zn) since the Roman Empire. It is included in Amdoun’s district that is located in the Tunisian Atlas (Rouvier 1977). It is located in the northwest region of Tunisia, 150 km N-W of Tunis, and its geographic coordinates are 36.9 and 9.11667 (in decimal degrees). The ores were logged in carbonate context. There are many mineralization types since the ore filled the diaclases, the fractures, and the dissolution cavities of the Campanian limestones of lenticular clusters. The ore paragenesis is formed by galena, calcite, siderite, sphalerite, cerussite, calamine, smithsonite, and iron oxides (Sainfeld 1952). The marcasite and pyrite are rare. Khanguet Kef Ettout exploitation technique was open sky. During the activity period (1888–1951), this mine produced 70,000 t of Zn-calamine, 15,000 t of Pb-calamine mixtures and 45,000 of galena, representing 38,000 t of Zn, and 34,000 t of Pb. The total ore production of Khanguet Kef Ettout was 91.238 t of concentrate zinc and 61.644 t of concentrate lead (Rapport of Mines Directorate 2005). The definitive cessations of the mining activities were in 1978.
The site is characterized by a humid climate with a heavy rainy season (average precipitation in winter is about 1000 mm year−1) (D.G.R.E. 2000), frequent winds, and a range temperature winter-summer − 1 to + 40 °C.
Sampling
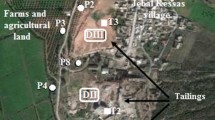
In Kef Ettout mining area, six important scattered tailings were identified. The sizes of these tailings are comparable and the total volume is about 600.000 m3. As well for the tailings and the agricultural soils, the topography is not flat and often we have crests and domes. So well the reliefs are low, the sampling following a regular mesh allows to have a nonrepresentative samples. So, it was felt that sampling of selected plots representative of its surrounding areas allows a better assessment of studied site. These pots were selected taking into account also the sediment texture, color, alteration and heterogeneity degrees of tailing sand soil sediments (estimated by macroscopic observation), and the culture nature for soil and the principal wind directions. In the tailings and soil, different plots were selected. For the six tailings, one representative plot was selected in each tailing. For soil sediments, the samples were taken from 19 plots areas (S1–S19) (Fig. 2) because macroscopically the agricultural soils of the site are comparable. The selected plots were different by the culture nature. For each plot, one square grid (5 m × 5 m) was used at a density of 10 soil points. Because of the clay soil texture and the heterogenetic composition of the tailings and to have representative results, each point was a composite (representative) of three or four samples distant about 15 cm. The locations of the sampling plots were recorded in field using a GPS. All soil samples were taken from cultivated lands. Always, 10 × 10 × 30 cm volume of sample was taken in polyethylene bags. In laboratory, the composite samples were homogenized and passed through 2 mm stainless steel sieve, lyophilized (freeze-drying), stored in plastic bags, and kept in a cooler room (about 4 °C) for further analysis.
Tables presented the statistic of element and parameter concentrations and values, respectively, obtained from each plot (about 10 composite samples). The minimum, maximum, standard deviation, and p (range values 0.001–0.05) were not presented to have more clear tables and figures.
Methods
For granulometric analysis, the Robinson pipette method was used. The mineralogical analysis of total rock (powder) was carried out by X-ray diffraction (XRD) SIEMENS D-5000 type with a scanning speed of 1°/2θ min and Cu-Kα radiation (40 kV, 20 mA) from 0° to 70° of the total fraction (Schultz 1964; Van der Marel 1966). The microscopic-metallographic analyses were based on polished sections. A raw de-carbonated sample following a 10% hydrochloric acid attack in order to eliminate the total carbonates was done. These de-carbonate powders were used for polished sections preparation and microscopic studies such as the petrography (alteration degree) and the mineralogy of tailings sediments. The pH and electric conductivity were measured in soil/water ratio of 1:5. After 1 h, mechanical shaking was done by pH meter mode l3305 and conductivity meter model WTW LF91 (Rayment and Lyons 2011; Montoroi 1997). The total organic carbon (TOC) percentage was achieved according to Walkly-Black method (1934), total carbonate equivalent determined by Bernard calcimeter, and the cation exchange capacity (CEC) using copper ethylenediamine complex (Bergaya and Vayer 1997).
To better evaluate the bioavailable metal quantity still in tailing and agricultural soil, the used sequential fractionation procedure was the BCR (The European Community Bureau of Reference) schemes since this procedure was applied and accepted by a large group of specialists (Salomons 1993; Fiedler et al. 1994; Ho and Evans 1997; Lopez-Sanchez et al. 1998; Usero et al. 1998; Martin et al. 1998; Agnieszka and Wieslaw 2002; Yuan et al. 2004). The BCR extraction procedures were presented in Table 1. It is very important to ensure the accuracy and reproducibility of results. Therefore, for each extraction, a blank sample was analyzed with sample extractions to test the reaction quality and possible contamination. In order to evaluate the stability and accuracy of the procedure in all samples, the reference material BCR-701 was used with the same procedure and the results were replicated three times. When the reproducibility was unacceptable, the analysis was replicated again to be sure that the results were representative. The recovery percentage of sequential extraction was calculated using the equation presented by Davidson et al. (1998).
The contents of Zn, Pb, and Cd in the total sediments and in the residual fractions were determined by digestion of 0.5 g of sediments with 5 mL mixture of HF (40%), 1.5 mL HClO4, 3.75 mL (70%), HCl (37%), and 1.25 mL (65%) HNO3 (Normes AFNOR 1979) in a sand bath at 250 °C. The final solutions were diluted with 100 mL of ultrapure water.
The concentrations of these elements were measured by atomic absorption spectrophotometry (AAS) (PerkinElmer type) with a graphite furnace. For all analyzed samples, each obtained result was accepted only when the deviation standard value was at least equal to the detection limits that are 5, 3.6, and 0.75 pg for Pb, Zn, and Cd, respectively. To simplify the presentation and limit the space, in the tables and figures, we reported only the mean values of each plot. For tailings and soil metal contents, the standard deviations were added. All calculations in this study were made using XLSTAT. The sphalerite and galena were the exploited minerals in Kef Ettout mine that contain significant traces of Cd (about 14 g). The chemical analyses showed that the strontium (Sr), manganese (Mn), nickel (Ni), copper (Cu), chromium (Cr), and cadmium (Cd) existed but their concentrations had not relatively an environmental impact. So, only the Pb, Zn, and Cd were presented.
To evaluate the stability degrees and contamination level of these tailings and pollution degrees of Kef Ettout area, different indexes were calculated.
Sure enough, among our objectives was to assess numerically the stability degrees (Utf and IR indices) of different metals in the tailings after this long exposure period. The fractional redistribution index Utf was proposed by Banin et al. (1990) to assess the metal mobility evolution in amended soils with incubation time. Utf was defined as follows:
Fa was the percentage of the total amount of metal that was bound to a given component in the amended soil and Fc was the percentage of the total amount of metal that was bound to the same component of control soil. For the application of Banin et al. (1990) equations to the tailings, the Fc was considered the percentage of the total amount of metal that was bound to each sediment fraction in initial wastes and Fa was the percentage of the total amount of metal that was bound to a same sediment fraction in tailings. Thus, the Utf ratio assesses the alteration degree of the abandoned wastes. When the metal quantities bounded to four fractions in the tailings (abandoned wastes) were similar to those of the initial wastes of valorization metal plant (quasi-equilibrium state), the Utf values were equals 1. Following the effects of external parameters such as the high temperature variations, high rainfall periods, and redox conditions, the tailings sediments were altered and their metal forms (speciation) and their distributions among the different fractions of tailings sediments had changed. These evolutions were displayed by an increase of Utf values. More the quantities of mobile metals were important and more the Utf values are high.
The IR was also introduced by Banin et al. (1990) to define quantitatively the binding intensity of metals to sediments. This index gives an idea about long-term effects of ambient conditions on the evolution of metal stabilities in abandoned wastes. IR was defined as follows (Banin et al. 1990):
In our case, Fi was the percentage of the total amount of metal that was bound to component i in tailings. The choice of n was arbitrary (equal 2) because the square relationship (n = 2) clearly expresses the increasing binding strengths of metals with increasing i in the sequential selective dissolution process and k = 4 (number of sequential extractions steps) (Banin et al. 1990). IR = 1 (equilibrium sate) if the total metal quantity was bound to the residual fraction of sediments. The IR value decrease indicated that the mobile and mobilizable metal quantities had increased.
For an evaluation of environmental risk and the classification of the sites according to their potential toxicities, several indices, factors, and equations were widely used (Trefrey and Presley 1976; Alexander et al. 1993; Sutherland 2000; Mil-Homens et al. 2006; Müller 1969; Hakanson 1980; Ouchir et al. 2016). In this work, the contamination factor (CF), geoaccumulation index (Igeo), and MF were used to evaluate the level of agricultural soil contamination. The CF and Igeo index were calculated, for each potentially toxic metal, using the following equations, respectively:
where Cm is the mean concentration of the metal in the soil, Cr is the geochemical background value of the concern trace metal (Table 8), and the constant 1.5 is the geochemical background matrix correction factor. It is used to minimize possible variations in the background values due to natural sources. Hakanson (1980) defined four levels of the CF: CF < 1, low; 1 < CF < 3, moderate; 3 < CF < 6, considerable; and 6 < CF, very high.
As described by Müller (1969), the Igeo values were classified into seven classes: Igeo < 0, practically uncontaminated; 0 < Igeo < 1, uncontaminated to slightly contaminated; 1 < Igeo < 2, moderately contaminated; 2 < Igeo < 3, moderately to highly contaminated; 3 < Igeo < 4, heavily contaminated; 4 < Igeo < 5, highly to very strongly contaminated; and Igeo > 5, very strongly contaminated.
Results and discussion
Mineralogical and physicochemical characterizations
The microscopic observation of the initial wastes and the tailings polished sections prepared from the de-carbonated samples. In the tailings, the pyrite where the grain size range was > 10 to < 100 μm (FeS2), galena (PbS) and sphalerite (ZnS) were identified. This matrix contained also calcite, barite, quartz, and essentially carbonates gangue and neoformed CaCO3.
Photo
The carbonate and the sphalerite were the more altered minerals in the studied tailings. The alteration degree progressed from the external border towards the center of different grains of minerals (Photo 1). The results of XRD analysis of tailings materials confirmed the presence of the main minerals of wastes (Fig. 3). The mine tailings contained small amounts of calcite, quartz, and barite. Because of the percentages of galena and sphalerite were lower than 5%, these minerals were not detected. This mineralogical composition was comparable to that of the initial wastes (Sainfeld 1952).
The grain size distribution of the tailings sample analysis in the texture triangle showed that the tailings sediments had a sandy-loam texture (sands from 24 to 70%, loams from 22 to 60%, clay ≤ 18%). The pH and organic matter (M) ranges were 7.1–8.2 and 0.69–1.4, respectively. The pH variation closely depended on the carbonate content of tailings sediments (50–80%) (Table 2). Thus, it can be stated that the alkaline pH values of Kef Ettout sediments (tailing and soils) could be attributed to strong buffering capacity of carbonates (Papadopoulou-Vrynioti et al. 2014). Such as the case of several tailings in the North of Tunisia, Khanguet Kef Ettout wastes had a neutral pH to slightly basic and contained relatively important amounts of organic matter (Sliti 2013; Mseddi 2013; Othmani 2013; Sebei 2007). This alkaline pH probably favored the metal mobility decrease (Plassard et al. 2000). The conductivity values varied from 500 to 580 μS cm−1.
Compared with tailings sediments, the texture of the sediments of the initial wastes stayed stable. However, the initial physicochemical parameters of initial wastes were different from those of tailings (Table 3). Probably, the carbonate precipitation in the tailings, compared to initial wastes, increased the mean pH about 0.9. In initial wastes, the organic matter concentrations were relatively high (Charef and Sheppard 1991). The long exposition of these wastes had contributed to the organic matter mineralization of the ore deposit and decreased the TOC in the tailings (from 2.9 to 1%) and the CEC (from 17.9 to 10.7 meq 100 g−1). An increase of the EC about 106 μS cm−1 was, in part, due to the release of ions adsorbed by organic compounds.
The granulometric, alkalinity, EC, CEC, and TOC values of surrounding agricultural soils were presented in Table 4. The plotting of granulometric results in the texture triangle showed that agricultural soils were classified as silty-clay and silty soils with small amounts of sand. The ranges of pH values and carbonate percentages were 7–8.1 and 15–25%, respectively. The mean soils EC varied from 400 to 530 μS cm−1. These values were relatively low probably, thanks to the high capacity of cationic exchange of these soils. The studied soils contained relatively high organic matter content (0.7–7.7%). Probably, this respectable percentage of organic matter was derived from the large vegetation area. The minimum and the maximum values of soils CEC in the mine site were from 13 to 22 meq 100 g−1 and 18 to 29 meq 100 g−1, respectively. The important cation exchange capacity of the soils was probably due to the high percentages of clay and organic matter or the high CaCO3 content as proposed by Papadopoulou-Vrynioti et al. (2014). However, these CEC values of mine wastes and soils were low compared with other contexts where the CEC were three times more important (Papadopoulou-Vrynioti et al. 2014).
The soil texture has an important role in the potentially toxic metal distributions. In the studied mine site, the planted soils had a high yield of clay (illite, montmorionite, and kaolinite), loam, and silt fractions that could contribute to the adsorption of important potentially toxic metal quantities (Li and Li 2000). The alkaline soil pH and the carbonate abundance contents that favored the metal and anions complexing decreased the metal mobility and bioavailability (Sherlock et al. 1995; Moncur et al. 2005). In addition, the organic matter concentrations dropped also the soil adsorption capacity of potentially toxic metals (Bosmans and Paenhuys 1980). All these parameters indicated that the agricultural soils of Kef Ettout could contain significant amounts of Pb, Zn, and Cd. These pollutants could stay in soils for a long time since the intensity of root uptake of metals by plants decreased with increasing soil pH to about pH 6.5–7.5 (Prasad and Freitas 2003; Kucharski et al. 2008; Fijałkowski et al. 2012). However, the relatively low CEC values seemed to suggest that the mobility of traces elements would be expected to be high (Papadopoulou-Vrynioti et al. 2014).
Potentially toxic metal contents and distribution
The minimum, the maximum, and the mean concentrations and standard deviations of Pb, Zn, and Cd of different analyzed samples from Initial wastes and tailings of the ore valorization plant were presented in Table 5.
The plant wastes were highly contaminated with Pb, Zn, and Cd. The highest obtained mean concentrations were of Zn (23,177.00 mg kg−1). Its mean concentrations were 1.5 times higher than of Pb. The high concentrations of Pb and Zn reflected the main components of the minerals extracted from the mine site (Sainfeld 1952; Obiora et al. 2016). The relative small variations of standard deviations (SD) of Pb, Zn, and Cd showed that wastes were homogeneous (Table 5).
In the tailings, the mean Pb, Zn, and Cd contents and their standard deviations in the six plots were summarized in Table 6. The range values were from 3250.00 to 37,400.00 mg kg−1 for Pb, from 2165.00 to 15,000.00 mg kg−1 for Zn, and from 12.50 to 90.00 mg kg−1 for Cd. The data indicated that the significant variations of metal concentrations were due essentially to the variations of tailings topography, the residue textures, the particle sizes, and the storage locations (shape, vegetation density, etc.). Compared to the metal contents of initial wastes, only 70% of Zn and 22% of Pb had left these pollution sources. Thus, these tailings continue posing environmental problems to the surrounding agricultural soils and water resources. However, the almost entire Cd was leached (about 98%).
In agricultural soils, the ranges of total contents of Pb, Zn, and Cd were from 6.00 to 230.00 mg kg−1, from 6.00 to 698.00 mg kg−1, and from 0.1 to 7.7 mg kg−1, respectively (Table 7). Almost all potentially toxic metal concentrations in the closest soils of the mine were extensively higher than the corresponding soil backgrounds (Table 8), particularly for Cd where its concentrations were higher than the corresponding controlled soils. Thus, the Nefza soil is severely polluted.
These results showed that we had a distribution of the potentially toxic metals in all studied areas. Several mechanisms and parameters could control the potentially toxic metals dispersion such as wind, rainfall (Ettler et al. 2011; Escarre et al. 2011), nature sediments (soils and tailings), and their physicochemical characteristics.
In the studied area, the maximum and the mean monthly speeds of the wind directions are about 2.4 and 11.8 mS−1, respectively. The Rose of the winds and the dynamic resultant of these winds show that there are two preferred directions, which are west to northwest directions with average speed about 10 mS−1 and east to southeast directions with average speed about 7 mS−1. The spatial distribution maps of the three potentially toxic metals of Kef Ettout (Fig. 4) indicated that, unsurprisingly, the tailings were the most polluted in this sector. Also compared to the tailings position, the east area where the wind forces were important showing the most polluted area. However, the west and southeast areas where the wind forces were less important we had moderately polluted agricultural soils. Thus, the winds carried significant amounts of Pb, Zn, and Cd. But, if we looked the details, there was on the one hand a local variation of potentially toxic metals and on the other hand there was any tendency to decrease away from the mine wastes (Huynh 2009). Thus, a second transport vector of pollutants had been involved in the distribution of potentially toxic pollutants which could be the water erosion. Besides in agricultural soils of east area, the effect of runoff from the tailings had remained limited. Probably, the forests had played the role of filter since the abundant organic matter adsorbed the pollutants from water erosion of tailing.
Despite the initial wastes were richer in Zn (1.5 times) than in Pb, the tailings kept many more Pb (1.6 times) than Zn. In addition to wind and water erosion, this selective leaching of potentially toxic metals could be due to the property of the two elements or physicochemical parameters of tailings and soils sediments. The principal component analysis (PCA) was used to define the relationship among the different physicochemical parameters and potentially toxic metals in Kef Ettout tailings and agricultural soils. The obtained results showed that the Cd was well correlated with Pb (> 0.76) and Zn (> 0.72) in the tailings and soils. However, the Zn and Pb were not well correlated in the tailings (0.46) and well correlated (> 0.86) in agricultural soils. The correlation orders of potentially toxic metals with physicochemical parameters were for Zn pH (0.63) > TOC (0.48) > CEC (0.39) > EC (0.35) in the tailings and CaCO3 (0.58) > CEC (0.42) > pH (0.29) > TOC (0.25) in the soils. However, for Pb, the order was pH (0.51) > CaCO3 (> 0.39) > CEC (> 0.28) > EC (0.24) > pH (0.1) in the tailings and in soils it was CaCO3 (0.61) > EC (0.34) > pH (0.26) > CEC (0.19) and inversely correlated with pH (− 0.11). The correlation order of Cd in the tailings was pH (> 0.37) and EC (> 0.28) and inversely correlated with CaCO3 (− 0.68), OM (− 0.07), and CEC (− 0.01). In soils, the order was CEC (0.63) > CaCO3 (0.56) > TOC (0.45) > pH (0.29) > EC (0.11).
The TOC and CEC were well correlated (> 0.73) in tailings and soils. This result confirmed the good affinity between CEC and TOC.
The pH was moderately correlated with Pb (0.51) and Zn (0.63), but it was weakly correlated with Cd (0.37), TOC (0.35), and EC (0.31).
The factor analysis of data set revealed three factors explaining 82.83 and 84.42 of the total variability of tailings and soil sediment parameters, respectively: F1, contributing with 44.4 and 46.71% of inertia in tailings and soils, respectively. The F1 was defined by Zn (0.876), Cd (0.841), Pb (0736), pH (0.773), and EC (0.534) for tailings and by Cd (0.936), Zn (0.910), Pb (0.786), CaCO3 (0.726), and pH (0.514) for soils. The F2, contributing with 28.18% (tailings) and 25.70% (soils) had well positive correlation (> 0.89) on TOC and CEC and moderate (0.420) negative on CaCO3 (tailings). The three components (F3) contribute with 10.25% for tailings and 12.05 for soils; it was defined by the pH (0.630) and TOC (0.284) and pH (0.801) in tailings and soils, respectively.
In the correlation circles (Fig. 5), the first component axis 1 was expressed towards its positive pole by pH, EC, and Pb, and Zn and Cd contents show good correlations between them in the tailings. However, for soils, it was expressed towards its positive pole by the pH, Pb, Zn, Cd, and CaCO3 contents showing also good correlations with each other and the EC towards its negative pole. The axes 2 were defined, in tailings and soils, by the TOC and CEC towards their positive poles and by the CaCO3 towards its negative pole for tailings.
These statistic values showed that the three potentially toxic metals in the tailings and agricultural soils had the same origin. Thus, we had any external origin in Kef Ettout pollution. It was also clear that the specific properties of the Pb, Zn, and Cd and physicochemical parameters had controlled in part the distribution of Pb, Zn, and Cd (Kabala and Singh 2001; Karczewska 1996; Narwal et al. 1999; Trifi et al. accepted). But these parameters differently controlled the distribution of the potentially toxic metals since the values and the correlation coefficients orders of the various physicochemical parameters between them and between the physicochemical parameters and the potentially toxic metals were variable. The abundant clay particularly in soil that had an important adsorption capacity must have an important effect on the distribution and mobility of potentially toxic metals leached from the tailings. Probably, another pollutant part was retained in soil. We added to that the heterogenic spatial distributions of pollutants which were also partly induced by the metal complexing under different forms such as Fe-Zn oxide co-precipitants (McLean and Bledsoe 1992) and the irregular topography of the surface soil.
Metal mobility
The information obtained about the total contents of heavy metals had permitted to have an idea about their pollution degrees. Thus, it was important to complete the balance about the impact of metals in tailings and their toxicity degrees. The BCR extraction methods were proposed to determine the bioavailable metals quantities that are bounded to the different sediment fractions.
In initial wastes, the Pb, Zn, and Cd proportions bounded to residual fractions followed the descending order: Pb = 99.8% < Zn = 99.3% < Cd = 97.9. Since the ore deposit was for a long time (geologic time) in quasi-equilibrium state, about 97% of Pb, Zn, and Cd quantities were bounded to residual fractions.
In tailings, the mean obtained values were illustrated in Fig. 6. The percentage ranges of Pb bounded to exchangeable and reducible fractions were 2–35 and 0.9–9%, respectively. However, the highest fractions of the Pb were bounded to organic matter (28–83%). From 9 to 30% of lead was in residual minerals. The percentages of Zn bounded to organic matter and reducible (3–80%) fractions were relatively high (from 3 to 51%). The exchangeable fractions involved between 0.8 and 4% of Zn. The lowest percentages of this metal were in residual fractions (4–10%) (Fig. 6).
Thus, in the tailings, 9–30, 4–10, and < 6% of Pb, Zn, and Cd, respectively, were in stable forms and could be released towards the surrounding environment only after important rock weathering. Consequently, the long exposition of the tailings to the local severe climatic conditions had destabilized the metals stock and increased their danger levels. Consequently, an important metal quantity was available to be leached towards the agricultural soils and local surface and groundwater. Also, these important metal quantities could be probably transferred from soil to crops (Ma and Rao 1997; He et al. 2005; Rodriguez et al. 2009; Chai et al. 2015). These results showed that Kef Ettout tailings continued to be a source of potential danger for the environment.
In agricultural soils, the percentages of Pb, Zn, and Cd bounded to exchangeable, reducible, oxidizable, and residual soil fractions were summarized in Fig. 7. As it can be seen from the obtained results, the highest percentages of Pb and Zn were always closely associated with organic matter fractions. The percentage ranges of Pb and Zn in F3 were 49 to 85% and 45 to 68%, respectively. The residual fractions bounded respectable Pb (5–31%) and Zn quantities (10–46%). However, the Pb and Zn bounded to exchangeable and reducible fractions were relatively low. F1 fixed between 2 and 17% of Pb and between 2 and 22% of Zn. F2 bounded from 0.5 to 23% of Pb and from 1 to 25% of Zn. The Cd distribution among the different soil fractions was different. The Cd was significantly bounded to the exchangeable fractions (20 to 55%) and only about 20% was involved in organic matter fractions. The proportions of this element bounded to the residual fractions (6–58%) were relatively more important than those associated with the reducible fractions (12–41%).
The potentially toxic metals existed as loosely bound fractions such as exchangeable and deductible forms; they tended to be easily moved and dispersed. Thus, the Zn and Pb quantities associated with exchangeable fractions posed serious threats because they were mobiles and potentially bioavailable for either plants or organisms (Huang et al. 2012; Halim et al. 2013; Chai et al. 2015; Fang et al. 2016). However, the residual fraction solid should mainly contain primary and secondary minerals that held metals within their crystal structures. Therefore, it did not seem reasonable to assume that these metals may be released under the conditions normally encountered in nature (Dang et al. 2002; Esshaimi et al. 2013). The organic matter could immobilize heavy metals or works as a factor which causes their releases. Indeed, the heavy metals associated with organic ligands could be complexes by soil organic matter, forming an organometallic complex (Lamy 2002; Chotpantarat et al. 2015) or bounded by organic matter by a complex process, due to the diversity of its connections with the mineral phase. Thus, it was often considered that the increasing of the amount of organic matter actively retains the metallic elements. It was also accepted that the metals bounded to the F3 fractions were immobile especially when the solubility degree of the organic matter was low. Also, the sorption capacity of organic matter was well above the mineral sorption capacity of the soil (Gworek et al. 2000; Van Loon and Duffy 2007; Ociepa et al. 2010; Fijałkowski et al. 2012). The increase of soil organic carbon percentages was accompanied by an increase in the CEC of the soil and a reduction of the metal absorption capacity of plants (Haghiri 1974). Usually, the acceptable solubility of organic matter led to its dissolution enabled the transition of metal elements to the aqueous phase (Bodar et al. 2006; Vamerali et al. 2010; Fijałkowski et al. 2012). Thus, the oxide-, carbonate-, and organic matter-bound fractions may be potentially bioavailable and the metal fractions are directly available to either plants or microorganisms (Ma and Rao 1997; He et al. 2005; Rodriguez et al. 2009). We can add that in Nefza region the climate is semiarid and the humidity degree is high.
Risk assessment
The metal quantity bounded to the different fractions of tailings sediments provides important information on the danger degrees of these metals that could be leached towards Nefza region. It is also important to know the stability degrees of these pollutants because more of these fractions are stable and more the danger of these metals is low. The pollution levels (contamination factor, geoaccumulation index, and mobility factor) gave, also, an idea about the risk degree of the pollutants. The results could be determinant to suggest, perhaps, some solutions that protect the surrounding soils.
The calculated values Utf1, Utf2, and Utf3 for Pb and Zn (Fig. 8) were comparable because of their range values were 0–15. The mean values of Utf1, Utf2, and Utf3 were 0.01, 0.15, and 0, respectively, for Pb and 0.02, 0.01, and 0.06, respectively, for Zn. However, the Utf4 ranges and mean values were 3.23–10.25 and 6.11 for Pb and 21.9–112.5 and 49.5 for Zn, respectively. These results showed that the distribution evolution of the Pb and Zn was comparable. However, the Cd behavior was totally different. The Cd quantities bounded to exchangeable (Utf1 = 24.13), reducible (Utf2 = 264), and oxide fractions (Utf3 = 36.11) were widely higher than of those of Pb and Zn, and the Utf1 and Utf3 of Cd were comparable. However, the Utf2 was about 8.8 times higher than of Utf1 and of Utf3 values and the Utf4 = 0.03 of residual fraction was near zero. This order was quite different in other mining contexts (Obiora et al. 2016; Daldoul et al. 2015). Globally, the Utf values indicated that the environmental conditions had destabilized the Pb, Zn, and Cd of abandoned wastes. According to the metal stock of initial wastes and their speciation, the calculated mean values of Utf1, Utf2, and Utf3 were near zero. However, the Utf4 values indicating after the long exposition the tailings continue to be a source of the Pb and Zn pollutants. The danger of the Zn is more important than the Pb since the mean Utf4 value of Zn is 8.2 times higher than of Pb.
The IR that determined the binding intensity of metals among the solid phases of the Kef Ettout and consequently the stability degrees of the tailings were calculated. The mean IR values for Pb, Zn, and Cd were 0.35, 0.17, and 0.29 (Fig. 8). Thus, the increasing order of the binding intensity of metals to sediments was Zn < Cd < Pb. These results showed that after the long-term effects of ambient conditions, the Pb remained the more stable element since it had the highest IR mean value (0.35). It was the closest value to the quasi-equilibrates state (IR = 1). While the Zn had the lowest binding intensity value with the tailings minerals and the lowest stable metal. The obtained values of IR confirmed that the IR of Pb was the closest to the equilibrium state. Thus, the results had confirmed that the tailings continued to provide for the surrounding environment more Zn quantity than the Pb and Cd since the increasing order of metal quantities leached from the tailings was Zn > Cd > Pb.
In agricultural soils of Kef Ettout, the minimum CF values were 0.7, 0.85, and 2 for Pb, Zn, and Cd, respectively. These factors showed that the studied soil had a very low level of contamination for Pb and Zn and moderate contamination for Cd. However, the maximum CF values (23, 99, and 154 for Pb, Zn, and Cd, respectively) indicated that Kef Ettout soils had a very high level of contamination. Thus, the calculated CF proved that the studied soils were enriched with Pb, Zn, and Cd.
The obtained mean Igeo values for Pb showed that 42% of the samples had a moderate contamination class, 36% had a practical uncontamination class, and 21% were moderately to highly contaminated. For Zn, Igeo values indicated that 42% of the mean samples had moderate contamination class, 21% were practically uncontaminated, 15% were moderately to highly contaminated, 10% were heavily contaminated, 5% had a high to very strong contamination class, and 5% were moderately to highly contaminated. Igeo values for Cd showed that 42% of the mean samples had a heavy contamination class, 15% were moderately to highly contaminated, 15% had a moderate contamination, 15% were practically uncontaminated, and 10% had a high to very strong contamination class. Thus, the Igeo values showed that in Kef Ettout soils, 78% of soils had moderate to high contamination status with Pb, 79% had a moderate to high and very strong contamination with Zn, and 85% of analyzed soils had moderate to high and very strong contamination with Cd. Globally, these agricultural soils could be considered contaminated with mobiles Zn and Cd and lesser with Pb.
To assess the degree of heavy metal danger in soils, MF were also calculated. As it is discussed above, we noted that the F3 was considered a mobile fraction especially in arid and humid climate such as the case of the Nefza site. The minimum, the maximum, and the mean calculated values of mobility factors were 53, 90, and 73, respectively, for Pb, 70, 94, and 78, respectively, for Zn, and 42, 94, and 66, respectively, for Cd (Fig. 9). Thus, the mean decreasing mobility order of studied metals was Cd < Pb < Zn. This order was also proposed by Lei et al. (2010). The maximum mean observed mobility variation was 12. We noticed that the mobility order of the soils was different from that of the tailings.
The physicochemical parameters and the mineralogy of the soil sediments controlled the solubility (mobility) of metal fractions and their biodisponibility. Probably, the organic matter, the clay minerals, and other active soil compounds contributed to the metal mobility degree in the soil. Sure enough, the basic soil pH normally promoted the precipitation of different species in soil that could limit the solubility and bioavailability of all ionic species such as metals (Deneux-Mustin et al. 2003). Also, the organic matter in Kef Ettout soil favored the stability increase of the formed organometallic complexes (Blanchard 2000) and hydroxides and immobilized them. Compared with tailings, the soil was richer in TOC (about two times), in clay fractions (about three times). Thus, the organic matter and the clay fraction induced an increase of the number and the variety of functional groups, of CEC, specific areas, specific behavior that strongly affected by their interactions and/or competition for the available surface sites and the surface precipitation-adsorption in soils (Neal and Sposito 1986; Kalbitz et al. 1998; Mouni et al. 2017) compared with the tailings. These parameters were decisive factors for potential mobility and bioavailability of Zn, Cd, and Pb in this soil kind. These soil characteristics were the major reasons of the increase of metal mobility in soils compared with the tailings.
Protection strategy
In Kef Ettout, the mine exploitation was not associated with appreciable changes in the geomorphologic characteristics of the area and the alterations in vegetation cover. Thus, the appropriate restoration for Kef Ettout concerns the tailings and soil. In order to minimize the potentially toxic metal impacts on the environment, it is recommended to select tailings management practices. The possibility of the tailings conservation could be a measure to prevent the potential loss of pollutants by wind and water erosion. The choice of tailings conservation methods and technologies can be based on the action on the physical and chemical parameters of wastes (e.g., Perel’man 1986) or stopping the transfer of tiny particles by wind and water ways (e.g., Alekseenko et al. 2017). The alkaline tailings pH let us think that the beneficial outcomes can be achieved by the tailings conservation. From the analysis of existing methods of polluted dust suppression of tailings, we proposed the use of the screen by clay layers, coated with polyethylene and polypropylene wastes. To limit the possibility of perforation of putted screen, it is necessary maintaining the isolation material.
For the agricultural soils, the possibilities of limiting the impact of the important quantity of labile potentially toxic metals, present a health danger, are more difficult. The possible scenario is the depollution of the agricultural soil by phytoremediation. However, taking into account the socioeconomic conditions of this area, it is imperative to track the quality of crops because the contaminated soils could introduce a significant amount of Pb, Zn, and Cd into human diets.
Conclusion
The abandoned mine wastes pose a serious threat to environment and human health since they contained to be often potentially pollutants. The toxic contaminants were dispersed by wind that often cause a threat to the ecosystem and leached from the waste rock piles by natural drainage with surface water. The selective potentially toxic metal distribution was controlled in part by the specific property of each metal and the physicochemical parameters of tailings and soils. Despite the basic pH, an important part of metal left the tailings. Probably, the organic matter content and the sediment granulometry of tailings contributed to these metal dispersions. Kef Ettout poses also a serious problem for the surrounding agricultural soils. An assessment of this type of pollution source was essential to carry out the danger degree of these tailings. The physicochemical parameter, the total concentrations of Pb, Zn, and Cd, and the BCR extraction were conducted. Based on the Utf and IR indexes, the CF, Igeo, and MF, the metal availability was evaluated.
The results showed that the initial wastes were highly contaminated with Pb, Zn, and Cd. The long period of initial waste exposition to severe climatic conditions (frequent torrential rainfall, high humidity, high wind speed, hot summer, etc.) had caused their alteration. During this long exposition period, one part of the metals was leached with water and wind and distributed towards the surrounding areas. About 78% of Pb, 30% of Zn, and 2% of Cd remained in tailings. It was clear that the Zn alteration was higher than of Pb because the initial wastes were richer in Zn than in Pb. However, the inverse concentrations order was detected in the tailings. The sequential extractions had proved that in initial wastes, more than 97% of metals were immobile. However, in the tailings, a small metal quantity was bounded to residual fractions. The calculated Utf and IR showed that Pb was the more stable element in these tailings. These important quantities of Pb, Zn, and Cd that left the tailings were deposed in agriculture soils. The mean concentrations of Pb, Zn, and Cd were 69, 141, and 1.8 mg kg−1, respectively, and the calculated CF and Igeo had proved that more than 75% of sampled soils were considered contaminated and the sequential extraction of soil metals showed that an important quantity of metals was mobile in soil. The organic matter and the high clay fraction of soils were the principal compounds that retained the metal in soils where their mobility order was Cd < Pb < Zn. Thus, the Kef Ettout tailings were always an important polluting source because of an important quantity of metals were in mobile forms. These potentially toxic metals were susceptible to be dispersed towards the surrounding areas and posed serious risks to crops. Since an important part of the potentially toxic metals remained is always in these tailings, it must be protected by the use of a screen of clay associated with polyethylene and polypropylene wastes to stop their dispersion. For agricultural soil, a strict control of produced vegetables is necessary. The phytoremediation could be a solution for these polluted soils.
Change history
06 May 2019
The original version of this paper was published with error.
References
Abdallah S, Al-Hobaib KQ, Al-Jaseem HMBA, Ahmed HA (2012) Environmental impact assessment inside and around Mahd Adh Dhahab gold mine, Saudi Arabia. Arab J Geosci 5:985–997
AFNOR, (1979) France (norms AFNOR, www.afnor.fr)
Agnieszka S, Wieslaw Z (2002) Application of sequential extraction and the ICPAES method for study of the partitioning of metals in fly ashes. Microchem J 72:9–16
Alekseenko VA, Pashkevich MA, Alekseenko AV (2017) Metallisation and environmental management of mining site soils. J Geochem Explor 174:121–127
Alexander CR, Smith RG, Calder FD, Schropp SJ, Windom HL (1993) The historical record of metal enrichments in two Florida estuaries. Estuaries 16:627–637
Baize D (1997) Teneurs totales en éléments traces métalliques dans les sols (France). Références et stratégies d’interprétation. INRA Éditions, Paris, p 410
Banin A, Gerstl Z, Fine PN, Metsger Z, Newzella D (1990) Minimizing soil contamination through control of sludge transformations in soil, Joint German-Israel Research. Projects Report No Wt 8678/458
Batik P (1980) Carte géologique de la Tunisie; feuille n°11: Hédil. Service Géologique, Office National des Mines
Bergaya E, Vayer M (1997) CEC of clays: measurement by adsorption of a copper ethylenediamine complex. Appl Clay Sci 12:275–280
Bian Z, Miao X, Lei S, Chen S, Wang W, Struthers S (2012) The challenges of reusing mining and mineral-processing wastes. Science 337:702–703
Blanchard C (2000) Caractérisation de la mobilisation potentielle des polluants inorganiques dans les sols pollués. Thèse spécialité: Science et technique du déchet Ecole doctorale de chimie de Lyon France, 241p
Bodar CW, Pronk ME, Sijm DT (2006) The European Union risk assessment on zinc and zinc compounds: the process and the facts. Integr Environ Asses 1(4):301–319
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham MB, Scheckel K (2014) Review remediation of heavy metal(loid)s contaminated soils: to mobilize or to immobilize? J Hazard Mater 266:141–166
Bosmans H, Paenhuys J (1980) The distribution of heavy metals in the soils of the Kempen. Pédologie 15:191–223
Bowen HJM (1979) Environmental chemistry of the elements. Academic Press, London
Buccolieri A, Buccolieri G, Dell’Atti A, Strisciullo G, Gagliano-Candela R (2010) Monitoring of total and bioavailable heavy metals concentration in agricultural soils. Environ Monit Assess 168:547–560
Camden-Smith BPC, Tutu H (2014) Geochemical modelling of the evolution and fate of metal pollutants arising from an abandoned gold mine tailings facility in Johannesburg. Water Sci Technol 69(5):1108–1114
Canadian Council of Ministers of the Environment (1991) Canadian water quality guidelines for the protection of aquatic life: guidance on the site-specific application of water quality guidelines in Canada: procedures for deriving numerical water quality objectives. In: Canadian environmental quality guidelines, Canadian Council of Ministers of the Environment, Winnipeg
Chai Y, Guo J, Chai S, Cai J, Xue L, Zhang Q (2015) Source identification of eight heavy metals in grassland soils by multivariate analysis from the Baicheng–Songyuan area, Jilin Province, Northeast China. Chemosphere 134:67–75
Charef A, Sheppard SMF (1991) The diapir related Bou Grine Pb–Zn deposit (Tunisia): evidence for role of hot sedimentary basin brines. In: Pagel M, Leroy J (eds) Source, transport and deposition of metals. Balkema, Rotterdam, pp 269–272
Chotpantarat S, Chunhacherdchai L, Wikiniyadhanee R, Tongcumpou C (2015) Effects of humic acid amendment on the mobility of heavy metals (Co, Cu, Cr, Mn, Ni, Pb, and Zn) in gold mine tailings in Thailand. Arab J Geosci 8:7589–7600
D.G.R.E (2000) Annuaire de l’exploitation des nappes. Rapport D.G.R.E, Tunis 282p
Daldoul G, Souissi R, Souissi F, Jemmali N, Chakroun HK (2015) Assessment and mobility of heavy metals in carbonated soils contaminated by old mine tailings in North Tunisia. J Afr Earth Sci 110:150–159
Dang Z, Liu C, Haigh MJ (2002) Mobility of heavy metals associated with the natural weathering of coal mine spoils. Environ Pollut 118:419–426
Davidson CM, Duncan AL, Littlejohn D, Ure AM, Garden LM (1998) A critical evaluation of the three-stage BCR sequential extraction procedure to assess the potential mobility and toxicity of heavy metals in industrially-contaminated land. Anal Chim Acta 363:45–55
Deneux-Mustin S, Roussel-Debet S, Mustin C, Henner P, Munier-Lamy C, Colle C, Berthelin J, Garnier-Laplace J, Leyval C (2003) Mobilité et transfert racinaire des éléments traces : influence des micro-organismes du sol. Pref Elisabeth Leclerc-Cessac Paris, Tec et Doc
Escarre J, Lefebvre C, Raboyeau S, Dossantos A, Gruber W, Cleyet Marel JC, Frerot H, Noret N, Mahieu S, Collin C, Van Oort F (2011) Heavy metal concentration survey in soils and plants of the Les Malines Mining District (Southern France): implications for soil restoration. Water Air Soil Poll 216:485–504
Esshaimi M, Ouazzani N, El Gharmali A, Berrkhis F, Valiente M, Mandi L (2013) Speciation of heavy metals in the soil and the tailings, in the zinc-lead Sidi Bou Othmane abandoned mine. Environ Earth Sci 3(8):138–147
Ettler V, Mihaljevic M, Kribek B, Majer V, Sebek O (2011) Tracing the spatial distribution and mobility of metal/metalloid contaminants in Oxisols in the vicinity of the Nkana copper smelter, Copperbelt province, Zambia. Geoderma 164:73–84
Fang ZQ (2016) Pollution Characteristics of Heavy Metal in Soil from Lead and Zinc mine and its Stabilization Study. China University of Mining & Technology, Beijing
Fiedler HD, Lopez-Sanchez JF, Rubio R, Rauret G, Quevauviller PH, Ure AM, Muntau H (1994) Study of the stability of extractable trace metal contents in a river sediment using sequential extraction. Analyst 119:1109–1114
Fijałkowski K, Kacprzak M, Grobelak A, Placek A (2012) The influence of selected soil parameters on the mobility of heavy metals in soils. Inż Och Środ 15:81–92
Gworek B, Barański A, Czarnowski K, Sienkiewicz J, Porębska G (2000) Risk assessment in contaminated land management. Rocz Gleboznawcze 3:101–110
Haghiri F (1974) Plant uptake of cadmium as influenced by cation exchange capacity, organic matter, zinc and soil temperatures. J Environ Qual 3:180–183
Hakanson L (1980) An ecological risk index for aquatic pollution control, a sedimentological approach. Water Res 14:975–1001
Halim MA, Majumder RK, Zaman MN, Hossain S, Rasul MG, Sasak K (2013) Mobility and impact of trace metals in Barapukuria coal mining area, Northwest Bangladesh. Arab J Geosci 6(12):4593–4605
He ZL, Yanga XE, Stoffellab PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19:125–140
Ho D, Evans GJ (1997) Operational speciation of cadmium, copper, lead and zinc in the NIST standard reference materials 2710 and 2711 (Monatna soil) by the BCR sequential extraction procedure and flame atomic absorption spectrometry. Anal Commun 34:363–364
Huang SW, Jin JY (2008) Status of heavy metals in agricultural soils as affected by different patterns of land use. Environ Monit Assess 139(1–3):317–327
Huang H, Li T, Gupta D, He Z, Yang XE, Ni B, Li M (2012) Heavy metal phytoextraction by Sedum alfredii is affected by continual clipping and phosphorus fertilization amendment. J Environ Sci 24:376–386
Huynh TH (2009) Impacts des métaux lourds sur l’interaction plante/verre de terre/microflore tellurique. Université Paris-Est, Océan 170p
Kabala C, Singh BR (2001) Fractionation and mobility of copper, lead and zinc in soil profiles in the vicinity of a copper smelter. J Environ Qual 30:485–492
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants. CRC Press Inc. 3ème Ed Kalbitz K, Wennrich R. Sci Total Environ, Boca Raton, pp 209–227
Kalbitz K, Rupp H, Meißner R, Braumann F (1998) Veränderungen in der Stoffdynamik eines Niedermoorgebietes durch Renaturierungsmaßnahmen. In: Geller W et al (ed) Gewässerschutz im Einzugsgebiet der Elbe. UFZ, Umweltforschungszentrum Leipzig-Halle GmbH. Vieweg+Teubner Verlag
Karczewska A (1996) Metal species distribution in top- and sub-soil soil in an area affected by copper smelter emissions. Appl Geochem 11:35–42
Khamseh A, Shahbazi F, Oustan S, Najafi N, Davatgar N (2017) Impact of tailings dam failure on spatial features of copper contamination (Mazraeh mine area, Iran). Arab J Geosci 10:244
Kossoff D (2014) Mine tailings dams: characteristics, failure, environmental impacts, and remediation. Appl Geochem 51:229–245
Kucharski R, Sas-Nowosielska A, Małkowski E, Japenga J, Kuperberg JM (2008) Phytoremediation technologies used to reduce environmental threat posed by metal-contaminated soils: theory and reality. In: Barnes I, Kharytonov MM (eds) Simulation and assessment of chemical processes in a multiphase environment. NATO Science for Peace and Security Series C: Environmental Security. Springer, Dordrecht
Lama EJ, Cánovasb EJM, Gálveza ME, Montofréb ÍL, Keithc BF, Fazd Á (2017) Evaluation of the phytoremediation potential of native plants growing on a copper mine tailing in northern Chile. J Geochem Explor 182:210–217
Lamy I (2002) Réactivité des matières organiques des sols vis-à-vis des métaux. J Natl l’étude Sols 22
Lei M, Zhang Y, Khan S, Qin PF, Liao BH, Liao BH (2010) Pollution, fractionation, and mobility of Pb, Cd, Cu, and Zn in garden and paddy soils from a Pb/Zn Mining area. Environ Monit Assess 168:215–222
Li LY, Li RS (2000) The role of clay minerals and effect of H+ ions on removal of heavy metal (Pb2+) from contaminated soil. Can Geotech J 37:296–307
Liakopoulos A, Lemiere B, Michael K, Crouzet C, Laperche V, Romaidis I, Drougas I, Lassin A (2010) Environmental impacts of unmanaged solid waste at a former base metal mining and ore processing site (Kirki, Greece). Waste Manag Res 28:996–1009
Lopez-Sanchez JF, Sahuquillo A, Fiedler HD, Rubio R, Rauret G, Muntau H, Quevauviller P (1998) CRM 601, a stable material for its extractable content of heavy metals. Analyst 123:1675–1677
Ma LQ, Rao GN (1997) Chemical fractionation of cadmium, copper, nickel, and zinc in contaminated soils. J Environ Qual 13:372–376
Martin R, Sanchez DM, Gutierrez AM (1998) Sequential extraction of U, Th, Ce, La and some heavy metals in sediments from Ortigas River, Spain. Talanta 46:1115–1121
McLean JE, Bledsoe BE (1992) Behaviour of metals in soils. U.S. Environmental Protection Agency /540/S92/018
Mil-Homens M, Stevens RL, Abrantes FF, Cato I (2006) Heavy metal assessment for surface sediments from three areas of the Portuguese continental shelf. Cont Shelf Res 26(10):1184–1205
Moncur MC, Ptacek CJ, Blowes DW, Jambor JL (2005) Release transport and attenuation of metals from an old tailings impoundment. Appl Geochem 20:639–659
Montoroi JP (1997) Electric conductivity of soil solution and aqueous. Etude Gest Sols 4:279–298
Mouni L, Belkhiri L, Bouzaza A, Bollinger JC (2017) Interactions between Cd, Cu, Pb, and Zn and four different mine soils. Arab J Geosci 10:77
Mseddi H (2013) Caractérisation des rejets miniers des sédiments et des sols d’El Akhouat (bassin versant aval de l’oued Siliana) phytoremediation des sols pollués -Tunisie-. Thèse de Doctorat de l’Université de Tunis El Manar, Tunisie. 221p
Müller G (1969) Index of geoaccumulation in sediments of the Rhine River. J Geol 2:109–118
Narwal RP, Singh BR, Salbu B (1999) Association of cadmium, zinc, copper and nickel with components in naturally heavy metal rich soils studied by parallel and sequential extractions. Commun Soil Sci Plant 30:1209–1230
Neal NH, Sposito G (1986) Effect of organic matter on the distribution, extractability and uptake of cadmium in soils. Soil Sci 44:641–650
Nganje TN, Adamu CI, Ugbaja AN, Ebieme E, Sikakwe GU (2011) Environmental contamination of trace elements in the vicinity of Okpara coal mine, Enugu, Southeastern Nigeria. Arab J Geosci 4(1–2):199–205
Obiora SC, Chukwu A, Davies TC (2016) Heavy metals and health risk assessment of arable soils and food crops around Pb–Zn mining localities in Enyigba, southeastern Nigeria. J Afr Earth Sci 116:182–189
Ociepa E, Kisiel A, Lach J (2010) Effect of fertilization with sewage sludge and composts on the change of cadmium and zinc solubility in soils. J Environ Stud 2:171–175
Othmani MA (2013) Caractérisation des rejets miniers de Touiref (Nord-Ouest de la Tunisie) et dynamique des métaux lourds dans les conditions superficielles et impact sur l’environnement. Thèse de Doctorat de l’Université de Tunis El Manar, Tunisie, 243p
Ouchir N, Ben Aissa L, Boughdiri M, Aydi A (2016) Assessment of heavy metal contamination status in sediments and identification of pollution source in Ichkeul Lake and rivers ecosystem, northern Tunisia. Arab J Geosci 9:539
Papadopoulou-Vrynioti K, Alexakis D, Bathrellos GD, Skilodimou HD, Vryniotis D, Vasiliades E (2014) Environmental research and evaluation of agricultural soil of the Arta plain, western Hellas. J Geochem Explor 136:84–92
Parizanganeh A, Hajisoltani P, Zamani A (2010) Assessment of heavy metal pollution in surficial soils surrounding Zinc Industrial Complex in Zanjan-Iran. Procedia Environ Sci 2:162–166
Perel’man AI (1986) Geochemical barriers: theory and practical applications. Appl Geochem 1(6):669–680
Plassard F, Winiarski T, Petit-Ramel M (2000) Retention and distribution of three heavy metals in a carbonated soil: comparison between batch and unsaturated column studies. J Contam Hydrol 42:99–111
Prasad MNV, Freitas H (2003) Metal hyperaccumulation in plants biodiversity prospecting for phytoremediation technology. Electron J Biotechnol 6:275–321
Rao SC, Northup BK (2008) Forage and grain soybean effects on soil water content and use efficiency. Crop Sci 48(2):789–793
Rauret G, López-Sánchez JF, Sahuquillo A, Barahona E, Lachica M, Ure AM, Davidson CM, Gomez A, Luck D, Bacon J, Yli-Halla M, Muntau H, Quevauviller P (2000) Application of a modified BCR sequential extraction (three-step) procedure for the determination of extractable trace metal contents in a sewage sludge amended soil reference material (CRM483), complemented by a three-year stability study of acetic acid and EDTA extractable metal content. J Environ Monit 2:228–233
Rayment GE, Lyons DJ (2011) Soil chemical methods—Australasia. CSIRO Publishing, Melbourne 495+20 pp
Rodriguez L, Ruiz E, Alonso-Azcarate J, Rincon J (2009) Heavy metal distribution and chemical speciation in tailings and soils around a Pb-Zn mine in Spain. J Environ Manag 90:1106–1116
Rouvier H (1977) Géologie de l'extrême Nord tunisien: Tectonique et paléogéographies superposées à l'extrémité orientale de la chaîne nord-maghrébine. Doctorat d'Etat. 2 Volumes, Univer Paris-Orsy 703 p
Sainfeld P (1952) Les gites plombo-zincifères de la Tunisie. Ann Mines Géol 9 Tunis:285
Salbu B, Krekling T, Oughton DH (1998) Characterization of radioactive particles in the environment. Analyst 123:843–849
Salomons W (1993) Adoption of common schemes for single and sequential extractions of trace metals in soil and sediments. Int J Environ Anal Chem 51:3–4
Salomons W, Stigliani W (1995) Biogeodynamics of pollutants in soils and sediments. Springer-Verlag, Berlin, 352p
Schultz LG (1964) Quantitative interpretation of mineral composition from X-ray and chemical data for the Pierre Shale U. S. Geol. Survey Prof. Paper 391C, United States Government Printing Office, Washington, D.C., C1-C31
Sebei A (2007) Impact des rejets miniers sur l’environnement. Cas de bassins versants des Oueds Mellègue et Tessa (Tunisie septentrionale). Thèse de Doctorat de l’Université deTunis El Manar, Tunisie, 256p
Sherlock EJ, Lawrence RW, Poulin R (1995) On the neutralization of acid rock drainage by carbonate and silicate minerals. Environ Geol 25:43–54
Sliti N (2013) Minéralogie et composition chimiques des rejets miniers et des sols dans l’ancien district de Khanguet Kef Ettout. Mastère de Recherche de l’Université de Tunis El Manar, Tunisie, 131p
Staunton S (2002) Direct and indirect effects of organic matter on metal immobilisation in soil. Dev Soil Sci 28(Part A):79–97
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ Geol 39:611–627
Trefrey JH, Presley BJ (1976) Heavy metals in sediments from San Antonio Bay and the Northwest Gulf of Mexico. Environ Geol 1(5):283–294
Trifi M, Dermech M, Charef A, Azouzi R, Hjiri B (2018) Extraction procedures of toxic and mobile heavy metal fraction from complex mineralogical tailings affected by acid mine drainage. Arab J Geosci 11:328
Ure AM, Quevauviller P, Muntau P, Greipink B (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int J Environ Anal Chem 51(1–4):135–151
Usero J, Gamero M, Morillo J, Gracia I (1998) Comparative study of three sequential extraction procedures for metals in marine sediments. Environ Int 24:478–496
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal – contaminated land. A review. Environ Chem Lett 8:1–17
Van der Marel HW (1966) Quantitative analysis of clay minerals and their admixtures. Contrib Mineral Petrol 12(1):96–138
Van Loon GW, Duffy SJ (2007) Chemia środowiskowa, Wyd. Naukowe PWN, Warszawa
Wang L, Li Y, Haoran Wang H, Cui X, Wang Xing LA, Wang X, Wang C, Gan D (2017) Weathering behavior and metal mobility of tailings under an extremely arid climate at Jinchuan Cu-Ni sulfide deposit Western China. J Geochem Explor 173:1–12
Yadav HL, Jamal A (2015) Impact of mining on water resources in India. Int J Adv Res 3(10):1009–1015
Yuan CG, Shi JB, He B, Liu JF, Liang LN, Jiang GB (2004) Speciation of heavy metals in marine sediments from the East China Sea by ICP-MS with sequential extraction. Environ Int 30:769–783
Zhong L, Liu L, Yang J (2012) Characterization of heavy metal pollution in the paddy soils of Xiangyin County, Dongting lake drainage basin, central south China. Environ Earth Sci 67(8):2261–2268
Acknowledgments
The constructive and thorough reviews of anonymous reviewers are warmly acknowledged. Additionally, we would like to thank Pr. Simon Sheppared for the English language revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sliti, N., Abdelkrim, C. & Ayed, L. Assessment of tailings stability and soil contamination of Kef Ettout (NW Tunisia) abandoned mine. Arab J Geosci 12, 73 (2019). https://doi.org/10.1007/s12517-018-4204-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-018-4204-0