Abstract
Mining activity is one of the most important sources of heavy metals in the environment. In NE Tunisia, the former Jebel Ressas mine represents a great hazard due to huge amounts of waste deposited in waste dumps and tailings often with high concentration of heavy metal pollution. The aim of this study was to determine total heavy metal contents in the mining wastes and in the soil samples collected in the vicinity of the former Jebel Ressas mine and to evaluate the mobility of heavy metals in the surrounding agricultural soils. The pH, CEC, organic matter content and total carbonate content in all the samples (soil and tailings) were also measured using the standard methods. The mine tailings are characterized by high levels of Cd (18–89 mg kg−1), Pb (433–5845 mg kg−1) and Zn (1682–40970 mg kg−1). The adjacent soils were also highly contaminated with metals. These toxic metal concentrations exceed those environmental standards proposed by the Off J Eur Communities L181: 6–12 (1986) for agricultural soils (3, 300, and 300 mg kg−1 for Cd, Pb and Zn, respectively). Selective extractions used to estimate the risks of toxic element mobilization show that a very low proportion of heavy metals is water soluble and exchangeable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining is one of the most important sources of heavy metals in the environment. In Northern Tunisia, there are ~50 metallic mines (Pb, Zn, F, etc.), >98 % of which are now closed and may be long-term sources of environmental pollution (Bouhlel 1993). One of the main concerns is the tailing materials that are generally enriched in heavy metals and consequently they are toxic. The composition of tailings is directly dependent on the composition of the ore and gangue as well as the process of mineral extraction used on the ore (Lottermoser 2007). Exposure of tailings to air, oxidation, and climatic conditions favored the release of heavy metals (Smuda et al. 2008) which are often a threat to the environment (Chiu et al. 2006; Chakroun et al. 2010; Concas et al. 2006). They have a negative impact on the concepts of sustainable development. In fact, many heavy metals remain in quantities that exceed the environmental standards in mining areas having a potential to contaminate soil and water. The tailing materials can be dispersed and accumulated in plants and animals and taken in by human beings as a consumer (Clemente et al. 2007; Wang et al. 2008).
Mining activity in Jebel Ressas (1869–1956) generated important quantities of wastes (two million tons). Under the semi-arid Mediterranean climate, wind and water erosion triggers the transport of highly Cd, Pb, and Zn contaminated dust towards the adjacent agricultural soils and the former miners’ village located near the waste dumps (Boussen et al. 2010; Mlayah et al. 2009). In this concern, few studies have been done in this area to determine the heavy metal concentration around mine areas and their impact on surrounding soil and water resources (Ghorbel et al. 2010). Thus, the aims of this study were: (1) to carry out geochemical and mineralogical studies of the tailing materials and the surrounding agricultural soils and (2) to estimate the heavy metals availability in soil samples collected in the vicinity of the Pb–Zn abandoned mine of Jebel Ressas.
Materials and methods
Description of the site investigated
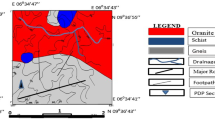
The abandoned Jebel Ressas mine is localized at 30 km in the South of the Tunisian capital and is characterized by a semi-arid climate. Jebel Ressas mine is located close to a rural district and surrounded by agricultural lands (Fig. 1). The soil is calcareous, deriving from Triassic carbonated bedrock. On the eastern side, the dump DIII is adjoined by the village of Jebel Ressas and on the western side by agricultural lands (orchards, cereal crops). The annual rainfall in the region is 450 mm, and average temperature is 25 °C. The prevalent winds in the region are from W to NW (Ghorbel et al. 2010).
The Jebel Ressas Pb–Zn deposits occur mainly as open-space fillings (lodes and tectonic breccia cements) in bioclastic limestones of the Upper Jurassic Ressas formation and along the contact of this formation with Triassic rocks. The galena–sphalerite association and their alteration products (cerussite, hemimorphite, and hydrozincite) are set within a calcite gangue (Jemmali et al. 2011). From 1869 to 1956, the Jebel Ressas mine produced approximately 122,000 tons of Pb and 165,000 tons of Zn (Direction Générale des Mines 2005). However, approximately two million tons of untreated waste materials dumped in three flattop dumps DI, DII, and DIII, rich in heavy metals (such as Cd, Pb, and Zn), remained following mining (Fig. 1). The lack of vegetation development on the mine tailings and the tailing pile slopes causes instability of the material and allows wind and rain erosion to occur (Fig. 2).
Mine wastes and soil sampling and characterization
In this study, there were three types of samples: composite tailing samples designated as T1, T2, and T3; composite soil samples from areas in the vicinity of the mine assumed as polluted (each sample was a mixture of three sampling corners forming a triangle in the centers of the field and having edges of two meters length) (P1–P10); and soil control sample (C sample), obtained about 1 km north from the tailing dams, representing the local geochemical background of the analyzed elements (Fig. 1). Soil samples were taken from 0 to 20 cm after removing the first layer of surface soil (2 cm). The sampling of the tailings was carried out in three tailing dams (DI, DII, and DIII) by using a cylindrical corer. The average sample of each tailing reservoir is considered after mixture of the various subsamples. After collection, the soil and tailing samples were carefully transferred to clean and dry self-sealing polyethylene bags and transported to laboratory. After being air-dried in paper lined propylene trays at room temperature and disaggregated with a wooden roller, all samples were sieved through <2 mm sieve.
The physical–chemical characterization of tailing materials and soil samples consisted in the determination of pH, organic matter content (OM), cation exchange capacity (CEC), and the carbonate content of the samples according to standard methods. Soil pH was determined using 1:5 soil/H2O (Thomas 1996). Cation exchange capacity was determined using the ammonium-saturation and distillation method (Sumner and Miller 1996). Equivalent calcium carbonate (% CaCO3) was determined by the volumetric method using a Bernard calcimeter (Aubert 1978). Estimation of total concentrations of heavy metals for all samples was determined by flame-atomic absorption spectrometry (AAS) and furnace-AAS after digestion of 2 g of sample at 80 °C for 2 h with aqua regia. The quality of analytical method (QA/QC) for total metal concentration was assessed using the reference contaminated soil sample (Standard Reference Material 2586) that was digested and analyzed in the same conditions as the soil samples from the study area. The agreements between the certified reference values and those determined by the analytical method were in the range of 90–110 %.
Selective extractions were performed individually to determine the water-soluble, exchangeable, and carbonate-bound fractions.
-
1.
Water soluble fraction: a 10 mL volume of deionized water was added to 1 g of dried soil or tailing sample. The mixture was shaken for 1 h at room temperature on a mechanic shaker. The extract was separated from the solid residue by centrifugation and stored at 4 °C before analysis (Kabala and Singh 2001; Lebourg et al. 1996).
-
2.
Exchangeable cations at the soil pH: a 25 mL volume of sodium nitrate 0.1 M was added to 10 g of dried soil or tailing samples. The mixture was shaken for 2 h at room temperature on a mechanic shaker. The extract was separated from the solid residue by centrifugation and stored at 4 °C before analysis (Gupta and Aten 1993).
-
3.
Associated to the carbonates: a 25 mL volume of sodium acetate (1 M) adjusted to pH 5.5 with acetic acid was added to 10 g of dried soil or tailing sample. The mixture was shaken for 6 h at room temperature on a mechanic shaker. The extract was separated from the solid residue by centrifugation. The liquid was stored at 4 °C before analysis (Han and Banin (1995). Heavy metal contents in the supernatant solutions were quantified with flame-atomic absorption spectrometry (AAS) and furnace-AAS and blanks were used for quality control. The proportions of each fraction are defined as the ratio of the bound heavy metal in each fraction to the total heavy metal in the soil. All reagents used to prepare the extracting solutions were of analytical grade (Merck pro-analysis). All solutions and dilutions were prepared with double deionized water. Duplicate analyses were performed on all samples and reagent blanks were used for quality control assurance. Blanks were measured in parallel for each analysis batch, and the appropriate extracting reagents were used to verify the quality of the reactants and to detect contamination. The accuracy of the selective chemical extraction was determined by comparing the sum of the concentrations obtained at different stages of extraction with the contents obtained by total decomposition (Cuong and Obbard 2006). The accuracy values ranged between 70 and 110 %, which are of the same order as those obtained by other researchers (Cuong and Obbard 2006; Li and Thornton 2001; Umoren et al. 2007; Cornu and Clozel 2000).
Diffraction analyses by X-ray (XRD) were performed on powdered bulk soil samples after the grinding of representative sub-samples using a Philips X’Pert-PRO diffractometer equipped with a diffracted beam monochromator and an X‘Celerator multichannel detector. The analyses were performed under the following conditions: Cu Kα radiation, 40 kV, 30 mA, step scanning at 0.01°2θ/s in the range 1–30°. Mineral identification was made by reference to the International Center for Diffraction Data (ICDD) and the International Crystal Structure Database (ICSD) patterns.
Results and discussion
Physical–chemical properties of soils and tailings
The results of chemical analysis of the tailings and soils that are taken from topsoil in the vicinity of the lead–zinc abandoned mine of Jebel Ressas and control soil are presented in Table 1.
The results obtained for the soil pH measurements revealed that the pH of the mine tailings varies from 7.1 to 7.3 with a mean value of 7.2. The pH values of the surrounding soils vary from 7.6 to 7.9 with a mean value of 7.8. The neutral and alkaline pH values in soil and mining residues are related to the presence of high concentration of carbonates (values ranging from 38.6 to 45.5 % for tailings, and from 31.4 to 45 for soils). In Tunisia, the soils that are developed on sedimentary rocks are mainly calcareous in the North and gypseous in the South. All the soil samples presented very high organic matter content (OM) ranging from 4.6 to 6.1 %, similar to background samples. These values are due to the nature of soil (arable land and cultivated). The lack of a vegetation cover in some parts of the mining area could explain the low values of OM content in samples corresponding to the tailings (0.78–1.1 %). The cation exchangeable capacity (CEC) varied between 9.4 and 13.2 cmol kg−1 with a mean value of 11.9 cmol kg−1. High CEC indicated that some soils have a natural potential for immobilizing toxic trace elements (García-Sánchez et al. 2010). In general, these results are correlated with previous studies carried on Tunisian soils (Boussen et al. 2010).
According to mineralogical analysis, in the Jebel Ressas soil samples the primary non-metallic phases are largely dominated by calcite (CaCO3) along with quartz. Pb is present as cerussite (PbCO3) and galena (PbS) (associated with calcitic gangue). Other minerals like hemimorphite (Zn4Si2O72H2O) and sphalerite [(Zn,Fe)S] could also occur.
Total heavy metal levels in soils and tailings
Total heavy metal contents in the soils and the mine tailings are shown in Tables 1. The mine tailings are characterized by high levels of Cd (18–89 mg kg−1), Pb (433–5845 mg kg−1), and Zn (1682–40,970 mg kg−1). The adjacent soils were also highly contaminated with metals. The highest heavy metal contents were 230, 12,825, and 42,368 mg kg−1 with median concentrations of 19, 5157, and 1842 mg kg−1 for Cd, Pb, and Zn, respectively. These high concentrations were also reported near mining sites in Tunisia reaching 15, 12,000, and 1000 mg kg−1 Cd, Pb, and Zn, respectively) (Sebei et al. 2006). Similar studies, on carbonated context and in semi-arid climates showed enrichment of heavy metals in soils near mining sites. For example, the heavy metal concentrations at the Jebal Ressas site were similar to those in Morocco, which ranged between 2.89 and 13, 74 and 6706, and 233 and 22,641 mg kg−1 for Cd, Pb, and Zn, respectively (Esshaimi et al. 2013). In Spain, Navarro et al. (2008) reported concentrations in soils near mining sites as high as heavy metal contents in the Tunisian soils reaching 104 for Cd, 1.9 % for Pb, and 4.6 % mg kg−1 for Zn. In Morocco, Iavazzo et al. (2012a, b) reported high Cd, Pb, and Zn contents reaching, respectively, 1020, 13,300, and 182,000 mg kg−1, in the high Moulouya Valley district.
According to the European standards (Council of the European Communities 1986), the majority of the soil samples located around the tailing D1, DII, and DIII exceed the maximum Cd, Pb, and Zn allowable concentrations of heavy metals for agricultural soils. This fact suggests the common origin of all the metals analyzed and, therefore, the mining activity can be pointed out as the source for the metal pollution of the studied area. In semi-arid environments, Aeolian erosion is the dominant natural mechanism in which pollution is spread from mines to agricultural soils (Boussen et al. 2010; Ghorbel et al. 2010; Jacob and Otte 2004; Conesa et al. 2007). Soils located in the prevalent wind direction (W to NW) from the mine tailings (Fig. 1) were enriched in heavy metals due to the Aeolian deposition of heavy metal-rich particles. In addition, hydric transport is a local cause of heavy metal contamination. For example, soils are flooded during flood periods and receive substantial quantities of tailings materials, which lead to the heavy metal contamination of the surrounding agriculture soils.
Solubility and extractability of heavy metals
The sequential extraction was used in this study to indirectly assess the potential phytoavailability of metal contaminants in soils. Water soluble and exchangeable fractions represent the immediately mobile and the carbonate-bound fraction (Adamo and Zampella 2008; Ure and Davidson 2002). Selective extraction results are presented in Table 2.
Based on our results, the amount of Cd, Pb, and Zn present in the water soluble and exchangeable fraction (i.e. the most mobile and bioavailable fraction present in soils) is very low. These results are in agreement with those observed by Li and Thornton (2001) and Rodriguez et al. (2009) in a contaminated environment near industrial activities and mines. After the oxidation of galena in alkaline conditions, when exposed to atmospheric oxygen and rain, Pb precipitates as cerussite (PbCO3) (Ramos Arroyo and Siebe (2007). According to Iavazzo et al. (2012a, b), the small contribution of water soluble and exchangeable forms to the total metals extracted sequentially could be explained by the fact that the small solubility of cerussite (logK = −13.1) and hemimorphite (logK = −24) enhances metal precipitation and cation retention processes in alkaline conditions.
On the contrary, the carbonate fraction is the most important fraction with approximately 68–77 % of total Pb, 9–15 % of total Zn, and 50–83 % of total Cd. Heavy metals show the following affinity for carbonates: Cd > Pb > Zn. Based on our results, the proportions of Cd associated to carbonates are high. Similarly, Yusuf (2007) observed that carbonate fraction of Cd represents more than 70 % of total Cd. Some authors, such as Iavazzo et al. (2012a, b), reported that Pb carbonate accounted for 53–82 % of the total Pb. Similarly, Li and Thornton (2001) showed that Pb is strongly associated with carbonates (24–55 %) and reducible fractions (Fe and Mn oxides phases) in contaminated soils from historical Pb mining and smelting areas in Derbyshire (England). The geochemical repartitioning of lead in the studied soils agrees with the mineralogical analysis, which shows the presence of lead carbonates such as cerussite and, to a lesser extent, galena (PbS). According to Lindsay (1979), cerussite is the most stable and dominant form of Pb in alkaline mineral soils. Thus, the small proportion of the most mobile Pb fraction suggests that Pb is stable in natural conditions, and consequently, mobilization risks are limited. Mineralogical analysis of the mine tailings and soils showed that zinc occurs as sphalerite, hemimorphite, and as a Fe-associated oxyhydroxide. According to Ramos Arroyo and Siebe (2007) the affinity of heavy metals to Fe oxyhydroxides decreases in the following order: Zn > As > Pb. In alkaline conditions, the association of Zn with oxyhydroxides and the residual fraction has been reported by several authors (Banin et al. 1990; Xiang et al. 1995). The predominant form of Zn is in the reducible fraction up to 54 % (Yobouet et al. 2010) or up to 25 % (Li and Thornton 2001). Xiang et al. (1995) reported that the percentage of zinc that was associated with Fe and Mn oxyhydroxides increased with increasing pH.
Conclusions
Jebel Ressas mine area has been characterized by determining various physico-chemical parameters including pH, CEC, CaCO3 content, and organic matter content. The studied wastes and soils of the mining areas showed that in general, all samples presented a neutral to alkaline pH similar to background sample, the alkaline pH could be attributed to the presence of carbonates. The data indicate that the abandoned tailings and soils are all highly enriched in Cd, Pb, and Zn. According to the European standards (86/278/EEC, 1986), most of the studied wastes and soils represent a potential source of contamination for the surrounding environmental compartments. The sequential extraction data show that a small fraction of Cd, Pb, and Zn was labile, which is attributed to the carbonate content of the soils, the low solubility of the major metallic mineral phases and to the alkaline pH conditions.
References
Adamo P, Zampella M (2008) Chemical speciation to assess potentially toxic metals (PTMs) bioavailability and geochemical forms in polluted soils. In: De Vivo B, Belkin HE, Lima A (eds) Environmental geochemistry: site characterization, data analysis and case histories. Elsevier, Amsterdam, The Netherlands, pp 175–212
Aubert G (1978) Méthodes d’analyses des sols. C.R.D.P, Marseille
Banin A, Gerstl Z, Fine P, Metzger Z, Newrzella D (1990) Minimizing soil contamination through control of sludge transformations in soil. Joint German-Israel research projects report. N°: Wt 8678/458
Bouhlel S (1993) Gîtologie, minéralogie et essai de modélisation des minéralisations à F-Ba-Sr-Pb-Zn-(S°) associées aux carbonates (jurassiques et cretacés) et aux diapirs triasiques: gisements de Stah-Kohol, Zriba-Guebli, Bou Jaber et Fej Lahdoum (Tunisie Septentrionale) Thèse d’Etat Es-Sciences géologiques. Université de Tunis, FST 293p
Boussen S, Sebei A, Soubrand-Colin M, Bril HF, Chaabani Abdeljaouad S (2010) Mobilization of lead-zinc rich particles from mine tailings in northern Tunisia by aeolian and run-off processes. Bull Soc Géol Fr 181:371–379
Chakroun HK, Souissi F, Bouchardon JL, Souissi R, Moutte J, Faure O, Remon E, Abdeljaoued S (2010) Transfer and accumulation of lead, zinc, cadmium and copper in plants growing in abandoned mining-district area. Afr J Environ Sci Technol 4:651–659
Chiu KK, Ye ZH, Wong MH (2006) Growth of Vetiveria zizanioides and Phragmities australis on Pb/Zn and Cu mine tailings amended with manure compost and sewage sludge: a greenhouse study. Bioresour Technol 97:158–170
Clemente R, Paredes C, Bernal MP (2007) A field experiment investigating the effects of olive husk and cow manure on heavy metal availability in a contaminated calcareous soil from Murcia, Spain. Agric Ecosyst Environ 118:319–326
Concas A, Ardau C, Cristini A, Zuddas P, Cao G (2006) Mobility of heavy metals from tailings to stream waters in a mining activity contaminated site. Chemosphere 63:244–253
Conesa HM, Faz A, Arnaldos R (2007) Initial studies for the phytostabilization of a mine tailing from the Cartagena-La Union Mining District (SE Spain). Chemosphere 66:38–44
Cornu S, Clozel B (2000) Extractions séquentielles et spéciation des éléments traces métalliques dans les sols naturels. Etude et Gestion des Sols 7:179–189
Council of the European Communities (1986) Directive (86/278/EEC) on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Off J Eur Communities L181:6–12
Cuong DT, Obbard JP (2006) Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Appl Geochem 21:1335–1346
Direction Générale des Mines (2005) Annuaire statistique, mines et dérivés. Ministère de l’Industrie (1997–2005), p. 30. Tunis
Esshaimi M, Ouazzani N, El Gharmali A, Berrekhis F, Valiente M, Mandi L (2013) Speciation of heavy metals in the soil and the tailings, in the zinc-lead Sidi Bou Othmane Abandoned Mine. J Environ Earth Sci 3:138–146
García-Sánchez A, Alonso-Rojo P, Santos-Francés F (2010) Distribution and mobility of arsenic in soils of a mining area (Western Spain). Sci Total Environ 408:4194–4201
Ghorbel M, Munoz M, Courjault-Radé P, Destrigneville C, Souissi R, Souissi F, Ben Mammou A, Abdeljaouad S (2010) Health risk assessment for human exposure by direct ingestion of Pb, Cd, Zn bearing dust in the former miner’s village of Jebel Ressas (NE Tunisia). Eur J Mineral 22:639–649
Gupta SK, Aten C (1993) Comparison and evaluation of extraction media and their suitability in a simple model to predict the biological relevance of heavy metal concentrations in contaminated soils. Int J Environ Anal Chem 51:25–46
Han FX, Banin A (1995) Selective sequential dissolution techniques for trace metals in arid zone soil: the carbonate dissolution step. Comm Soil Sci Plant Anal 26:553–576
Iavazzo P, Adamo P, Boni M, Hillier S, Zampella M (2012a) Mineralogy and chemical forms of lead and zinc in abandoned mine wastes and soils: an example from Morocco. J Geochem Explor 113:56–67
Iavazzo P, Ducci D, Adamo P, Trifuoggi M, Migliozzi A, Boni M (2012b) Impact of past mining activity on the quality of water and soil in the High Moulouya Valley (Morocco). Water Air Soil Poll 223:573–589
Jacob DL, Otte ML (2004) Influence of Typha latifolia and fertilization on metal mobility in two different Pb-Zn mine tailings types. Sci Total Environ 333:9–24
Jemmali N, Souissi F, Vennemann TW, Carranza EJM (2011) Genesis of the Jurassic Carbonate-Hosted Pb–Zn deposits of Jebel Ressas (North-Eastern Tunisia): evidence from mineralogy, petrography and trace metal contents and isotope (O, C, S, Pb) geochemistry. Resour Geol 61:367–383
Kabala C, Singh BR (2001) Fractionation and mobility of copper, lead, and zinc in soil profiles in the vicinity of a copper smelter. J Enviro Qual 30:485–492
Lebourg A, Sterckeman T, Ciesielski H, Proix N (1996) Intérêt de différents réactifs d’extraction chimique pour l’évaluation de la biodisponibilité des métaux en traces du sol. Agronomie 16:201–215
Li X, Thornton I (2001) Chemical partitioning of trace and major elements in soils contaminated by mining and smelting activities. Appl Geochem 16:1693–1706
Lindsay WL (1979) Chemical Equilibria in Soils. Wiley, Chichester, p 450
Lottermoser BG (2007) Mine wastes, characterization, treatment, environmental impacts, 2nd edn. Springer, New York
Mlayah A, Ferreira da Silva E, Rocha F, Ben Hamza Ch, Charef A, Noronha F (2009) The Oued Mellègue: mining activity, stream sediments and dispersion of base metals in natural environments. North-western Tunisia J Geochem Explor 102:27–36
Navarro MC, Perez-Sirvent C, Martinez-Sanchez MJ, Vidal J, Tovar PJ, Bech J (2008) Abandoned mine sites as a source of contamination by heavy metals: a case study in a semiaridzone. J Geochem Explor 96:183–193
Ramos Arroyo YR, Siebe C (2007) Weathering of sulphide minerals and trace element speciation in tailings of various ages in the Guanajuato mining district, Mexico. Catena 71:497–506
Rodriguez L, Ruiz E, Alonso-Azcarate J, Rincon J (2009) Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J Environ Manage 90:1106–1116
Sebei A, Chaabani F, Ouerfelli K, Abdeljaoued S (2006) Evaluation de la contamination des sols par les métaux lourds dans la région minière de Fedj Lahdoum (NW de la Tunisie), Rev Médit Environ. Tunisie, pp.1–13
Smuda J, Dold B, Spangenberg JE, Pfeifer HR (2008) Geochemistry and stable isotope composition of fresh alkaline porphyry copper tailings: implications on sources and mobility of elements during transport and early stages of deposition. Chem Geol 256:62–76
Sumner ME, Miller WP (1996) Cation exchange capacity and exchange coefficients. In: Chao TT, Sanzolone RF (1992) Decomposition techniques. J Geochem Explor 44: 65–106
Thomas GW (1996) Soil pH and soil acidity. In: Sparks DL et al (eds) Methods of soil analysis part 3. Chemical method. American Society of Agronomy, Madison, WI, pp 475–490
Umoren IU, Udoh AP, Udousoro II (2007) Concentration and chemical speciation for the determination of Cu, Zn, Ni, Pb, and Cd from refuse dump soils using the optimized BCR sequential extraction procedure. Environmentalist 27:241–252
Ure AM, Davidson CM (2002) Chemical speciation in soils and related materials by selective chemical extraction. In: Ure AM, Davidson CM (eds) Chemical speciation in the environment. Blackwell Science, Oxford, pp 265–300
Wang X, Liu Y, Zeng G, Chai L, Xiao X, Song X, Min Z (2008) Pedological characteristics of Mn mine tailings and metal accumulation by native plants. Chemosphere 72:1260–1266
Xiang HF, Tang HA, Ying QH (1995) Transformation and distribution of forms of zinc in acid, neutral and calcareous soils of China. Geoderma 66:121–135
Yobouet YA, Adouby K, Trokourey A, Yao B (2010) Cadmium, copper, lead, and zinc speciation in contaminated soils. Int J Eng Sci Technol 2:802–812
Yusuf KA (2007) Sequential extractions of lead, copper, cadmium, and zinc in soils near Ojota waste site. J Agron 6:331–337
Acknowledgments
The authors wish to thank Prof. Baghdad Ouddane of the University of Sciences and Technology of Lille (France) for his help in chemical analysis. Also, thanks to the anonymous reviewers for their constructive comments that led to an improved manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elouear, Z., Bouhamed, F., Boujelben, N. et al. Assessment of toxic metals dispersed from improperly disposed tailing, Jebel Ressas mine, NE Tunisia. Environ Earth Sci 75, 254 (2016). https://doi.org/10.1007/s12665-015-5035-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-5035-x