Abstract
The potential sources of recharge of both water and solutes to the Quaternary aquifer in the area between Ismailia and El Kassara canals in northeastern Egypt include seepage from the irrigation canals and conduits, return flow after irrigation in the cultivated fields, local precipitation, and the upward flow of groundwater from the underlying Miocene aquifer system. Water isotopes, solute concentrations, and sulfate isotopes were used to investigate the geochemical sources, reactions, and the impacts of the hydraulic connections among recharge sources. The obtained results indicate a minimal influence of the underlying Miocene aquifer as a water and solute source while old and new contributions from the irrigation canals represent the main sources of recharge. The chemical reactions responsible for the chemical constituents and salinity in the aquifer include silicate weathering, evaporite dissolution, and carbonate precipitation. Most of groundwater samples appear to lie at/or close to equilibrium with montmorillonite, kaolinite, and illite where clay minerals are quite common in the local soils of the Quaternary aquifer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quaternary aquifers are of great importance for reclaimed land projects in arid and semi-arid regions like Egypt where The Nile water supply is not sufficient to meet all demands for the agricultural activities, and groundwater resources represent the alternative supply. El Salhia El Giddida occupies one of the new reclaimed projects using groundwater for irrigation purposes. Many hydrological, hydrochemical, and stable isotope studies have been conducted in and nearby the study area (El Dairy 1980; Salluma 1983; Salem 1996; Hamza et al. 1987; Al-Gamal et al. 2005; Salem and Sadek 2006; Dahab et al. 2007 and Ahmed et al. 2013). These studies indicated the presence of old Nile water and mixing between recent Nile with old Nile in most of the localities, yet they did not study the sources of groundwater for the study area in detail. Also they did not study the different geochemical reactions responsible for the chemical constituents. The chemical composition of groundwater is controlled by many factors that include composition of precipitation, geological structure, and mineralogy of the watersheds and aquifers. The interaction of all these factors leads to various water compositions (Murray 1996; Rosen and Jones 1998).
Tracing groundwater by means of environmental isotopes offer unique and supplementary information on the origin and movement of groundwater and its dissolved solutes and allows a quantitative evaluation of mixing and other physical processes such as evaporation and isotopic exchange in geologic systems (Mook 2000).
The inverse geochemical modeling is commonly used to illustrate and reconstruct the chemical evolution of groundwater along a flow path from an initial point in an aquifer to a final point along a groundwater flow path (Sharif et al. 2008). The soundness and validity of inverse modeling results depends on a valid conceptualization of the groundwater system, the validity of basic concepts of aqueous geochemistry, accuracy of data, and an understanding of geochemical processes occurring in an aquifer (Güler and Thyne 2004).
To better understand the hydrological and geochemical properties of the aquifer system, we sought to answer three main questions in detail, which are as follows:
-
What are the sources of groundwater to this aquifer?
-
What chemical reactions are responsible for the chemical constituents and salinity?
-
Is there any connection between the Quaternary aquifer and the underlying Miocene aquifer, and if so, to what degree?
In order to answer these questions, stable isotope ratios of 2H/1H and 18O/16O were used to identify the possible sources of recharge to the groundwater system and zones of mixing. Also, the dual isotope approach of δ34S and δ18O of sulfate was used to identify sulfate sources. A Na+-normalized Ca++ versus HCO3 − and Na+ versus Cl− plots was used to identify the potential geochemical reactions controlling the chemical evolution and salinity of the groundwater in the Quaternary aquifer. Additionally, stability field diagrams of the Na2O–SiO2–Al2O3–H2O, K2O–SiO2–Al2O3–H2O and CaO–SiO2–Al2O3–H2O systems were used to identify the secondary clay minerals produced from the geochemical weathering of silicate minerals. Inverse model (Netpath-win) was also used to identify the geochemical reactions along two flow paths in the study area.
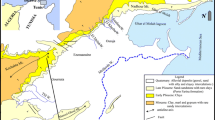
The area of study is located to the east of the Nile Delta, between Ismailia Canal to the south and El Kassara canal to the north. It is bounded by latitudes 30° 32′ 24 and 30° 40′ 12 N and longitudes 31° 49.2′ and 32° 11.4′ E (Fig. 1). It is characterized by a hot summer and a short rainy winter. The relative humidity is higher in winter than in summer, and evaporation intensity is generally higher in summer than that in winter. Annual rainfall ranges between 4.2 and 37 mm/year (Dahab et al. 2007) across the study site.
Geologic setting
The Quaternary aquifer in the studied area is composed of aeolian and old deltaic deposits which consist of sand and sandstone with intercalations of clay (Moussa 1990). The sedimentary succession in the study area is strongly affected by faulting and folding processes (El Dairy 1980) which are dominantly represented by NE–SW normal faults with downthrown side towards southeast and northwest and NW–SE normal faults with strike dip towards northeast direction. The vertical displacement along these faults ranges from a few meters to hundreds of meters (Gad 1995). The western and middle parts are strongly affected by deep-seated normal faults where saline water of Miocene aquifer moves upward along the fault planes (Gad 1995).
Hydrogeological conditions
The Quaternary water bearing deposits rest directly on a Pliocene clay and Miocene sandy limestone (Fig. 2). The thickness of the Quaternary aquifer is strongly affected by a set of normal faults with downthrown sides towards the northeast. This faulting increases the aquifer thickness towards the north and northeast, where it ranges from 300 to 400 m in the study area while it reaches more than 900 m near the Mediterranean Sea. The Quaternary aquifer occurs under unconfined conditions. Semi-confined and confined conditions occur at the eastern and northeastern parts (East Abu Sweir area) of the aquifer. This pattern is attributed to the presence of shale and clay intercalation cap beds overlying the aquifer in these localities. Depths to groundwater show a wide range, with relatively large values at high elevations at the western and middle parts of the study area, ranging from 20 to 34 m below the ground surface. Depths to groundwater are small at the low topography areas representing the new reclaimed areas located northeast of Abu Sweir ranging from 6 m to less than one meter below the ground surface (Dahab et al. 2007) (Fig. 3).
Hydrogeological cross section DD′, showing the composition of the Quaternary aquifer and the underlying aquifers (Dahab 2007)
Water levels and flow net map of the Quaternary aquifer in El Salhia El Gidida–Abu Sweir area, east Delta, Egypt (Dahab 2007). It shows that the net flow is towards the middle of the study area
Materials and methods
Field investigations were carried out during March and June 2013. A total of 103 water samples were collected at the head wells for chemical analyses. All wells had been in use before sampling until the pH and specific conductance stabilized to provide a representative in situ sample for groundwater. Physical parameters such as pH and EC were measured in the field. Samples were unfiltered and stored in pre washed propylene bottles thoroughly rinsed with the sample. The chemical analyses were performed after 5 days from collection with precision of less than 5 % E.B, at the hydrogeochemistry department, Desert Research Center, Cairo, Egypt. Alkalinity, calcium, magnesium, and chloride were analyzed by volumetric titration. Na and K were measured by a flame photometer (Jenway model PFP7). Nitrate, phosphate, and silica were analyzed by colorimetry with a UV–visible spectrophotometer (Thermo-Spectronic model 300). All the isotopic analyses were conducted at the University of Arizona Laboratory of Isotope Geo-chemistry. Deuterium and 18O were measured on mass spectrometer (Finnigan Delta S) with a precision of 0.9 per mil. δ34S was measured on mass spectrometer (ThermoQuest Finnigan Delta PlusXL). δ18O of sulfate was measured on mass spectrometer (Thermo Electron Delta V) with precisions estimated to be ±0.15 or better.
NETPATH is a geochemical model that interprets the net geochemical mass balance reactions between the initial and final waters that accounts for the changes in the chemical and isotopic compositions along a hydrological flow path. The reactant phases used in mass-transfer modeling include the minerals determined by Moussa (1990) which are as follows: albite, orthoclase, microcline, muscovite, Na-montomorellinite, kaolinite, illite, chlorite, calcite, pyroxenes, amphiboles, and quartz.
Results and discussion
Stable isotopes
The stable isotope results reflect various mixing patterns between different water sources, namely fresh water from Ismailia canal and El Kassara canal with average values of δ18O and δ2H‰ +3.426 and +24.48 ‰, respectively, and old Nile water prior to the construction of the Aswan High Dam with an average value of −0.6 and 4.3 for δ18O and δD. The underlying Miocene paleowater has average values of −6.76 and −33 ‰ for δ18O and δ2H‰ (Table 1). Mixing of these different kinds of water is clearly shown (Fig.4). Whenever contour lines of δ18O and δ2H‰ show decreases in the direction of groundwater flow, recharge from surface water which is enriched in heavy stable isotopes and mixed with other water of less enriched heavy isotopic composition is occurring.
The most enriched water has δ18O and δ2H‰ values equal to +3.4 and +24.95 ‰, respectively, which is located at the southeastern part of the study area. The enrichment is attributed to aridity conditions. On the other hand, it can be seen that the most depleted closed lines (δ18O varies from +0.9 to 1.1 ‰ and δ2H‰ varies from +7 to +9 ‰) represent an isolated formation with low permeability which has remained away from recent recharge.
As evident from Fig. 5, most of the groundwater samples plot below the local meteoric water line (LMWL), suggesting a meteoric origin. It is observed that both the surface water and some groundwater samples plot away from the LMWL showing evidence of isotopic enrichment by evaporation on the surface or in the unsaturated zone before recharge. The relationship between δ18O and Cl− can interpret the geochemical mechanism of groundwater caused by the evaporation effect. If salinity is due to evaporation, a plot of chloride content or total dissolved solids against δ18O or δ2H‰ will be positively correlated, as increased evaporation would result in isotopic enrichment as well as an increase in Cl−. If salinity is due to leaching of evaporate salts by rapid percolation through preferential pathways, then groundwater should retain δ18O or δ2H‰ similar to that of the original rainfall with high content of Cl− or TDS. On the other hand, recharge from surface water would result an increase in heavy isotope content and low content of Cl−. The relationship between δ18O and Cl− (Fig. 6) indicates three main types of water are observed. The first group is waters in which leaching of salts lead to salinization, while the second group is those affected by both evaporation and leaching of salts together. The third group are those waters affected by the contribution of recharge from recent Nile water.
The relationship between δ18O‰ versus chloride indicates three main types of water. The first group is waters in which leaching of salts lead to salinization, while the second group is those affected by both evaporation and leaching of salts together. The third group is those waters affected by the contribution of recharge from recent Nile water
Piper diagram is also a good tool to check the water sources along with stable isotopes. It shows that some groundwater samples are plot close to the surface water which indicates the contribution from recent Nile water (Fig 7), while few samples plot close to the Miocene water which reflects the contribution from the underlying Miocene aquifer. Piper diagram also shows that the majority of groundwater samples (76 %) lie in the sub area of Na–Cl water type, where primary salinity exceeds 50 %. Twenty percent of the groundwater samples lie in the sub area of Na–HCO3. The rest of the samples lie in the subarea of Ca–HCO3 water type.
Sources of groundwater and salinity
There are four poles of water and solutes sources in the study area; the old recharge by Nile water, recent recharge by Nile water, the Miocene water, and the evapotranspiration as shown in Fig. 8 which reveals that the major contribution is coming from a water whose O-18 is more or less homogenous with varying salinities due to dissolution of different minerals. In addition, three different groups of waters are recognized as the following:
-
Group A (low TDS)
Samples of this group plot on the mixing line between the old recharge by Nile water (before construction of Aswan High Dam) and recent recharge by Nile water (Fig. 8). Location of each sample on this line indicates its relative degree of mixing. The isotopic content ranges between +1.02 and +2.76 ‰ with respect to δ18O and +9.62 and +23.35 ‰ with respect to δ2H‰. The TDS ranges between 437 and 910 ppm, indicating short residence time.
-
Group B (moderate TDS)
This group includes samples which are deviated slightly from the mixing line of the recent old Nile water, suggesting an increase of salinity (1107 to 2100 ppm) due to leaching and dissolution of salts (Fig. 8). It shows an isotopic content ranging from +1.51 to +3.4 and +9.3 to +24.95 ‰ for δ18O and δ2H‰, respectively.
-
Group C (high TDS)
Samples of group C plot between the four end members showing mixing of different sources. This group includes samples with an isotopic content ranging from +0.92 to +2.47 and +8.84 to +17.13 ‰ for δ18O and δ2H‰, respectively. The TDS ranges from 2000 to 6000 ppm.
Sources of sulfates
Sulfate can be of atmospheric, pedospheric, and lithospheric origin. In addition, sulfate derived from anthropogenic sources, such as industrial wastes, fertilizers, and soil amendments. Commonly, their distinctive isotope compositions allow one to clearly distinguish the sulfur sources in the groundwater and surface water (Gu, 2005). The analysis of coupled δ34S and δ18O (SO4) data concentrations reveal that there are three sulfate sources in groundwater (Fig. 9). The first sulfate source is from the terrestrial sulfate, where δ34S values vary from 9.1 to 13.1 ‰; 18O (SO4) values are −1.8 ‰. Bacterial sulfate reduction results in a positive correlation of δ34S and δ18O with a slope near 1 (Clark and Fritz 1997); where bacteria catalyze this reaction, it is easier for bacteria to break 32S–O bonds than 34S–O bonds.
The second source of sulfate is atmospheric deposition, where δ34S values vary from 8.5 to 13.2 ‰; 18O (SO4) values vary from 11.1 to 12.7 ‰, emphasizing the process of water recharge from recent Nile water to the Quaternary aquifer. The third source is from leaching and dissolution of sulfate from the Quaternary aquifer, where δ34S values vary from 13.7 to 16.8 ‰; 18O (SO4) values vary from 11.1 to 16.9 ‰. Two samples plot close to the Miocene field indicating the upward contribution from the Miocene aquifer.
Mechanisms of the hydrogeochemical evolution
The general ranges of the chemical parameters for water from the Quaternary aquifer (Table 2) are as follows: electric conductance, 499–9200; pH, 7.4–8.84; sodium, 45–1453 mg/l; potassium, 1–28 mg/l; calcium, 8–492 mg/l; magnesium, 2–247 mg/l; bicarbonate, 173–666 mg/l; sulfate, 12–1865 mg/l; nitrate, 0–297 mg/l; and silica, 2–70 mg/l. A Na+-normalized Ca++ VS HCO3 − plot (Fig. 10) (Gaillardet et al. 1999) shows that all groups of samples plot in and between the fields of evaporite dissolution and silicate weathering. These processes were emphasized by the relationship between Na+ and Cl− (Fig. 11) showing that some samples plot along the 1:1 trend-line, indicating that halite dissolution is responsible for increased sodium, whereas most of the samples lie above the equi-line and they are typically interpreted as Na+ released from a silicate weathering reaction (Meybeck 1987) or due to returned irrigation water. The major component activities of the water samples plot on stability field diagrams of the Na+ 2O–SiO2–Al2O3–H2O, K2O– SiO2–Al2O3–H2O and Ca++O–SiO2–Al2O3–H2O systems. In these diagrams (Fig.12a–c), most groundwater samples appear to lie at/or close to equilibrium with montmorillonite, kaolinite, and illite. Such clay minerals are quite common in the local soils (El Nahry 2007). The behavior of carbonate minerals in a near surface environment is primarily controlled by the equilibrium reaction:
Any process that increases the amount of CO2 promotes the production of carbonic acid and therefore the system shifts to use up the increased carbonic acid and starts to dissolve calcite. So as the CO2 increases, the reaction shifts to calcite dissolution. On the other hand, any increase of calcium or bicarbonate shifts the reaction to precipitate calcite. In the Quaternary aquifer, the released bicarbonate and calcite from silicate weathering and evaporate dissolution shift the reaction to calcite precipitation.
Hydrogeochemical inverse modeling
The results of the geochemical modeling (Netpath-win), the water-rock and mass-transfer reactions for two hydrological flow paths, are represented in the following chemical reaction (Tables 3 and 4):
-
The first flow path (Fig. 1):
-
The second flow path (Fig. 1):
The model shows the incongruent dissolution of silicate minerals, the precipitation of clay minerals and calcite in the Quaternary aquifer. Based on geological, structural, hydrochemical, and isotopic data, a conceptual model for the Quaternary aquifer has been proposed (Fig. 13). The model summarizes the sources of waters and the hydrochemical processes in the aquifer.
Conclusion
Three different sources of water and solutes in the aquifer were investigated including the following: recent recharge by Nile water at the recharge area which is enriched in heavy isotopes and old recharge by Nile water which is more developed and depleted in heavy isotopes and a minor contribution comes from the underlying Miocene aquifer due to excessive pumping, which is the most enriched water in δ34S and δ18O of sulfate. The δ18O versus Cl relationship reflects that different hydrogeochemical processes account for the chemistry of the groundwater. They comprise the following: leaching and dissolution of salts, evaporation of shallow groundwater, and recharge from surface water. Sodium and calcium ions in the Quaternary aquifer are coming from the incongruent dissolution of plagioclase (albite and anorthite) to form clay minerals which increase along the flow path. Additional potential sources of Na+ come from evaporite deposits and cation exchange of divalent cations for Na+. Magnesium and potassium ions result from the incongruent dissolution of biotite, K-feldspars, and ferromagnesian minerals (pyroxenes and amphiboles) or dolomite/high magnesium calcite.
References
Ahmed MA, Abdel Samie SG, Badawy HA (2013) Factors controlling mechanisms of groundwater salinization and hydrogeochemical processes in the Quaternary aquifer of the Eastern Nile Delta. Egypt Environ Earth Sci 68:369–394
Al-Gamal SA, Korany EA, Awad SA, Ahmed MA, Abdelwahab S (2005) Recharge mechanisms for the Quaternary and the Miocene aquifers, East Cairo District, Egypt, using conventional and non conventional techniques. Isotope & Rad Res 37(3):711–735
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, New York
Dahab KA, El Abd EA, Fattah MK, El Osta MM (2007) Assessment of groundwater problems of the Quaternary aquifer in the area between El Salhia El Gidida-Abu Sweir, East Nile Delta. Egypt GREMNA 2:329–353
El Dairy MD (1980) Hydrogeological studies on the eastern part of Nile Delta using isotope techniques. A master thesis submitted to Zagazig Uni., Egypt
El Nahry AH (2007) Using geostatistical analysis and spectral signature for identifying shale distribution and type in El-Salhia project, Ismaillia, Egypt. Int J Geol 1:22–26
Gad MI (1995) Hydrogeological studies for groundwater reservoirs, east of the Tenth of Ramadan city and vicinities. a master thesis submitted to Ain Shams Uni, Cairo, Egypt
Gaillardet J, Dupre B, Louvat P, Allegre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chemical geology. 159:3–30
Gu A (2005) Stable isotope geochemistry of sulfate in groundwater of Southern arizona: implications for groundwater flow, sulfate sources, and environmental ignificance. A PhD dissertation submitted to the University of Arizona, Geosciences
Güler C, Thyne GD (2004) Delineation of hydrochemical facies distribution in a regional groundwater system by means of fuzzy c-means clustering. Water Resour Res 40. doi:10.1029/2004WR003299
Hamza MS, Aly AI, Swailem FM, Nada A (1987) Environmentally stable isotopes and groundwater recharge in the eastern Nile delta. International Journal of Water Resources Development 3(4):228–232
Meybeck M (1987) Global chemical weathering from surficial rocks estimated from river dissolved loads. American Journal of Science. 287:401–428
Mook WG (2000) Environmental isotope in the hydrological cycle; principles and application, vol IHP-V, Technical Documents in Hydrology | No. 39. UNESCO/IAEA, Paris
Moussa BM (1990) Petrology and soil genesis of the surface Quaternary deposits, East of the Nile Delta, Egypt. A PhD thesis submitted to the Uni. of Ain shams. Faculty of sci.
Murray KS (1996) Hydrology and geochemistry of thermal waters in the Upper Napa valley, California. Ground Water 34(6):1115–1124
Rosen M, Jones S (1998) Controls of the chemical composition of groundwater from alluvial aquifers in the Wanaka and Wakatipu basins, central Otago. New Zealand Hydrogeology J 6:264–281
Salem WM (1996) Application of isotopic and hydrochemical techniques to study groundwater in eastern Nile Delta and assessment of pollution in Lake Manzala. A PhD thesis submitted to Cairo Uni. Fac. of Engineering
Salem WM, Sadek MA (2006) Chemical and isotopic signature for mapping zones of Sea water intrusion and residual saline pockets in coastal aquifers of the Egyptian Nile Delta. Arab J Nucl Sci Appl 39:111–127
Salluma M (1983) Hydrogeological and hydrochemical studies east of the Nile Delta. A PhD dissertation submitted to Ain Shams University, Egypt
Sharif MU, Davis RK, Steele KF, Kim B, Kresse TM, Fazio JA (2008) Inverse geochemical modeling of groundwater evolution with emphasis on arsenic in the Mississippi River Valley alluvial aquifer, Arkansas (USA). Hydrogeology J. 350:41–55
Acknowledgments
The authors would like to thank The Egyptian Cultural and Educational bureau in Washington DC for the funds provided to complete this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomaah, M., Meixner, T., Korany, E.A. et al. Identifying the sources and geochemical evolution of groundwater using stable isotopes and hydrogeochemistry in the Quaternary aquifer in the area between Ismailia and El Kassara canals, Northeastern Egypt. Arab J Geosci 9, 437 (2016). https://doi.org/10.1007/s12517-016-2444-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-016-2444-4