Abstract
The recharge sources and groundwater degradation in the coastal plain of Oussja Ghar el Melah were investigated using geochemical and isotopes tools. We demonstrate that the water quality of shallow groundwater in the phreatic aquifer is impacted by long-term intensive irrigation. We show that the factors controlling the salinity and composition of groundwater are the return flow of the irrigation water, the water–rock interaction in the aquifer and salts formed on the surface by marine sprays. In the low plain where NO3 − content is of up to about 140 mg/L, groundwater is affected by diffuse pollution caused by intensive agricultural activities. In the upstream part of the plain and in the area situated in the SW, stable isotopes and tritium values indicate recent recharge by local precipitation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal areas in the Mediterranean basin are characterized by large water demand, caused by continuous growth in population, intensive agricultural practices and developing of industrial and touristic activities. Intensive exploitation of coastal aquifers to satisfy the important demand especially for domestic and agriculture uses may generate problems due to resources scarcity, quality degradation and marine intrusion (Barale and Folving 1996; Custodio and Bruggeman 1987; Ma et al. 2007; Custodio 2010). The most severe problems related to groundwater quality in these areas are rapid salinization of water resources and nitrate pollution. A combination of natural causes (low aquifers recharge due to low and variable precipitation) and anthropogenic factors (high water demand and wide fertilizer use caused by intensive agriculture practice, extension of urban areas, wastewater use in irrigation, etc.), have caused significant water quality degradation in the last few decades (Stigter et al. 1998; Re and Zuppi 2011).
The abundance of water, the fertility of the soil and mild climate in eastern coastal plains of Tunisia have induced continued migration to these areas (75 % of the Tunisian population live in coastal cities). As a consequence to the population progressive growth and the increasing of economic and touristic activities, the demand for water is increasing progressively. Intensive pumping of groundwater for several years, with concomitantly increasing in agricultural activities has led to qualitative and quantitative degradation of groundwater resources. Declining of groundwater level, excessive salinization of water resources and sea water intrusion are the common results of increase in groundwater abstraction observed in recent years (e.g., Fedrigoni et al. 2001; Trabelsi et al. 2007; Kouzana et al. 2009).
The plain of Oussja Ghar el Melah (OGM) is an example of the largest agricultural production area in the NE part of Tunisia. Groundwater from the shallow aquifer provides almost all water supply for irrigation. For many years ago, the growth over time of agricultural activities, has led to an ever-rising increase in water demand. Intensive groundwater exploitation, carried out without careful planning, has, however, brought about severe deterioration of water resources.
In the present study, environmental isotopes (18O, 2H and 3H) in conjunction with hydrochemistry (major ions and Br−) were employed to understand the groundwater recharge process and to determine sources of quality deterioration under the influence of intensive agricultural activities in the coastal plain of Oussja Ghar el Melah.
Geological setting
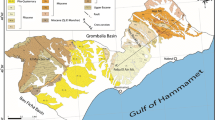
The OGM plain (135 km2) is located in the NE part of Tunisia. It is bounded on the north and in west sides by mountainous relief: Nadhour Mountain in the north (300 m) and Kechabta Mountain in the west (400 m). In the east, the boundary is formed by the Mediterranean Sea and by the Wadi of Medjerda and Menzel Ghoul Mountain in the South (Fig. 1).
The study area is a part of the molasse basin related to the major subsidence period during the Mio-Pliocene. Miocene formations represented by clay, marl and gypsum alternating with rare sandy intercalations are the oldest rocks outcropping in the surrounding mountains of the study area (Burrolet and Dumon 1952; Melki et al. 2011). These formations are covered by clay sequences attributed to Early Pliocene (Fig. 1). A thick Upper Pliocene formation (Porto Farina formation) made of sand and sandstone with clays intercalations overlies the impervious clay layers of Miocene and Late Pliocene (Chelbi et al. 1995; Oueslati et al. 2006; Mejri et al. 2010; Melki et al. 2011; Mejri 2012). The thickness of the Porto Farina formation is about 300 m (Burrolet and Dumon 1952; Melki et al. 2011). As Menzel Ghoul mountain situated in SW part of the study area, Nadhour and Kechabta mountains correspond to anticlines structures continuing in the south with a syncline structure forming the OGM plain (Figs. 1, 2). This depression constitutes the old delta of Medjerda. The plain was historically occupied by the Ghar el Melah lagoon (5000 years ago) and then filled with recent fluvial deposits composed of alternation of sand, silt and clay attributed to Holocene period (Burrolet and Dumon 1952; Oueslati et al. 2006). The thick sequence formed by Upper Pliocene and Quaternary deposits hosts the OGM phreatic aquifer. Seismic reflection study carried out in the studied area (Melki et al. 2011) shows that the thickness of the Plio-Quaternary formations varies from 300 m in the SW part of the plain (Gournata) to more than 600 m in the East part (between Oussja and the lagoon of Ghar el Melah).
The plain is characterized by low altitudes that rarely exceed 20 m in the foot of the mountains and are close to 3 m in the central part of the plain. These low topographic slopes favored the extension of wet and marshy lands in the eastern part around the Wadi Medjerda and in the west of the lagoon of Ghar el Melah.
Climatic and hydrogeological settings
The study area is characterized by a semi-arid climate with approximately 500 mm of mean annual precipitation, with the majority of precipitation occurring during the winter months between October and April (90 %). The mean annual temperature is of 18.8 °C with a minimum of 11.3 °C in February and a maximum of 26.8 °C in August. As estimated by means of empirical formula such as those of Thornthwaite, Turc, and Penman The mean annual ETP is of 1346 mm/year.
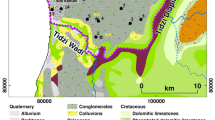
The plain of OGM corresponds to a depression (syncline structure) filled by recent Plio-Quaternary heterogeneous alluvial deposits represented essentially by sands, silts and clays. These sediments that have locally high infiltration capacity constitute the phreatic aquifer in the plain. This coastal aquifer is recharged by water flowing from the surrounding hills, where Plio-Quaternary sediments outcrop, as well as by local infiltration on the plain (central and downstream part) and from the different rivers crossing the plain: Wadi Saadane and el Kherba in the north, wadi el Melah in the central part and Wadi Tlil in the south–west.
Groundwater flow determined from well records is from the NW to SE, where it discharges to the Ghar el Melah lagoon and to the wet lands (called Sebkha) crossed by Wadi Medjerda (Fig. 3). The static water level varies between about 60 and 70 m below the ground in the northwestern areas near the mountains to less than 1 m in the downstream part of the plain around the Ghar el Melah lagoon. Pumping tests showed low transmissivity values varying from 3 to 9 × 10−4 m2/s in SW part (Gournata) to 1 to 5.5 × 10−4 m2/s in the central part of the plain (Oussja).
In the plain of OGM the groundwater is used essentially for irrigation. Groundwater abstraction is about 13 millions m3 by the means of 1450 hand-dug wells (about 11 wells/km2) equipped with pumps.
During recent decades, the phreatic aquifer of OGM, suffers from an excessive exploitation due to an increase in water demand to satisfy agriculture sector and to the extension of cultivated lands. Pumped groundwater reaching 13 millions m3 in 2009 is superior to estimated resources (7 millions m3). As shown in the piezometric contour map (Fig. 3), there is no severe drawdown of the piezometric levels observed near the sea. In the current state of knowledge, the overexploitation of groundwater resources seems not affecting the hydrodynamic of the water table and there is no seawater intrusion process declared. The anthropic effect caused by intensive pumping for irrigation in the dry season (from May to August) is offset by an excess in precipitation and limitation of pumping in the rainy season from October to April.
Methods
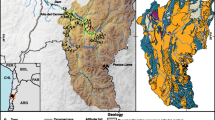
Sixty three samples (including Mediterranean Sea water, Ghar el Melah lagoon water and one spring: sample number 10) were collected for geochemical analysis (major elements) and isotopes (2H, 18O and 3H) between March and May 2010 (Fig. 4). Well locations were chosen to achieve a diverse spatial representation with different depths. The static water level in sampled wells varies from 0.5 to 59.5 m below the ground surface. Samples were collected from pumped wells in a polyethylene bottles. All samples for chemical analyses were filtered in the field through 0.45 μm filters to remove all suspended solids.
Chemical analyses were done with ion chromatography method in the National Centre for Nuclear Sciences and Technology (CNSTN) using a Dionex DX120 ion chromatograph. The precision and accuracy of analyses based on the electro-neutrality of samples were better than 5 %. Electrical conductivity (EC), temperature and pH were measured in situ.
Samples for stable isotope analysis were collected according to the procedures described by Clark and Fritz (1997). Stable isotopes were carried out on an Isoprime–GV Instruments mass spectrometer in the LAMA (Montpellier—France). Hydrogen isotope composition was measured by water reduction over metallic chrome, while δ18O was analyzed by water-CO2 equilibration; both results are expressed in δ ‰ V-SMOW (IAEA 1995) with an overall precision of 0.05 ‰ for 18O and 0.8 ‰ for 2H. Tritium concentration was determined by a scintillation counter following electrolytic enrichment in the LRAE–Sfax. The concentrations are expressed as tritium units (TU). Analytical uncertainty of tritium analyses was ±0.4 TU.
Results
The hydrochemical and isotopes results for groundwater samples from the OGM plain are shown in Table 1. The pH values range from 7 to 8.9 with a neutral average value of 7.5. Independently of well characteristics (depth, geographic situation) the temperatures measured in the field show homogeneous values varying from 15 to 22.8 °C. They seem to be influenced only by the surface air temperature.
The hydrochemical data show that fresh groundwater zones are mainly in the upstream part of the basin (piedmont of mountains) and the SW area influenced by wadi Tlil. In these regions the EC is less than 3.5 mS/cm. The EC increases (EC >3.5 mS/cm) in the lower alluvial plain with the proximity of the wells to the sea and Ghar el Melah lagoon (Fig. 5).
A wide range of salinities and chemical compositions characterize the study area. The TDS values vary between 710 and 5150 mg/L. The correlations established between the TDS and concentrations of major elements (Table 2) show that the TDS is well correlated with the concentrations of chloride and sodium (respective r 2 = 0.96 and 0.91). This correlation reflects the contribution of these elements to the mineralization of groundwater in the studied area. The TDS is also correlated with Br− (r 2 = 0.88) that is in good correlation with Cl− (r 2 = 0.93). A very large variability was also found in the nitrate concentrations, ranging from 4.51 to 136 mg/L.
The Piper diagram (Fig. 6) illustrates two chemical facies that directly related to the nature of the aquifer and the proximity of the sampled wells from the sea. Groundwater is of a Cl-–Na+/Ca2+ type in the border of the basin and in the region situated in the west of Gournata (Fig. 4). In these areas, the aquifer is made of gravel and sandy formations. In the downstream part of the plain near the Ghar el Melah lagoon and the sea, the groundwater is of Cl-–Na+ type.
Saturation index (SI) calculation using PHREEQC indicate that the groundwater is saturated with respect to carbonate minerals (calcite, aragonite and dolomite) and unsaturated with respect to evaporated minerals (gypsum and anhydrite).
Groundwater samples show isotopic content ranging from −5.66 to −2.97 ‰ vs V-SMOW for δ18O and from −32.3 to −19.1 ‰ vs V-SMOW for δ2H. The mean values are, respectively, −4.66 and −28.1 ‰ vs V-SMOW for δ18O and δ2H. Compared to values measured in the Tunis station (situated at 30 km in the south) (IAEA/GNIP; Celle-Jeanton et al. 2001) stable isotopes content and the tritium data (ranging from 0.4 to 5.1 TU) advocate for a recent groundwater renewal with two different recharge modes: (1) fast recharge due to the infiltration of irrigation water and runoff water through the Plio-Quaternary formation in the plain, and (2) diffuse recharge by direct infiltration of precipitation in the upstream part of the plain where oxygene-18 values decrease with depth (between 15 and 40 m) and groundwater shows a low tritium levels indicating different recharge timing.
Discussion
Groundwater salinization and degradation in coastal areas around the Mediterranean basin is one of the most problems related to groundwater resources treated by several studies recent years (Custodio and Bruggeman 1987; Custodio 2010; Re and Zuppi 2011; Fedrigoni et al. 2001; Trabelsi et al. 2007; Fukada et al. 2004; Kass et al. 2005; Grassi and Cortecci 2005; Grassi et al. 2007). Salinization is generally related to: (1) seawater intrusion caused by overexploitation of resources, (2) return of irrigation water due to intensive irrigation and low depth of the water table and (3) intensive use of fertilizers by farmers to increase crop productivity.
In the study area, vegetable cropping systems under intensive irrigation are the main agriculture activities. However, arboriculture and grain farming predominated in the foothills area. Around the most important villages (Gournata, Ezzouaouine, Oussja, and Ghar el Melah), cultivated areas are sometimes greatly reduced by increasing urbanization of local agglomerations. This expansion of urban areas is due to an increase in the local population and the suburbanization phenomenon around the capital Tunis. Due to this changing the pressure on water resources in the study area has increased dramatically in recent decades because of the extension of agricultural and urban lands. This has led to a deterioration of water quality.
The important salinity of the groundwater observed in the central part of the plain and around Oussja and the Ghar el Melah lagoon (Fig. 5) is the consequence of multiple phenomena:
-
The first one is related to aquifer lithology that changes from a sandy one in the upper stream part of the basin to a most clayey and silty one in the lower part of the plain.
-
The second one is the return of irrigation water to the aquifer that characterizes the lower part of the plain intensively cultivated and where the water table levels are less than 10 m. Indeed, in semi-arid regions, intensive irrigation by groundwater causes recycling of salts accumulated in surface and their transfer through the unsaturated zone and their accumulation in the aquifer: salts accumulated in the soil during dry periods are flushed to the aquifer during rainy season. Figure 7a clearly shows that the shallower horizons (depth ≤10 m) present the highest values of EC. Excess irrigation water return flow to the aquifer is confirmed also by high NO3 − contents characterizing the shallowest levels (depth ≤10 m). This contamination comes certainly from the surface by return of irrigation water or/and by the precipitation as highlighted in Fig. 7b.
-
The third one is the effect of marine sprays. In coastal areas, salts resulting from marine sprays are accumulated on the surface and then recycled by precipitation or by irrigation water to the aquifer (Cruz and Silva 2000). This is clearly illustrated by the evolution of the Cl− and Br− concentrations versus the depth of the water table (Fig. 8).
Major elements geochemistry
Considering the ion concentrations, a classification into two groundwater types was made of the 61 samples collected in the area: Cl-–Na+ and Cl-–Na+/Ca2+ type. The Cl-–Na+ type dominates the low part of the plain, whereas the Cl-–Na+/Ca2+ type characterizes the upstream and the NW parts (except wells number 58, 59 and 69).
As shown in the Table 2 a well-defined correlations characterize the relationship of the TDS values and Na+ and Cl− contents (respective r 2 = 0.91 and 0.96). These correlations indicate that the inferred elements are the major contributor to the groundwater mineralization.
The Na+/Cl− relationship (Fig. 9) shows that this two elements are strongly correlated (r 2 = 0.91) and that the low Na+/Cl− ratio (0.78) closely approach sea and lagoon water values (respectively, 0.85 and 0.86) and typical seawater value (0.86) (Vengosh et al. 1999; Fedrigoni et al. 2001). In the current state of knowledge, there is no seawater intrusion detected by hydrodynamic measurements (Fig. 3), so high Cl− values can be attributed to the effect of marine sprays and halite dissolution. Indeed, in the semi-arid regions, salts accumulated on ground surface by evaporation of meteoric water, irrigation water and marine sprays, are dissolved in the next rainy event and flushed to the aquifer. This phenomenon characterizes especially the lower area of the plain near the coast wherein lowest depths of the water table and highest Cl− contents (500–2500 mg/L) were measured.
In addition, the observed general decreasing trend in Na with increasing in Cl− (Na+/Cl− <1) along the flow direction could be due to ion-exchange process. The hypothesis of cation exchange that affects groundwater is showed by the Ca2++Mg2+−SO4 2−+HCO3 − relationship in Fig. 10. During the cation exchange process clay particles of the aquifer exchange Ca2+ and Mg2+ against Na+. This process implies a decrease of Na+ in the groundwater against an increase in Ca2+ and Mg2+ (Appelo and Postma 1994; Hem 1992). If the cation exchange is the only process controlling the composition of groundwater, the relation between Ca2++Mg2+ and SO4 2−+HCO3 − should be linear with a slope of 1 (Vengosh et al. 1999). The Fig. 10 shows that the Ca2+ and Mg2+ are in excess with respect to SO4 2−+HCO3 −. This can be explained by the dissolution of carbonate (calcite, dolomite and aragonite) and evaporate mineral (gypsum and anhydrite). Indeed the saturation indexes (SI) of dissolved minerals revealed that the groundwater is generally saturated in carbonate minerals (calcite, aragonite and dolomite) and undersaturated with respect to gypsum and anhydrite.
Br−/Cl− relationship
In natural water, Br− and Cl− are considered conservative tracers and the Br−/Cl− ratio is not influenced by base-exchange reaction (Vengosh et al. 1999; Fedrigoni et al. 2001, Knuth et al. 1990; David and William 1997; Freeman 2007). This characteristic has led to the use of Br− concentrations and Br−/Cl− mass ratios to infer the geochemical provenance of different groundwater (e.g., dissolution of halite or sea intrusion) in coastal areas.
In the plain of OGM, bromide concentrations in groundwater are relatively high, mostly near the coast and around the lagoon of Ghar el Melah. Br− contents vary from 0.8 mg/L in NW part of the plain to 11 mg/L in the coastal region situated east of Ghar el Melah. Bromide concentrations show the same trend as the chloride against water table depth (Fig. 8) indicating their marine origin due to marine sprays. In Fig. 11, Br− and Cl− contents show good correlation (r 2 = 0.97) and Br−/Cl− ratio is constant (2.2 ‰) and is slightly higher than that of Mediterranean water (1.5 ‰) defined by Vengosh et al. (1999). High Br−/Cl− ratio in groundwater observed in the vicinity of the coast can be explained by the leaching of salts (derived from marine sprays) deposited on the ground, by rainwater and irrigation water. Similar findings are reported by Vengosh et al. (1999) in the Mediterranean basin.
Groundwater sampled in wells situated away from the coast and the Ghar el Melah lagoon in the upstream part of the plain and in the southwest part of the plain, present Br−/Cl− ratios close to 1.5 ‰ (marine ratio). This suggests a primary source for chloride and bromide related to salts formed on the surface and driving from sea sprays. Salts accumulated on the ground surface are then dissolved and flushed to the aquifer by rain water.
Nitrate concentration in groundwater
Nitrate is the most common water contaminant index for anthropogenic sources (Freeze and Cherry 1979). Irrigation and fertilizer use can have profound impact on the hydrology and quality of water resources in agricultural regions (Johnston et al. 1998; Gourcy et al. 2009). Excessive infiltration of irrigation water can introduce agriculture contaminants (e.g. nitrate) to shallow groundwater, increase recharge rates and significantly alter groundwater residence times (Böhlke 2002). Nitrate pollution of groundwater may stem from different sources, including fertilizers, animal waste and domestic and industrial discharge. The use of fertilizers in farming areas is, however, considered to be the main source of NO3 − groundwater contamination (Hill 1982; Grassi et al. 2007).
In the study area, synthetic fertilizers and waste animals are largely used by farmers. In the absence of system of wastewater treatment, domestic water is discharged directly into individual septic tanks. As shown in Fig. 7b, the most contaminated horizons are the shallowest levels (depth <10 m), which are characterized by NO3 − contents of up to about 140 mg/L. The most contaminated area is the lower part of the plain between Ezzouaouine and Oussja where NO3 − values range between 50 and 136 mg/L. In this part of OGM plain agriculture activities are more developed (Fig. 12).
In the upstream part of the basin and in the region situated in the eastern part of Ghar el Melah city, no recharge by water irrigation return was observed and the NO3 − values are less than 40 mg/L and ion concentration decreased as the well depth increased. This is related to the important depth of the water table and to agriculture practices (arboriculture).
Isotope study
In arid and semi-arid regions, groundwater recharge occurs subsequent to heavy and long-term precipitations characterizing the humid season. The short-term precipitations with small amounts do not contribute to groundwater recharge as evaporation exceeds infiltration rates. To understand the relationship between isotopic composition of groundwater and those of precipitation as input function, we refer to values measured at the station of Tunis situated at 30 km in the south from the plain of Oussja Ghar el Melah for both stable isotopes (18O and 2H) and tritium. Stable isotope values in this station for 18O and 2H, respectively, are −4.5 and 25.1 ‰ in the rainy season and −1.1 and −1.2 ‰, respectively, in the dry season (Celle-Jeanton et al. 2001). Local meteoric water line (LMWL) was defined by Celle-Jeanton et al. (2001) as: δ2H = 8δ18O + 11. Then, groundwater with a δ2H/δ18O slope of ≈8 may indicates origin from meteoric water with minimal past-precipitation effects, whereas a slope <8 may reflects evaporation during or after rainfall and/or mixing with an external water source with high δ18O and δ2H values (e.g., return of irrigation water). Tritium content of the rain water in recent years shows an annual natural level ranging between 5 and 10 TU (IAEA 1995; IAEA/GNIP).
Thirty four groundwater samples were analyzed for the isotope study. The results show isotopic content ranging from −5.66 to −2.97 ‰ vs V-SMOW for δ18O and from −32.3 to −19.1 ‰ vs V-SMOW for δ2H. The mean values are, respectively, −4.66 and −28.1 ‰ vs V-SMOW for δ18O and δ2H (Fig. 13). The average values of stable isotopes are similar to those of precipitations in the humid season suggesting that the groundwater derived from rainfall origin. However, in the detail groundwater isotopic composition varies into different groups according to geographical location in the plain, well depth and recharge mode.
An evaporative effect in stable isotopes is seen for groundwater sampled in the low part of the plain and around the village of Oussja. In these areas, isotope values within 10 m (Fig. 14) below the ground fall on the evaporation line (δ2H = 4.5δ18O − 6.9) indicating possible surface water contribution (irrigation water) to the aquifer. Isotope composition show enriched values varying between −4.26 and −2.97 ‰ for 18O, and between −26.9 and −19.1 ‰ for 2H. The evaporative effect could also be due to near-surface evaporation in the unsaturated zone during natural recharge for some samples. Isotope findings are corroborated by similar observations for EC, Cl−, Br− and NO3 − (Figs. 7, 8). The Tritium content of this type of water ranges between 1.43 and 3.81 TU indicating some modern recharge component probably between 1950 and 1980.
A second group of more depleted values of stable isotopes ranging between −5.66 and −5.15 ‰ for 18O and between −32.3 and −28.9 ‰ for 2H characterizes groundwater from the upstream part of the plain (well 15, 23, 26, 27), the region situated in the East of the village of Ghar el Melah (well 3, 6, 8 and 10) and the western part of the basin (well 39, 54, 65, 69). On the 18O/2H graph (Fig. 13), representative points of this type of water lie on and around the LMWL with a deuterium excess (d) values in the range of 10.2 to 13.2 ‰ indicating rapid recharge and a lack of evaporation effect prior to infiltration and reflecting groundwater replenishment under more humid climatic conditions during earlier times around 1950 or older. In fact tritium content of this type of groundwater is below 1 TU (Fig. 15). Carbone-14 dating is needed to confirm the age of this type of groundwater.
An intermediate isotope composition between the two previous groups and similar to that of the local meteoric water based on present-day precipitations is observed in the middle part of the plain between Oussja and Gournata and its southern part around the Wadi TliI. Stable isotopes are between −5 and −4 ‰ for 18O and from −32 to −27.0 ‰ for 2H with modern-day 3H levels ranging from 1.6 to over 5 TU (Fig. 15). The highest tritium values (between 4 and 5 TU) measured in central part of the plain between Oussja and Gournata (well 38, 50, 51, and 57) reflect recent recharge (posterior to 1980) by rapid infiltration of rainwater. Tritium content of rain water measured in Tunis and Sfax (situated, respectively, at 50 and 300 km in the south), was about 6.2 and 5.33 TU in 1997 (IAEA/GNIP).
Conclusion
This study investigated the salinization processes and degradation of the groundwater in the shallow coastal aquifer of Oussja Ghar el Melah using a dual geochemical and isotopic approach. Results of this investigation are expected to represent general conditions in many other coastal Tunisian and Mediterranean aquifers suffering joint hard natural conditions and severe anthropic constraints.
Based on different geochemical parameters (e.g. EC, Cl−, Br−, NO3 −, Na+/Cl− and Br−/Cl− ratios) and well characteristics (e.g., depth and location) different water types were distinguished and many sources of groundwater salinization were highlighted. Mineral saturation states and ionic relations indicate that the major geochemical process controlling hydrochemical evolution are dissolution of soil salts, dissolution to saturation of carbonate minerals, dissolution of gypsum and halite and cation exchange. In addition, in many area in the plain, the marine sprays accumulated as a salts on the surface and then leached and flushed to the aquifer by rainwater or return of irrigation water constitute a primary source of groundwater salinization. In the central and lower part of the plain where the water table levels are less than 10 m and agriculture activities are the most intense high concentration of nitrate were measured. Nitrates derived from fertilizers intensively used by farmers are transported to the aquifer by natural recharge process and by return flow from irrigation water (Fig. 16).
Hydrochemical observations are confirmed by isotopic study. Stable isotope signature and tritium contents of groundwater provide evidence for recent groundwater recharge by infiltration of local precipitations and return flow from irrigation. Low tritium values with depleted 18O and 2H in some areas of the plain suggest more earlier recharge (pre-nuclear period) that occurs probably under different climate conditions.
Finally, authorities charged for water management must take urgent necessary measures in the aim to encourage more rational use of fertilizers and to reduce the use of groundwater in irrigation to prevent seawater intrusion. If seawater intrusion takes place the restoration of the aquifer may take a very long time. For example, Appelo and Postma (1994) estimate about 40 years to refresh 2 m thick in the case of seawater intrusion.
References
Appelo CAJ, Postma D (1994) Geochemistry, groundwater and pollution. Balkema, Rotterdam
Barale V, Folving S (1996) Remote sensing of coastal interactions in the Mediterranean region. Ocean Coast Manag 30:217–233
Böhlke JK (2002) Groundwater recharge and agricultural contamination. Hydrogeol J 10:153–179
Burrolet PF, Dumon E (1952) Geological map of Porto Farina. ONM, Tunisia
Celle-Jeanton H, Zouari K, Travi Y, Daoud A (2001) Isotopic characterisation of the precipitation in Tunisia. Variations of the stable isotope compositions of rainfall events related to the origin of air masses. C R Acad Sci 333:625–631
Chelbi F, Paskoff R, Trousset P (1995) La baie d’Utique et son évolution depuis l’Antiquité: une réévaluation géoarchéologique. In: Antiquités africaines, 31, pp 7–51
Clark I, Fritz P (1997) Environmental isotopes in hydrogeology. CRC Press, Boca Raton
Cruz JV, Silva MO (2000) Groundwater salinization in Pico Island (Azores, Portugal): origin and mechanisms. Environ Geol 39(10):1181–1189
Custodio E (2010) Coastal aquifers of Europe: an overview. Hydrogeol J 18(1):269–280. doi:10.1007/s10040-009-0496-1
Custodio E, Bruggeman KA (1987) Groundwater problems in coastal areas, UNESCO. Stud Repo Hydrol 45:596
David CA, William BF (1997) Use of bromide:chloride ratios to differentiate potential sources of chloride in a shallow, unconfined aquifer affected by brackish-water intrusion. Hydrogeol J 5(2):17–26
Fedrigoni L, Krimissa M, Zouari K, Maliki A, Zuppi GM (2001) Origine de la minéralisation et comportement hydrogéochimique d’une nappe phréatique soumise à des contraintes naturelles et anthropiques sévères: exemple de la nappe de Djebeniana (Tunisie). C R Acad Sci 332:665–671
Freeman JT (2007) The use of bromide and chloride mass ratios to differentiate salt-dissolution and formation brines in shallow groundwaters of the western Canadian sedimentary basin. Hydrogeol J 15:1377–1385
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, Englewood Cliffs, p 604
Fukada T, Hiscock KM, Dennis PF (2004) A dual-isotope approach to the nitrogen hydrochemistry of an urban aquifer. Appl Geochem 19:709–719
Gourcy L, Baran N, Vittecoq B (2009) Improving the knowledge of pesticide and nitrate transfer processes using age-dating tools (CFC, SF, H) in a volcanic island (Martinique, French West Indies). J Contam Hydrol 108:107–117
Grassi S, Cortecci G (2005) Hydrogeology and geochemistry of the multilayered confined aquifer of the Pisa Plain (Tuscany–Central Italy). Appl Geochem 20:41–54
Grassi S, Cortecci G, Squarci P (2007) Groundwater resource degradation in coastal plains: the example of the Cecina area (Tuscany–Central Italy). Appl Geochem 22:2273–2289
Hem JD (1992) Study and interpretation of the chemical characteristics of natural water (3rd ed): US Geological Survey Water Supply. Paper, 2254, p 263
Hill AR (1982) Nitrate distribution in the groundwater at the Alliston region of Ontario, Canada. Ground Water 20:696–702
IAEA (1995) Reference and intercomparison materials for stable isotopes of light elements. In: Proceedings of a consultants meeting held in Vienna, 1–3/10/1993, IAEA, Vienna, TECDOC-825, p 165
IAEA/GNIP data. http://www-naweb.iaea.org/napc/ih/IHS_resources_gnip.html
Johnston CT, Cook PG, Frape SK, Plummer LN, Busenberg E, Blackport RJ (1998) Ground water age and nitrate distribution within a glacial aquifer beneath a thick unsaturated zone. Ground Water 36:171–180
Kass A, Gavrieli I, Echieli Y, Vengosh A, Starinksy A (2005) The impact of freshwater and wastewater irrigation on the chemistry of shallow groundwater: a case study from the Israeli coastal aquifer. J Hydrol 300:314–331
Knuth M, Jackson JL, Whittemore DO (1990) An integrated approach to identifying the salinity source contaminating a groundwater supply. Ground Water 28:207–214
Kouzana L, Ben Mammou A, Sfar Felfoul M (2009) Seawater intrusion and associated processes: case of the Korba aquifer (Cap-Bon, Tunisia). C R Geosci 341:21–35
Ma F, Yang YS, Yuan R, CaiS Z, Pan S (2007) Study of shallow groundwater quality evolution under saline intrusion with environmental isotopes and geochemistry. Environ Geol 51:1009–1017
Mejri L (2012) Tectonique quaternaire, paléosismicité et sources sismogéniques en Tunisie nord-orientale: Etude de la faille d’Utique. PhD Thesis: Univ. Toulouse, p 184
Mejri L, Regard V, Carretier S, Brusset S, Dlala M (2010) Evidence of Quaternary active folding near Utique (Northeast Tunisia) from tectonic observations and a seismic profile. C R Geosci 342:864–872
Melki F, Zouaghi T, Harrab S, Casas Sainz A, Bedir M, Zargouni F (2011) Structuring and evolution of Neogene transcurrent basins in the Tellian foreland domain, north-eastern Tunisia. J Geodyn 52:57–69
Oueslati A, Charfi F, Baccar F (2006) Presentation of the tunisian site: la basse vallée de oued Mejerda et la lagune de Ghar el Melah. Fifth International Meeting INCO-CT-2005-015226, p 26
Re V, Zuppi GM (2011) Influence of precipitation and deep saline groundwater on the hydrological systems of Mediterranean coastal plains: a general overview. Hydrol Sci J 56(6):966–980
Stigter TY, Van Ooijen SPJ, Post VEA, Appelo CAJ, Carvalho Dill AMM (1998) A hydrogeological and hydrochemical explanation of the groundwater composition under irrigated land in a Mediterranean environment, Algarve, Portugal. J Hydrol 208:262–279
Trabelsi R, Zairi M, Ben Dhia H (2007) Groundwater salinization of the Sfax superficial aquifer, Tunisia. Hydrogeol J 15:1341–1355
Vengosh A, Spivack AJ, Artzi Y, Ayalon A (1999) Geochemical and Boron, strontium and oxygen isotopic and geochemical constraints for the origin of the salinity in groundwater from the Mediterranean coast of Israel. Water Resour Res 35:1877–1894
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben Ammar, S., Taupin, JD., Zouari, K. et al. Identifying recharge and salinization sources of groundwater in the Oussja Ghar el Melah plain (northeast Tunisia) using geochemical tools and environmental isotopes. Environ Earth Sci 75, 606 (2016). https://doi.org/10.1007/s12665-016-5431-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5431-x