Abstract
The speciation of copper in seawater is greatly influenced by the presence of organic ligands that find their way from various sources. The occurrence of copper-complexing ligands in the coastal sediment of eastern Red Sea and their potential resuspension under the impact of physical parameters such as wind and currents are investigated. The competitive ligand exchange and detection of copper by adsorptive cathodic stripping voltammetry (ACSV) were used to determine the dissolved concentration of copper-complexing ligands and their conditional stability constants after suspension of marine sediments in UV-irradiated seawater. The laboratory experiments of suspended marine sediments in UV-irradiated seawater followed by the measurements of copper in the filtrate have indicated the presence of two ligands with concentrations in the range of 3.53–25.58 nM g−1 for L1 and 8.33–28.35 nM g−1 for L2 whereas the log conditional stability constants ranged between 12.59 and 13.87 for log K 1 and 11.79 and 12.96 for log K 2. Comparison of log K 2 with values for log K of copper complexes with thiols from previous studies suggests that thiols consist of the major part of copper-complexing ligands in the coastal marine sediments of eastern Red Sea. Relatively, positive and good correlations are found for copper-complexing ligands with total copper and organic content in the sediments. Calculation of the flux of copper-complexing ligands and their contribution to the total budget in the coastal water south of Jeddah indicate that the impact of sediments as potential source is less than 13 %. It is therefore that other processes such as in situ production and input from sewage effluents have to be considered to account for the majority of dissolved copper-complexing ligands in the area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Our understanding of trace metals biogeochemistry in the oceans increased in the mid-1970s due to the “new reliable data” that resulted from precautions in sampling and advanced analytical techniques (Chester 2003). The earlier data had focused on the total dissolved concentration of trace metals with a few studies oriented to study the chemical forms of trace metal “speciation.” Later on, studies on trace metal speciation attracted the attention of oceanographers, and it has been shown that the biogeochemical cycles of trace metals are related to their speciation rather than total concentration (e.g., Bown et al. 2012). Studies on copper speciation covered various marine environments from coastal (Laglera and van den Berg 2003; Buck and Bruland 2005; Muller and Batchelli 2013; Oldham et al. 2014) to oceanic waters (Coale and Bruland 1988, 1990; Donat and van den Berg 1992; Midorikawa and Tanoue 1998; Moffett and Dupont 2007). These investigations have shown that copper is highly bounded to organic ligands and organic complexation of copper consists up to 99 % of total dissolved copper in seawater. The high affinity of copper to organic ligands reduces the concentrations of free ionic copper to ultra low values (picomolar concentrations). Therefore, organic complexation is the main factor in controlling the levels of free ionic copper in seawater. Some other factors including the adsorption/complexation of copper on the surface of particulate organic matter and/or possibly on iron/manganese oxide particles are also believed to affect copper speciation (Kerner and Geisler 1995; Gerringa et al. 1998; Fernández Severini et al. 2009; Al-Farawati et al. 2011; Roussiez et al. 2011).
Most studies of the organic complexation of copper in seawaters have focused on the distributions and processes in the water column, where a significant fraction of copper-complexing ligands are likely to be produced through biological activities (Zhou and Wangersky 1989; Moffett and brand 1996; Croot et al. 2000). However, sediments could be a potential source of both trace metals and organic ligands (Skrabal et al. 1997, 2000; Chapman et al. 2009; Santos-Echeandía et al. 2013). In most studies on dissolved copper complexation with organic ligands, up to two classes of copper binding organic ligands have been detected. It was suggested that in situ production is the source of stronger copper organic ligands whereas riverine input and diffusion from shelf sediments are the major carrier of weaker copper organic ligands (Muller et al. 2001). Porewater measurements were used to evaluate the flux of copper-complexing ligands from Chesapeake Bay sediments, and the flux ranged between 0.3 and 1.2 μM m−2 day−1 whereas the flux of dissolved copper was less by 40-fold, indicating that most of the copper is complexed by organic ligands. The magnitude of the benthic fluxes of copper-complexing ligands in the Bay ranged between 10 and 50 % of the pool of copper-complexing ligands in Chesapeake Bay (Skrabal et al. 1997). Recently, the daily flux of copper-complexing ligands due to tidal currents was investigated in non-vegetated and vegetated area for Tagus Estuary, and it was found that the flux in vegetated area (48.2 μM m−2 day−1) was higher by around 15 orders of magnitude than the non-vegetated area (Santos-Echeandía et al. 2013).
Our aim is to study the potential releases of copper-complexing ligands from coastal sediments of eastern Red Sea. Since bottom currents in the marine environment are known to re-suspend the marine sediments, our laboratory experiments are based on simple suspension of marine sediments in UV-irradiated seawater. The copper-complexing ligands and conditional stability constants were evaluated in filtrate using adsorptive cathodic stripping voltammetry (ACSV) (Campos and van den Berg 1994). The supporting parameters such as organic carbon content, total copper, carbonate content, and grain size category in sediments were also measured and are correlated with copper-complexing ligands in order to understand their role on the biogeochemical cycle of copper.
Materials and methods
Study area
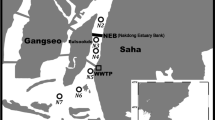
The sediment samples (five samples) were collected along the eastern coast of Red Sea (Fig. 1). One sample was collected from Haqel in the north (St. HQ1). Four samples were collected along Jeddah coast: North Corniche (St. NC2), Reayat Al-Shabab Lagoon (St. RS3), Al-Arbaeen Lagoon (St. AR4), and South Corniche (St. SC5). This geographical variation represents different depositional environments of various characteristics. The stations HQ1, NC2, and SC5 are coastal stations which are more open to the sea; therefore, the physical parameters (e.g., winds and currents) are expected to have major impact on the sediment characteristics. The other two stations, RS3 and AR4, are coastal lagoons with restricted water circulations. Settings of these stations also vary due to the impact of human activities on their environment. The human impact on coastal environment of Haqel (St. HQ1) is less than that of Jeddah coast (Sts. NC2, RS3, AR4, and SC5). This is due to a population of 30,000 people in Haqel compared to a population of 3.4 million people in Jeddah (www.jeddah.gov.sa and http://www.cdsi.gov.sa/english/). The environmental parameters in coastal environments of Jeddah indicate that the area is polluted at various levels. The most pronounced feature is the high levels of contaminants that were recorded in the coastal lagoons of Reayat Al-Shabab Lagoon (St. RS3) and Al-Arbaeen Lagoon (St. AR4) and are attributed to the discharge of untreated sewage and restricted water circulation (Basaham 1998; El-Rayis 1998; El-Rayis and Moammar 1998; El Sayed 2002a; Turki et al. 2002). The South Corniche receives a daily amount of 300,000 m3 of partially treated sewage from Al-Khumra Sewage Treatment Plant (El Sayed 2002b; Basaham et al. 2009; Al-Farawati 2010). However, the municipality of Jeddah has used a multiport diffuser pipe dispersing system which enhances the dispersion of the contaminants. The North Corniche suffers from discharges of untreated sewage from different sources due to recreational activities (e.g., hotels and chalets).
Sample collection and treatment
The surface sediment samples were collected from stations (Fig. 1) during April 2010 using grab sampler. The samples were placed in sealed polyethylene bags and transported to the laboratory in icebox. The samples were dried overnight at the room temperature on a clean bench and subsequently grounded using a gate pestle and mortar. Finally, subsamples were used for the analysis of various parameters.
Instrumentation and analytical procedures
The measurements of total dissolved copper and complexation parameters (copper-complexing ligands and the conditional stability constants of copper-organic complexes) were undertaken using a μAutolab voltammeter (ECO Chemie, The Netherlands) connected to a Metrohm VA 663 electrode (Metrohm, Switzerland) and controlled by a personal computer. The reference electrode (Metrohm, Switzerland) was a double-junction Ag/AgCl (3 M), and the counter electrode (Metrohm, Switzerland) was a glassy carbon rod. The interference of dissolved oxygen in the samples was removed after treatment of the samples with a high-purity nitrogen gas for a period of 5 min. Destruction of organic compounds was achieved using 500-W high-pressure mercury vapor lamp constructed at oceanography laboratories in Liverpool, UK, coupled with 30-ml silica tubes (Achterberg and van den Berg 1994). The pH measurements were undertaken using a Metrohm 744 pH meter and calibrated against a pH 4 and 9 on the NBS pH scale. The voltammetric cell was rinsed at the beginning of the measurement with diluted acid followed by Milli-Q waters. The measurement of dissolved organic carbon (DOC) was undertaken using Shimadzu TOC-VCPH analyzer. Carbonate content in the sediments was estimated by treating a known weight of the air-dried sediment with 0.2 M HCl (1/25 w/v). Carbon dioxide evolved was measured using a calcimeter. Carbonate concentration in the sample was calculated using standard pureCaCO3. Organic carbon content in sediments was measured using the sulfo-chromic wet oxidation method (Le Core 1983). Powdered sediment was first treated with phosphoric acid at 110 °C to get rid of carbonate and chloride ions; then, organic matter was oxidized with a mixture of potassium dichromate and sulfuric acid. The excess dichromate was then back titrated with sodium thiosulfate. The quantity of the thiosulfate corresponds to a definite quantity of total organic carbon, which is then attributed to the weight of the sediment sample. For the determination of total copper in sediments, powdered freeze-dried subsamples were digested using a nitric/hydrofluoric/perchloric acid mixture in the ratio 3:3:1 (v/v) (Basaham 1998). Digestion was carried out using a microwave digestion unit. The acid was evaporated to near dryness, and the residue was taken in 0.1 M HCl. Concentrations of total copper were determined using thermal atomization technique GFAA (PerkinElmer Analyst 800). Determination of grain size of the sediments was carried out using the standard dry sieving technique. Sediments were classified according to mud-sand-gravel ratio (Flok 1980).
Reagents
All reagent solutions were prepared in Milli-Q by weighing the appropriate salt into sterilized polystyrene tubes (30 ml) or Nalgene high-density polyethylene (HDPE) bottles. The final volume of the solutions was adjusted by weight. A stock solution of salicylaldoxime (SA) (0.01 M) was prepared in 0.1 M HCl. A stock solution of borate buffer solution (1.5 M) was prepared by dissolution of boric acid in NaOH solution (0.4 M) and was UV irradiated to eliminate organic compounds. Addition of 50 μl of buffer to 10 ml of seawater gave a pH of 8.2 (NBS pH scale). The decision of using borate buffer was taken after measurements of the pH of seawater from the study area (pH ~8.1). Copper standards were prepared by dilution of atomic absorption spectrometry standard solutions (BDH, Spectrosol grade) in Milli-Q water and acidified to pH ~2.5 by addition of HCl. Acids were purified by sub-boiling distillation in quartz still.
The impact of time on the releases of DOC
Various weights (0.5, 1, 2, and 5 g) of marine sediments were suspended in 130 ml of UV-irradiated seawater. The samples were placed on a shaker, and the concentration of DOC was measured at time intervals of 1, 2, 4, 8, 16, and 24 h.
Procedure to determine total dissolved copper using ACSV
ACSV was used for the determination of trace metals. In ACSV, the metals are chelated with a suitable complexing agent and then adsorbed or deposited on the hanging mercury drop electrode (HMDE) (van den Berg 1989). SA was used as complexing agent for determination of copper (Campos and van den Berg 1994). Acidified seawater was UV irradiated prior to the total dissolved copper determination. An aliquot of 10 ml seawater was pipetted into the voltammeric cell; 100 μl borate pH buffer (final concentration 0.01 M) and 25 μl SA (final concentration 25 μM SA) were added. The solution was deaerated by purging for 5 min with high-purity nitrogen gas. The deposition potential was set to −1.1 V; four mercury drops were discarded before a new mercury drop was extruded, and the solution was stirred for a preset period of 1 min. Then, the stirrer was stopped and a quiescent period of 10 s was allowed before switching the potential to −0.15 V to re-oxidize the deposited and plated copper. Then, the potential scan was initiated from −0.15 V and was terminated at −0.6 V. The peak height of copper was recorded and calibrated with standard copper. The accuracy of method was tested by analyzing nearshore seawater reference materials for trace metals (CASS-4). Our results were within 15 % of the certified values.
Copper-complexing ligand titrations
Acid-clean HDPE bottle (capacity of 250 ml) was used to suspend 2 g of a sediment sample in150 ml UV-irradiated seawater that has been collected from the coast of Jeddah. The concentration of total dissolved copper, initially present in the seawater, was determined (~2 nM copper) using ACSV. The sample was placed on a shaker for 24 h, and the seawater was filtered using acid-clean cellulose nitrate filter (0.45 μm). One hundred twenty milliliters of filtrate was transferred to acid-clean high-density polyethylene bottle (capacity of 250 ml), and borate buffer and SA were added at final concentration of 0.01 M and 5 μM, respectively. Copper was added to an acid-clean polystyrene bottles (30 ml) giving a concentration range between 0 and 800 nM (in 10 ml seawater) in ten steps; 10 ml aliquots of filtered seawater (of previous step) were then pipetted into the acid-clean polystyrene bottles (30 ml) and left overnight for equilibrium. The electrochemical signal of copper was measured using ACSV.
Evaluation of copper-complexing ligands
The concentrations of copper-complexing ligands and conditional stability constants of copper complexes with organic ligands were determined by titration of the samples with copper. ACSV takes the advantage of competition between the organic ligands released from the sediment and added complexing ligand (SA) to the added copper in the sample. Linearization of titration data is the key for the calculation of copper-complexing ligands and conditional stability constants of copper-organic complexes (van den Berg and Kramer 1979; Ruzic 1982; van den Berg 1982):
where [CuL] is the concentration of copper complexes with organic ligands L, [Culabile] is the labile copper concentration, CL is the concentration of copper-complexing ligand, α ’ is the α-coefficient of Cu2+ with inorganic complexes and SA, and K ’ CuL is the conditional stability constant of copper organic complex (CuL). In the presence of single ligand and using a linear least squares regression, the plot of [Culabile]/[CuL] as a function of [Culabile] is straight. However, the plot shows curvature in the presence of two or more ligands. Value for CL was obtained by the slope−1 whereas K'CuL was obtained from K ’ CuL = α ’ × slope / Y-axis intercept. Further details of theory and data treatment can be obtained elsewhere (Campos and van den Berg 1994; Laglera and van den Berg 2003; Chapman et al. 2009).
Results and discussion
The impact of time on the release of DOC
During preliminary experiments, marine sediments were suspended in UV-irradiated seawater and the total and labile copper (labile copper is denoted for electrochemically active copper species) was measured in the samples. These experiments have indicated the potential release of copper-complexing ligands to seawater due to the presence of non-labile fraction of copper, calculated by the difference between the concentration of labile copper and total copper. The copper-complexing ligands are believed to be of organic nature rather than inorganic. The correlations between copper-complexing ligands and DOC were strong and positive in the water column of western North Atlantic (Zhou and Wangersky 1989). Therefore, DOC can be used to predict the release of organic ligands as function of time under the circumstances of our experiments. Along the interval time of 24 h, with suspension of various amounts of sediments in UV-irradiated seawater (free of organic ligands), the maximum concentration of DOC was observed after 4 h whereas after this time interval, the concentration of DOC showed more or less constant value or even decrease (Fig. 2). This interesting finding indicates that some of DOC is likely to re-adsorb on the sediment and/or degradation of certain amount of easily oxidized DOC occurred after 4 h. For this study and in order to investigate the potential releases of copper-complexing ligands from coastal sediments, an equilibration time of 24 h and suspension of 2 g of the sample in UV-irradiated seawater were used to study copper speciation. The use of 24 h was selected to ensure the stability of organic ligands released from the marine sediments while selection of 2 g of the sample was arbitrary.
Sedimentary copper-complexing ligands and their conditional stability constants
The values of sedimentary copper-complexing ligands, conditional stability constants of copper with organic complexes, total copper, carbonate content, and organic carbon content in the coastal sediment of eastern Red Sea are shown in Table 1. The concentrations of copper-complexing ligands are reported as nanomolar per gram. Usually, concentrations of copper-complexing ligands in marine sediments for most studies are reported as nanomolar as these studies focus on the fluxes of copper-complexing ligands from porewater reservoir to the upper waters. Here, we assume that copper-complexing ligands are potentially released to the overlaying water by resuspension of marine sediments under the impact of waves and currents. The titration of samples followed by linearization has indicated the presence of two ligands: a strong (L1) and a weak ligand (L2) (Fig. 3). The concentration of L2 is significantly low at station HQ1 (8.33 nM g−1) whereas the values are almost close to 25 nM g−1 at the other stations with highest value recorded at Reayat Al-Shabab Lagoon (28.35 nM g−1). The concentrations of L1 show similar pattern with lowest value at station HQ1 (3.53 nM g−1) and highest value at Reayat Al-Shabab Lagoon (25.58 nM g−1). However, the variability of L1 values is evident in comparison to the values of L2. This may indicate that L1 is more sensitive to the environmental conditions or its production is enhanced by diagenetic processes in the sediments. In general, the value of L2 is higher than the value of L1 by a factor of 2–4. The only exceptional is observed at station RS3 as the value of L2 (28.35 nM g-1) is almost equal to the value of L1 (25.58 nM g−1). It is worthwhile to mention that the stability constants of the complexing ligands with copper from the present study are in good agreement with the stability constants obtained by Chapman et al. (2009)) for shallow lagoon waters. The authors have measured the concentrations of thiols and copper-complexing ligands (L1 and L2) and suggested, based on similarity of thiols and L2 concentrations, that L2 is dominated by thiols. It is therefore possible that thiols consist of major parts of L2 in the coastal sediments of eastern Red Sea. For most studies on copper speciation in seawater, almost one ligand was found as inferred from linearization technique developed by van den berg (1982) and Ruzic (1982). L1 seems to be more resistant to the oxidation, and/or its production is enhanced in situ by the activities of marine phytoplankton. The in situ production of L1 was suggested as a result of metabolism processes by marine phytoplankton (Zhou and Wangersky 1989; Moffett and brand 1996; Croot et al. 2000). Based on the results from the present study and as copper concentrations in seawater are usually less than the concentrations of copper-complexing ligands (Al-Farawati, unpublished data), we suggest that L1 could have substantial impact on the biogeochemical cycle of copper in the coastal waters of Red Sea compared to L2. The importance of L2 seems to appear in environments of high levels of copper such as polluted areas. Such speculations were also suggested in estuarine environments (Laglera and van den Berg 2003; Santos-Echeandia et al. 2008a). In addition, relatively high levels of copper and copper-complexing ligands were reported in estuarine waters and were attributed to resuspension of sediments (Santos-Echeandia et al. 2008a). However, in order to assess the impact of L1 and L2 on the biogeochemical cycle of copper in the Red Sea, the magnitude of the flux from the sediments has to be evaluated.
Complexing ligand titration of filtrate after suspension of sediment from station RS3 in UV-irradiated seawater for 24 h (using 5 μM SA). a Peak height versus total dissolved copper; the straight line is for calculated peak height in the absence of organic ligands. b Linearization of the data showing curvature indicating the presence of two ligands
Correlation of complexation parameters with total copper, organic carbon content, and carbonate content
Correlations of complexation parameters (copper-complexing ligands) with total copper, organic carbon content, and carbonate content in the marine sediments of eastern Red Sea are shown in Fig. 4. A strong and negative correlation was reported between the organic carbon content and carbonate content in the sediments of Red Sea (Basaham 1998; El-Sayed et al. 2002; Basaham 2008). One would expect that the correlation of the complexing ligand concentrations with organic carbon content to be positive whereas the correlation with carbonate content is expected to be negative. The correlation pattern for the copper-complexing ligand L2 with total copper and organic carbon content is identical showing positive correlation with an arch shape (Fig. 4a, b). The correlation for copper-complexing ligand L1 with total copper and organic carbon content is also positive but with relatively linear relationship (Fig. 4c, d). Surprisingly, the correlation of copper-complexing ligands with carbonate contents has not show negative pattern as it would be expected (Fig. 4e, f). Total copper is strongly correlated to organic carbon content (r 2 = 0.99), and that could be attributed to the simultaneous precipitation with particulate materials (Fig. 4g). Copper is considered as scavenging type element that is removed from the water column due to adsorption on particulate materials and eventually buried in the sediments (Bruland 1983; Chester 2003). Such behavior is reported in the coastal waters of Jeddah due to a strong and negative correlation that was found between dissolved copper and particulate organic materials (Al-Farawati et al. 2011). Additionally, copper is one of bio-limiting element nutrient that is utilized by the marine organisms. For example, the growth of the dinoflagellate Gonyaulax tamarensis was shown to be limited at cupric ion activities less than 10−13 M (Schenck 1984). After the death of marine organisms, copper accumulates in the sediments along with organic materials.
Potential impact of copper complexation data on dissolved copper
Dissolved copper speciation was investigated in coastal waters of Southern Corniche of Jeddah (Al-Farawati, unpublished data). The authors have modeled their data based on single complexing ligand, and they reported value of L1 in the range between 5 and 133 nM whereas the log K 1 was in the range between 12.26 and 13.65. Using these values, the authors were able to calculate the concentration of free copper in seawater which was found to be in the range 10−12.79 to 10−15.08 M. Comparison of the values of log K 1 obtained in the sediments (the range; 12.59–13.97) with seawater values that have been observed by Al-Farawati (unpublished data) shows close agreement. In order to evaluate the importance of the copper-complexing ligands from sediment on the dissolved copper speciation, the fluxes of copper-complexing ligands have to be estimated. To evaluate the impact of marine sediments as a source of copper-complexing ligands to seawater, the resuspension of the sediments in the coastal waters of Jeddah for the Sothern Corniche area (St. SC5) is estimated based on a model using the wind data to estimate the resuspension of the sediments in shallow coastal waters (Booth et al. 2000). The model is based on wind-induced wave phenomenon developed by coastal engineering Research Center, US Army Corps of engineering (SPM 1984). Furthermore, the grain size of the sediment is an important factor in the calculation of the flux. The grain size analysis of the sediment for the South Corniche shows that the sediment type is gravel sandy. For this study, it is assumed that the sea is fully developed. The wind data recorded at the Obhur Meteorological Station show that during April, the wind is mainly from N to NE. The Meteorological Station records various parameters at 15-min interval including the wind speed and direction. By utilizing the wind speed, direction, and grain size of the sediment, the resuspension of marine sediments in South Corniche area is estimated to be 361 g m−2 day−1. Using values of 6.98 nM g−1 for L1 and 21.83 nM g−1 for L2 in the area of South Corniche (Table 1), the daily flux of L1 and L2 is accounted to 2.52 and 7.88 μM m−2 day−1, respectively. The daily flux of L1 in South Corniche is comparable with the value for non-vegetated area in Tagus estuary (2.16 μM m−2 day−1, Santos-Echeandía et al. 2013) whereas the flux of L2 in South Corniche is higher by 3–4 orders of magnitude (2.31 μM m−2 day−1 for Tagus estuary). However, the fluxes of L1 and L2 for vegetated area in Tagus estuary are 11.5 and 64.7 μM m−2 day−1, respectively (Santos-Echeandía et al. 2013). These values exceed our values which indicate the importance of vegetation as a source of copper-complexing ligands in the marine environments. On the other hands, our values are higher by several orders of magnitude than the values reported for total the flux of copper-complexing ligands for Chesapeake Bay (0.3–1.2 μM m−2 day−1, Skrabal, et al. 1997). On the basis of daily presence of dissolved copper-complexing ligands in South Corniche and using average value of 21.7 nM (Al-Farawati, unpublished data), the contribution of sedimentary copper-complexing ligands to the pool of dissolved copper-complexing ligands in the upper seawater layers represents less than 13 % that indicates the potential contribution of other sources such as in situ production (Croot et al. 2000) and sewage effluent (Santos-Echeandia et al. 2008b).
Conclusion
Marine sediments from the coastal environments of eastern Red Sea were suspended in UV-irradiated seawater. Detection of copper using ACSV in the filtrate revealed the presence of non-labile fraction of copper that is attributed to complexation of copper by organic ligands released from the sediments. Copper-complexing ligands and their conditional stability constants are determined by competition of SA and organic ligands for copper. Two classes of copper-complexing ligands are detected. Thiols are likely to contribute to the majority of the weaker ligand (L2). The contribution of copper-complexing ligands from the sediments to the pool of dissolved copper-complexing ligands in the south coast of Jeddah is less than 13 %. This indicates that other sources have to be considered in order to evaluate their role in the biogeochemical cycle of copper in the coastal water of Red Sea.
References
Achterberg EP, van den Berg CMG (1994) In-line ultraviolet-digestion of natural water samples for trace metal determination using an automated voltammetric system. Anal Chim Acta 291(3):213–232
Al-Farawati R (2010) Environmental conditions of the coastal waters of Southern Corniche, Jeddah, eastern Red Sea: physico-chemical approach. Aust J Basic Appl Sci 4:3324–3337
Al-Farawati R, Gazzaz M, El Sayed M, El-Maradny A (2011) Temporal and spatial distribution of dissolved Cu, Ni and Zn in the coastal waters of Jeddah, eastern Red Sea. Arab J Geosci 4:1229–1238. doi:10.1007/s12517-010-0137-y
Basaham AS (1998) Distribution and behavior of some heavy metals in the surface sediments of Al-Arbaeen Lagoon, Jeddah, Red Sea coast. JKAU: Earth Sci 10:59–71
Basaham AS (2008) Mineralogical and chemical composition of the mud fraction from the surface sediments of Al-Kharrar, a Red Sea coastal lagoon. Oceanologia 50:557–575
Basaham AS, Rifaat AE, El-Mamoney MH, El Sayed MA (2009) Re-evaluation of the impact of sewage disposal on coastal sediments of the southern Corniche, Jeddah, Saudi Arabia. JKAU: Mar Sci 20:109–126
Booth JG, Miller RL, McKee BA, Leathers RA (2000) Wind-induced bottom sediment resuspension in a microtidal coastal environment. Cont Shelf Res 20:785–806. doi:10.1016/S0278-4343(00)00002-9
Bown J, Boye M, Nelson DM (2012) New insights on the role of organic speciation in the biogeochemical cycle of dissolved cobalt in the southeastern Atlantic and the Southern Ocean. Biogeosci 9:2719–2736
Bruland KW (1983) Trace elements in seawater. In: Riley JP, Chester R (eds) Chemical oceanography, vol 8, 2nd edn. Academic press, New York, pp 157–200
Buck KN, Bruland KW (2005) Copper speciation in San Francisco Bay: a novel approach using multiple analytical windows. Mar Chem 96:185–198. doi:10.1016/j.marchem.2005.01.001
Campos MLAM, van den Berg CMG (1994) Determination of copper complexation in seawater by cathodic stripping voltammetry and ligand competition with salicylaldoxime. Anal Chim Acta 284:481–496
Chapman CS, Capodaglio G, Turetta C, Berg CMG (2009) Benthic fluxes of copper, complexing ligands and thiol compounds in shallow lagoon waters. Mar Environ Res 67:17–24. doi:10.1016/j.marenvres.2008.07.010
Chester R (2003) Marine geochemistry. Blackwell Science Ltd
Coale KH, Bruland KW (1988) Copper complexation in the Northeast Pacific. Limnol Oceanogr 33(5):1084–1101
Coale KH, Bruland KW (1990) Spatial and temporal variability in copper complexation in the North Pacific. Deep-Sea Res 37(2):317–336
Croot PL, Moffett JW, Brand LE (2000) Production of extracellular Cu complexing ligands by eukaryotic phytoplankton in response to Cu stress. Limnol Oceanogr 45:619–627. doi:10.4319/lo.2000.45.3.0619
Donat JR, van den Berg CMG (1992) A new cathodic stripping voltammetric method for determining organic copper complexation in sea water. Mar Chem 38:69–90
El Sayed MA (2002a) Distribution and behavior of dissolved species of nitrogen and phosphorus in two coastal Red Sea lagoons receiving domestic sewage. JKAU: Mar Sci 13:47–73
El Sayed MA (2002b) Factors controlling the distribution and behaviour of organic carbon and trace metals in a heavily sewage polluted coastal environment. JKAU: Mar Sci 13:21–46
El-Rayis OA (1998) Environmental conditions of two Red Sea coastal lagoons in Jeddah. 2. Nutrients. JKAU: Mar Sci 9:49–59
El-Rayis OA, Moammar MO (1998) Environmental conditions of two Red Sea coastal lagoons in Jeddah: 1. Hydrochemistry. JKAU: Mar Sci 9:31–47
El-Sayed MA, Basaham AS, Gheith AM (2002) Distribution and geochemistry of trace elements in central Red Sea coastal sediments. Int J Environ Stud 59(1):1–31
Fernández Severini MD, Botté SE, Hoffmeyer MS, Marcovecchio JE (2009) Spatial and temporal distribution of cadmium and copper in water and zooplankton in the Bahía Blanca estuary, Argentina. Estuar Coast Shelf Sci 85:57–66. doi:10.1016/j.ecss.2009.03.019
Flok RL (1980) Petrology of sedimentary rocks. Hemphil Publishing Company, Austin, Texas
Gerringa LJA, Hummel H, Moerdijk-Poortvliet TCW (1998) Relations between free copper and salinity, dissolved and particulate organic carbon in the Oosterschelde and Westerschelde, Netherlands. J Sea Res 40:193–203. doi:10.1016/S1385-1101(98)00021-5
Kerner M, Geisler CD (1995) Dynamics of Cu release during early aerobic degradation in aggregated seston from the Elbe estuary. Mar Chem 51:133–144. doi:10.1016/0304-4203(95)00055-V
Laglera LM, van den Berg CMG (2003) Copper complexation by thiol compounds in estuarine waters. Mar Chem 82:71–89. doi:10.1016/S0304-4203(03)00053-7
Le Core P (1983) Dosage du Cabone Organique Particulaire. In: Aminot A, Chaussepied M (eds) Manuel des analyses chimiques en Mlieu Marin. CNEXO, Brest, pp 203–210
Midorikawa T, Tanoue E (1998) Molecular masses and chromophoric properties of dissolved organic ligands for copper(II) in oceanic water. Mar Chem 62:219–239. doi:10.1016/S0304-4203(98)00040-1
Moffett JW, Brand LE (1996) Production of strong, extracellular Cu chelators by marine cyanobacteria in response to Cu stress. Limnol Oceanogr 41:388–395
Moffett JW, Dupont C (2007) Cu complexation by organic ligands in the sub-arctic NW Pacific and Bering Sea. Deep Sea Res Part I 54:586–595. doi:10.1016/j.dsr.2006.12.013
Muller FLL, Batchelli S (2013) Copper binding by terrestrial versus marine organic ligands in the coastal plume of River Thurso, North Scotland. Estuar Coast Shelf Sci 133:137–146. doi:10.1016/j.ecss.2013.08.024
Muller FLL, Gulin SB, Kalvoy A (2001) Chemical speciation of copper and zinc in surface waters of the western Black Sea. Mar Chem 12:162–176
Oldham VE, Swenson MM, Buck KN (2014) Spatial variability of total dissolved copper and copper speciation in the inshore waters of Bermuda. Mar Pollut Bull 79:314–320. doi:10.1016/j.marpolbul.2013.12.016
Roussiez V, Ludwig W, Radakovitch O, Probst J-L, Monaco A, Charrière B, Buscail R (2011) Fate of metals in coastal sediments of a Mediterranean flood-dominated system: an approach based on total and labile fractions. Estuar Coast Shelf Sci 92:486–495. doi:10.1016/j.ecss.2011.02.009
Ruzic I (1982) Theoretical aspects of the direct titration of natural waters and its information yield for trace metal speciation. Anal Chim Acta 140:99–113
Santos-Echeandia J, Laglera LM, Prego R, van den Berg CMG (2008a) Dissolved copper speciation behaviour during estuarine mixing in the San Simon Inlet (wet season, Galicia). Influence of particulate matter. Estuar Coast Shelf Sci 76(2):447–453
Santos-Echeandia J, Laglera LM, Prego R, van den Berg CMG (2008b) Copper speciation in continental inputs to the Vigo Ria: sewage discharges versus river fluxes. Mar Pullut Bull 56:308–317
Santos-Echeandía J, Caetano M, Laglera LM, Vale C (2013) Salt-marsh areas as copper complexing ligand sources to estuarine and coastal systems. Chemosphere 90(2):772–781
Schenck RC (1984) Copper deficiency and toxicity in Gonyaulax tamarensis (Lebour). Mar Biol Lett 5:13–19
Skrabal SA, Donat JR, Burdige DJ (1997) Fluxes of copper-complexing ligands from estuarine sediments. Limnol Oceanogr 42:992–996
Skrabal SA, Donat JR, Burdige DJ (2000) Pore water distributions of dissolved copper and copper-complexing ligands in estuarine and coastal marine sediments. Geochim Cosmochim Acta 64:1843–1857
SPM (1984) US army engineer waterways experiment station vol 2, 4 edn. Coastal Engineering Research Center
Turki AJ, El Sayed MA, Basaham AS, Al-Farawati R (2002) Study on the distribution, dispersion and mode association of some organic and inorganic pollutants in a coastal lagoon receiving sewage disposal. Final Report, King Abdulaziz University Scientific Research Council
van den Berg CMG (1982) Determination of copper complexation with natural organic ligands in sea water by equilibration with MnO2. I. Theory. Mar Chem 11:307–322
van den Berg CMG (1989) The electroanalytical chemistry of seawater. In: Riley JP (ed) Chemical oceanography, vol 9, 1st edn. Academic Press Ltd, London, pp 197–245
van den Berg CMG, Kramer JR (1979) Determination of complexing capacities of ligands in natural waters and conditional stability constants of the copper complexes by means of manganese dioxide. Anal Chim Acta 106:113–120
Zhou X, Wangersky PJ (1989) Changes in copper-complexing organic ligands during spring blooms in the coastal waters of Nova Scotia, Canada. Mar Ecol Prog Ser 53:277–284
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia, under grant no. 550/150/1432. The authors, therefore, acknowledge with thanks (DSR) technical and financial support. Thanks are also extended to Prof. Dr. Fazel Shoudary and Dr Alaa Al-Barakati for their help in the treatment of physical data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Farawati, R., El Sayed, M.A., Shaban, Y.A. et al. Occurrence of copper-complexing ligands in the coastal sediments of eastern Red Sea. Arab J Geosci 9, 250 (2016). https://doi.org/10.1007/s12517-015-2283-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-015-2283-8