Abstract
Organic complexation of Cu in estuarine waters in Japan was investigated using reverse titration competitive ligand exchange-adsorptive cathodic stripping voltammetry (CLE-AdCSV). We detected very strong Cu-binding organic ligands (L1) with conditional stability constants (KʹCuL1) of more than 1017. These ligands were successfully determined throughout the water column in Otsuchi Bay as well as in three rivers flowing into the bay. Organic ligands in the rivers had a concentration range of 2.6 nM–5.0 nM and log KʹCuL1 values between 17.5 and 18.6. The use of reverse titration enabled the detection of high concentrations of a weaker ligand class (L2) in river waters that ranged from 339 to 354 nM with log KʹCuL2 values between 11.9 and 12.1. We estimated that L1 was not derived from rivers or open ocean. Our incubation results indicated that the detected ligands did not increase with phytoplankton growth in Otsuchi Bay. Rather, coastal and benthic sediments may be major suppliers of L1 due to the enrichment of these ligands close to the sediment source. In contrast, the possible sources of weak Cu-binding organic ligands in Otsuchi Bay were estimated to be humic substances.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In seawater, more than 99% of the dissolved Cu is complexed by strong organic ligands (Bruland et al. 2000; Buck et al. 2012; Buck and Bruland 2005; Jacquot et al. 2013; Sunda and Lewis 1978). This complexation decreases the concentration of Cu2+ from potentially toxic to low levels that are safe for some phytoplankton (Brand et al. 1986; Moffett and Brand 1996; Croot et al. 2000; Wong et al. 2021) and sometimes limits diatom growth (Semeniuk et al. 2009). These ligands can be categorized into two (or three) classes based on their conditional stability constants (KʹCuL). The strongest ligand classes are probably protein-based organic matter exuded by cyanobacteria Synechococcus sp. (Moffett and Brand 1996) while the weaker ligand classes may consist of humic substances (Kogut and Voelker 2001; Wong et al. 2018; Whitby and van den Berg 2015; Dryden et al. 2007), thiols (Laglera and van den Berg 2003; Whitby et al. 2017) and other phytoplankton exudates (Leal et al. 1999; Dupont and Ahner 2005). Since Cu-binding organic ligands control Cu toxicity and bioavailability in seawater, it is important to accurately determine the organic complexation of Cu in marine ecosystems.
Competitive ligand exchange-adsorptive cathodic stripping voltammetry (CLE-AdCSV) is the most suitable method for determining the parameters of organic complexation of Cu with high sensitivity and simple equipment in water samples (e.g., Buck et al. 2012; Campos and van den Berg 1994; Wong et al. 2018, 2019, 2021). The forward titration method can be used to determine the organic complexation of Cu with ligand titration followed by CLE-AdCSV employing salicylaldoxime (SA) as a competing ligand (Campos and van den Berg 1994; Sander et al. 2007). Generally, ligand concentrations are detected in excess (i.e., [L] > [Cu]) in seawater (Buck and Bruland 2005; Whitby et al. 2018; Wong et al. 2018, 2019, 2021). The chemical speciation parameters of Cu using forward titration were also obtained for the coastal regions. Only one class of ligand was detected in water samples from Bohai Bay (Li et al. 2015) with log KʹCuL of 12.7–13.6. Other studies in Thurso Bay (Muller and Batchelli 2013) and Scheldt Estuary (Laglera and van den Berg 2003) detected two classes of ligands with log KʹCuL values between 11.7–16.2 and 12.7–16.1, respectively. Similarly, two classes of ligands with log KʹCuL of 12.0–15.6, were found in Otsuchi Bay (Wong et al. 2018).
Recently, an alternate reverse titration method has been used to detect organic ligands with different KʹCuL values in seawater (Nuester and van den Berg 2005; Santos-Echeandía et al. 2008; Wiwit et al. 2021). Reverse titration is a method to determine the organic complexation of Cu by varying the concentrations of the artificial ligands (primarily, SA) at a constant concentration of Cu with detection CLE-AdCSV (Nuester and van den Berg 2005; Santos-Echeandía et al. 2008; Wiwit et al. 2021). It is useful for detecting low concentrations of ligands with high KʹCuL values (Nuester and van den Berg 2005; Santos-Echeandía et al. 2008). Only one class of ligand has been detected in the coastal water samples from the Vigo Ria with log KʹCuL values between 14.4 and 16.3 (Santos-Echeandía et al. 2008). On the other hand, the reverse titration method has also enabled the detection of Cu-binding organic ligands with vastly different KʹCuL values in seawater samples via the addition of Cu to the samples (Wiwit et al. 2021). Using this method, Wiwit et al. (2021) revealed the presence of a very strong class of Cu-binding organic ligands with log KʹCuL > 16 and very high concentrations of a weaker class of ligand with log KʹCuL value between 11.6 and 15.8 at the surface of Otsuchi Bay. However, the sources and identities of these ligands are currently unknown. Because organic ligands are mainly produced by phytoplankton or derived from terrestrial sources or sediments, investigation of these ligands in estuaries and coastal regions may provide clues to their sources and identities.
Otsuchi Bay is a semi-enclosed bay bordering the North Pacific Ocean in a scarcely populated area of northeast Japan. The bay receives minimal anthropogenic input and is fed by three rivers (Otsuchi, Kozuchi, and Unozumai), thus making it an ideal location for studying the natural sources of Cu-binding organic ligands. This study shows the very strong class of Cu-binding organic ligands with high conditional stability constants log KʹCuL1 > 17 and high concentrations of the weaker class (L2) in river water samples for the first time using reverse titration method, which was not achieved using forward titration method. In addition, this study also estimates the possible sources of Cu-binding organic ligands in estuarine region Japan revealed using forward and reverse titration methods.
2 Materials and methods
2.1 Sampling location and storage
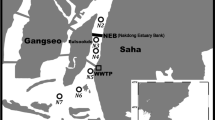
Seawater samples from Otsuchi Bay were collected in May 2017 using an acid-cleaned Teflon-coated Niskin-X sampler attached to a hydro-wire on a research boat Yayoi (International Coastal Research Center, Atmosphere and Ocean Research Institute (AORI), The University of Tokyo). Seawater samples were collected from three sampling sites in the bay: Stn. 02, Stn. 11, and Stn. 16, and from one site (Stn. 18) at the mouth of the bay (Fig. 1). A CTD sensor, which was lowered into the water from the research boat, was used to measure the temperature and salinity of the seawater at the sampling sites. Surface water was also collected from the Otsuchi, Kozuchi, and Unozumai rivers. In January 2020, river water samples were collected again from the Unozumai River. Water samples from the rivers were collected using an acid-washed polypropylene (PP) beaker and placed in acid-cleaned 5 L polyethylene (PE) containers. The sampling method described by Mashio et al. (2020) and Wong et al. (2018) was followed. Furthermore, seawater samples were also collected at a depth of 10 m from Station CV1003_S in the subarctic Pacific Ocean during the KH-17–5 cruise onboard R/V Hakuho-Maru in October 2017 (Fig. 1). Samples were collected using acid-cleaned Teflon-coated Niskin-X samplers attached to a Ti wire (Kim et al. 2015). Figures were created using the Ocean Data View developed by Schlitzer (2020).
Water samples that were used for measuring the total dissolved Cu concentrations were filtered through a 0.2 µm pore size filter cartridge (Acropak, Pall Industries) into acid-cleaned 500 mL low-density polyethylene (LDPE) bottles, whereas samples used for Cu speciation analysis were collected in acid-cleaned 500 mL fluorinated high-density polyethylene (FLPE) bottles while on the ship (Wong et al. 2018). The samples used for total dissolved Cu concentration measurements were acidified to a pH lower than 1.8 via the addition of ultrapure HCl (20%, Tamapure AA-100, Tama Chemicals) and those used for Cu speciation analysis were immediately frozen at − 20 °C.
2.2 Analytical methods
The measurement of total dissolved Cu concentration and Cu speciation analysis were performed using cathodic stripping voltammetry (CSV), as described in the Methods section of our previous study (Wiwit et al. 2021).
The total dissolved Cu concentrations were determined using a 797 VA Computrace (Metrohm) stripping voltammetry system. The working, reference, and counter electrodes were a hanging mercury drop electrode (HMDE); Ag/saturated AgCl, 3 M KCl electrode; and glassy carbon electrode, respectively. Before analysis, seawater samples were placed in Teflon beakers with quartz lids and irradiated with ultraviolet (UV) light using a high-pressure Hg vapor UV lamp (UM-453B-A, USHIO) for 1 h to destroy the interfering dissolved organic matter and metal-complexing organic ligands (Kim et al. 2015). Following UV irradiation, 10 mL of acidified sample was placed in a Teflon cell containing 25 µM SA, and the pH of the solution was adjusted to 8.35 using a 4% NH3 solution (Tamapure AA-100, Tama Chemicals) and 200 µL of a borate buffer (1 M boric acid in 0.35 M ammonia). The borate buffer was purified by passing it through a chelating resin (NOBIAS PA-1, Hitachi Hi-Tec) followed by UV irradiation for 1 h (Wong et al. 2018). The voltammetric parameters were as follows: initial purge, 180 s; deposition potential, − 0.05 V; deposition time, 120 s; equilibration time, 5 s; start potential, − 0.05 V; end potential, − 0.5 V; pulse amplitude, 0.025 V; pulse time, 0.05 s; voltage step 0.005 V, voltage step time, 0.25 s; sweep rate, 0.02 V/s; and the number of replications, 3. Subsequently, the concentration of total dissolved Cu in the seawater samples was calibrated using the standard addition method within the range of 0–6 nM Cu additions (Lohan et al. 2002). The accuracy of the method was determined by measuring the concentration of dissolved Cu in reference seawater SAFe and GEOTRACES inter-calibration samples. It was found that the Cu concentrations in the SAFe samples were 0.50 ± 0.02 (n = 2), which is close to the dissolved Cu consensus value (0.52 ± 0.05). Meanwhile, the Cu concentration in the GEOTRACES GS samples were 0.82 ± 0.04 (n = 2), which is also close to the dissolved Cu consensus value (0.84 ± 0.06). The procedural blank seawater samples (n = 7) were found to have a concentration of (0.24 ± 0.03) nM and the detection limit for this method is 0.09 nM, taken as 3 times the standard deviation of the blank samples.
The forward titration method was carried out according to Wong et al. (2018). Both [L] and log KʹCuL were calculated using the complete complexation fitting models of the ProMCC software (Omanović et al. 2015). Since we did not add excess copper to the incubation samples, this forward titration method was applied for investigating the production of organic ligands in the incubation samples. Meanwhile, the reverse titration method was used to the natural water samples for detecting the stronger and weaker ligands.
The reverse titration method was carried out according to our previous study (Wiwit et al. 2021) and included the following steps: 10 mL of seawater samples was added to 12–15 Teflon vials with lids. Subsequently, 200 µL of borate buffer and a set concentration of Cu were added to each vial. The samples were left to equilibrate for 2 h before the known SA concentrations of 1–350 µM were added to each vial. Next, the samples were allowed to equilibrate overnight. All experiments were conducted in a class–1000 clean area. This was done to “condition” the vials. After the samples were discarded, the procedure was repeated using the same vials. The concentrations of labile Cu in the samples were measured the next day using CSV. The voltammetric parameters used were same as those used for the measurement of the concentrations of the Cu-binding organic ligands using forward titration, but the deposition time was between 15 and 300 s. Subsequently, [L] and log KʹCuL were determined using the Ružić–van den Berg linearization (RvB) method, assuming one ligand system (Ružić 1982), and the least squares regression (LSR) method (Nuester and van den Berg 2005), respectively. The maximum peak current (ipmax) for each experiment was obtained by adding excess SA ([SA] = 1000 µM) to the seawater sample.
3 Incubation experiments
3.1 Sampling and incubation experiment setup
Seawater samples for the incubation experiments were collected in May 2019 at a depth of 1 m at Stn 02 in Otsuchi Bay (Fig. 1). Samples were collected using an acid-cleaned Teflon-coated Niskin-X sampler attached to a hydro-wire (Wong et al. 2018). Then, the samples were passed through a nylon mesh with pore size of 80 µm to filter out zooplankton into four acid-cleaned 15 L polyethylene cubic containers. On the same day, the seawater samples were stored at 4 °C and sent to AORI at the University of Tokyo, Kashiwa Campus. Subsampling was conducted in the laboratory the following day (approximately 24 h after sample collection). The seawater samples were transferred into an acid-cleaned 20 L FLPE tank and divided equally into acid-cleaned 1 L polycarbonate bottles.
The natural seawater samples were supplemented with nitrate and phosphate solutions (final concentrations: 16 µM NO3− and 1 µM PO43−, respectively) and borate buffer (1 M) to stabilize the pH of the seawater and were labeled “Nutrient”. Other bottles that contained seawater without any nutrient additions were labeled “Light” or “Dark” based on the incubation condition. Before transferring the seawater to carboys, the carboys were rinsed with the seawater samples three times to reduce contamination and ensure that minimal leachable sites remained on the wall. “Nutrient” and “Light” bottles were incubated for up to 2 weeks under 12 h light–dark cycle at 20 °C with an illuminance of 3,000 lx in an incubator (Multi Thermo Incubator MTI-202, EYELA), whereas “Dark” bottle was incubated at 20 °C in darkness. Each treatment was performed in duplicate.
3.2 Measurements of total dissolved Cu concentration, Cu speciation, and chlorophyll-a in the incubated samples
To measure the concentrations of total dissolved Cu and chlorophyll-a and analyze Cu speciation, subsamples were collected from the incubated bottles on days 0, 1, 3, 5, and 14. Samples for measuring the total dissolved Cu samples were collected from incubated samples (stored the 1 L polycarbonate bottles) in 125 mL low-density polyethylene (LDPE) bottles after passing through a 0.2 μm filter cartridge (Acropak, Pall Industries) using a peristaltic pump. Then, the samples were acidified to pH < 1.8 by adding ultrapure HCl (Tamapure AA–100, Tama Chemicals) and stored.
Samples for Cu speciation analysis were also collected in 500 mL fluorinated high-density polyethylene (FLPE) bottles after passing through a 0.2 μm filter cartridge (Acropak, Pall Industries) using a peristaltic pump from the incubation sample bottles. The samples were frozen immediately at – 20 °C. Cu speciation analyses were performed using forward titration method, as described in Methods section of our previous study (Wiwit et al. 2021).
Chlorophyll-a content was determined using 250 mL of incubation water samples. The samples were filtered through a pre-combusted 47 mm GF/F filter (Whatman) with a circular aspirator at a pressure of 0.016 MPa (120 mmHg). Prior to use, the filters were combusted at 450 °C for 3 h. The filters with the samples were extracted by placing them in 6.0 mL N, N-dimethylformamide (DMF, Wako Pure Chemical Industries) solvent (Suzuki and Ishimaru 1990) and stored in dark at 4 °C for 2–3 days before analysis. The chlorophyll-a concentration was measured using a Turner 10–AU–005 fluorometer (USA) (Holm-Hansen et al. 1965).
4 Results
4.1 Hydrography
The temperature–salinity (T–S) diagram at Stn 02, 11, 16, and 18 in Otsuchi Bay is shown in Fig. 2. The surface layer water system (SW) occupies a wide area of sampling sites in Otsuchi Bay flowing with temperature > 2 °C and salinity > 32.0. This type of water mass appeared in the spring and summer seasons in the upper layers of the Sanriku coastal area (Hanawa and Mitsudera 1986). The properties of seawater in Otsuchi Bay were influenced by the open-ocean waters, the Tsugaru Warm Current (TWC) from the Sea of Japan, the cold Oyashio Current from the subarctic North Pacific, and the Kuroshio Current from the subtropical North Pacific (Ishizu et al. 2017). The waters of Otsuchi Bay have short residence times between 1 week and 1 month (Fukuda et al. 2016).
T–S diagram of Otsuchi Bay waters. Surface layer water system (SW) occupied in the wide area of sampling sites in Otsuchi Bay. Water mass is classified according to Hanawa and Mitsudera (1986)
4.2 Distribution of dissolved Cu
The distribution of dissolved Cu in Otsuchi Bay is shown in Fig. 3 and Table S1. Total dissolved Cu concentrations were approximately 4.76 nM at the surface (1 m) of Stn. 16 and decreased toward Stn. 18 (2.87 nM). Furthermore, the concentration of total dissolved Cu decreased with depth at all stations (Fig. 3). The dissolved Cu concentrations in Otsuchi River (5.23 nM) and Unozumai River (5.08 nM) were higher than those in the bay (Table 1). The total dissolved Cu concentration in the CV1003_S sample, which was influenced by Oyashio Current, was 2.22 nM.
4.3 Ligand characteristic of organic complexes of Cu
4.3.1 Strong Cu-binding organic ligands ( L 1 )
The reverse titration method revealed the presence of low concentrations of very strong ligands with log KʹCuL1 > 15 at a depth of 1 m at the four stations in Otsuchi Bay which were shown in our previous study (Wiwit et al. 2021). The log KʹCuL1 values of the Cu-binding organic ligands in the Otsuchi Bay at Stn. 02; Stn. 11; Stn. 16; and Stn. 18 were 15.4; 15.4; 15.8; and 15.3, respectively. In this study, the Cu speciation parameters for the full water column at Stn. 18 obtained using the reverse titration method are shown in Fig. 4 and Table S2. At a depth of 1 m at Stn. 18, log KʹCuL1 was 15.3, whereas at a depth of 5 m from the surface to the bottom water, it was approximately 17.2.
The concentrations of strong ligands in the river water samples were also determined using the same reverse titration method (Table 1). The significant difference in salinity between seawater and river water produced different αSA values. The α coefficients for complexation of Cu by SA (\({\alpha }_{CuSA}+ {\alpha }_{Cu(S{A)}_{2}})\) were defined in a previous study (Wiwit et al. 2021). To confirm whether detectable organic ligands existed in the river water, we obtained the titration curves for UV-irradiated river water sample and natural water sample (Fig. 5). The river water sample used in this study was collected from the Unozumai River in January 2020. The titration and modeled data fit curves of the two water samples are illustrated in Fig. 5. The peak of the curve increased with increasing SA concentration. Further, the titration curve of the natural river water shifted toward higher SA concentrations than that of the UV-irradiated river water sample, which suggested the presence of natural Cu-binding organic ligands in the natural river water sample. To destroy all the organic ligands in the samples, it took 2 h to UV-irradiate the river water samples. This was longer than the time required (1 h) for the seawater samples. The log KʹCuL1 values in river water samples were much higher than those in seawater samples. The log KʹCuL1 values were between 17.5 and 18.6 in river water samples (Table 1), and the concentration of strong ligands L1 was in the range 2.60–5.04 nM.
4.3.2 Weak Cu-binding organic ligands ( L 2 )
The log KʹCuL values of Cu-binding organic ligands determined using the reverse titration method with the addition of 120 nM Cu in surface water samples were between 11.6 and 12.1, and these ligands were categorized as weak ligands (L2) (Wiwit et al. 2021). The titration curves of the UV-irradiated and non-UV-irradiated river samples were clearly distinguished, and no ligand was detected in the UV-irradiated river water samples when 350 nM Cu was added after 2 h of irradiation (Fig. 5). This suggests that the reverse titration method successfully detected high concentrations of weak Cu-binding organic ligands in river water samples.
The concentrations of L2 and the log KʹCuL2 values of weak Cu-binding organic ligands in river waters around Otsuchi Bay and open-ocean samples are summarized in Tables 1 and 2, respectively. The concentration of L2 in the surface water of Otsuchi Bay at Stn. 02; Stn. 11; Stn. 16; and Stn. 18 were previously reported as 89.74; 121.0; 89.86; and 116.3 nM, respectively (Wiwit et al. 2021). The concentration of weak ligands [L2] in the surface water of Otsuchi Bay exceeded 120 nM, indicating that all the added Cu was saturated by the L2 in the samples (Wiwit et al. 2021). In contrast, higher concentrations of L2 in river water samples from the Otsuchi, Kozuchi, and Unozumai rivers were 342, 339, and 354 nM, respectively (Table 1). In the open-ocean sample taken from Stn. CV1003_S with a salinity of 33.47, the concentration of weak ligand L2 was 112 nM (Table 2). The coastal seawaters in Otsuchi Bay contained very high concentrations of L2, which were not detected using the forward titration method in a previous study (Wong et al. 2018).
4.3.3 Cu 2+ concentrations
Free copper concentrations ([Cu2+]) of the river water and open-ocean samples were calculated using ProMCC software with a two-ligands system (Omanović et al. 2015). [Cu2+] was found to be in the range 10−15 –10−16 M in the rivers (Table 1). In contrast, in the open-ocean sample from Stn. CV1003_S, [Cu2+] was 6.74 × 10−15 M (Table 2). These concentrations are similar to those in the North Pacific Ocean (Moffett and Dupont 2007). [Cu2+] found in this study are below the toxicity threshold for most phytoplankton (Brand et al. 1986).
4.4 Incubation experiments
4.4.1 Chlorophyll-a concentrations
The chlorophyll-a concentrations in Otsuchi Bay seawater samples, which were collected in May 2019, on different days, i.e., from day zero to day 14, are shown in Fig. 6. The concentrations of chlorophyll-a for the initial conditions in Otsuchi Bay samples were approximately 0.89 µg L−1. This value agreed with those (approximately 0.5–3.0 µg L−1) obtained at the same sampling site in a previous study (Fukuda et al. 2016).
The chlorophyll-a concentration in “Nutrient” samples started increasing on the third day of incubation and reached 12.3 µg L−1, indicating a 14-fold increase from the initial concentration. It started increasing on day 3 for “Nutrient”. In “Light” samples, chlorophyll-a concentrations increased twofold (2.20 µg L−1) after 3 days and decreased to 0.65 µg L−1 at the end of incubation time because of depleted nutrients in the samples. The chlorophyll-a concentrations in the “Dark” samples decreased after 14 days and reached 0.06 µg L−1.
4.4.2 Organic complexation of Cu determined using forward titration method
The Cu speciation parameters and total dissolved Cu concentrations for the incubation samples obtained using the forward titration method are given in Table 3. Since dissolved Cu concentrations were almost constant in “Dark” condition, the Cu adsorption onto bottle wall should be small. Two classes of ligands were detected at the start of incubation (day zero). The ligand concentrations and log KʹCuL1 for strong ligands were 3.46 nM and 13.9, respectively. In contrast, the ligand concentrations and log KʹCuL2 for weak ligands were 13.4 nM and 12.6, respectively. Furthermore, in “Nutrient” samples, the concentration range of Cu-binding organic ligands from day 0 to day 14 was 3.46–12.85 nM, with the corresponding log KʹCuL values of 13.9. On the other hand, in “Light” samples, the concentration range of Cu-binding organic ligands from day 0 to day 14 was 3.46–9.62 nM and the corresponding range of log KʹCuL values was 13.9–13.7. In “Dark” samples, the range of ligand concentrations from day 0 to day 14 was 3.4 –4.41 nM and the corresponding range of log KʹCuL values was 13.9–14.2.
Excess ligands ([L] > [Cu]) were detected in the incubated samples using the forward titration method. The production of ligands in the seawater samples is related to the increase in the complexation coefficient of Cu complexes (αCuL) values with incubation duration. The αCuL values represent the complexation coefficients for all organic ligands (i.e., both L1 and L2) that exist in seawater samples (van den Berg and Donat 1992; Wong et al. 2021). We observed no clear increase in the αCuL value (with an error of 6.0%) in the “Nutrient” samples (Fig. 7).
5 Discussion
5.1 The possible source of Cu in Otsuchi Bay
The total dissolved Cu concentration data in this study were compared to a previous study that reported the total dissolved Cu concentrations at the same sampling sites in Otsuchi Bay (Wong et al. 2018). Due to the small number of published data for Cu concentrations in Otsuchi Bay, we compared our data with those data in Wong et al. (2018). Although some seasonal variations may affect the two datasets, the difference was too small to discuss. These data were plotted against salinity (Fig. 8). Cu concentrations in Otsuchi Bay were mostly higher than those determined by Wong et al. (2018) and they reported that there were additional sources of dissolved Cu in Otsuchi Bay other than rivers. Vertical profiles of temperature and salinity at Stn. 18 show that the temperature decreased and salinity increased steeply from 1 to 5 m depth, indicating strong stratification (Fig. 4). The strong stratification will inhibit the upward diffusion of dissolved Cu from the deep layer to surface layer. In addition, the concentration of dCu decrease with the salinity, which indicates that the river is a possible source of dCu in the surface water. Atmospheric deposition and coastal sediments or both have been suggested as the additional sources of dissolved Cu (Achterberg et al. 1999; Kremling and Hydes 1988). As previously discussed for the East China Sea (Wong et al. 2019), the atmospheric deposition of Cu would be small in Otsuchi Bay. Therefore, coastal sediments may be the other source of Cu. At depths below 5 m where the temperature and salinity profiles become constant, sediments may play an important role. A high concentration of dissolved Cu was observed at the bottom depth, which indicates that the bottom sediment is also a possible source of Cu.
The concentration of total dissolved Cu ([Cu]) versus salinity in Otsuchi Bay compared to a previous study in Wong et al. (2018)
5.2 Possible source of L1
L1 concentrations and log KʹCuL1 of Cu-binding organic ligands at Stn. 18 were measured near the sea surface and a depth of 72 m (Fig. 4). The concentration range of L1 in this study was lower than that found in the estuarine waters of Vigo Ria using the reverse titration method (in the range of 4.1–7.1 nM); high log KʹCuL values in the range of 15.5–16.7 were reported in low salinity areas of Vigo Ria (Santos-Echeandía et al. 2008). The concentrations and log KʹCuL of the Cu-binding organic ligands in Otsuchi Bay were further compared with those reported by Wong et al. (2018) at the same sampling sites; they calculated the Cu speciation parameters using a forward titration method with a 5 µM SA detection window. L1 concentrations obtained using the forward titration method were higher than the total dissolved Cu concentrations and had a log KʹCuL1 of 14.8. However, the KʹCuL1 obtained using the reverse titration method in this study was higher than that obtained using forward titration in Wong et al. (2018). Furthermore, the complexing capacity αCuL obtained in this study was one order of magnitude higher than that reported by Wong et al. (2018). This suggested that the detected ligand groups (strong and weak ligands) in this study were different from those reported by Wong et al. (2018).
Ligand concentrations in Otsuchi Bay were similar to the total dissolved Cu concentrations throughout the water column. Ligands found at a depth of 1 m had log KʹCuL1 of 15.3, whereas ligands below the water surface had log KʹCuL1 of 17.4. This implies that the surface water at Stn. 18 had different L1 ligands from the samples collected in deeper waters. If the Cu in deep layer was supplied from the sediments, the L1 ligands might have a similar origin.
To investigate the possible source of L1 in Otsuchi Bay, the chemical speciation data of Cu at both the rivers flowing into the bay and Stn.CV1003_S were obtained (Fig. 1, Table S2, Table 1, and Table 2). High concentrations (2.6–5.04 nM) of ligands with very high log KʹCuL1 values (17.5–18.6) comparable to those at Stn. 18 were observed in all three rivers. Moreover, the log KʹCuL1 values in low-density surface water were lower than those in the rivers, thus, the riverine strong ligand is unlikely to be the source of the strong ligands that are found in Otsuchi Bay. However, we could not elucidate the seasonal variation of river water discharge because we collected the river water samples only at one time. In Stn. CV1003_S, the very strong ligand had concentration of 1.75 ± 0.04 nM and log KʹCuL1 of 16.8 ± 0.38. Further, the strong ligands were also detected in one station in the subtropical western North Pacific Ocean with concentration of 1.06 ± 0.03 nM and log KʹCuL1 of 16.4 ± 0.14 (Wiwit et al. 2021). However, the concentration of strong ligands below a depth of 5 m at Stn. 18 are higher than those in the open ocean, which indicates that the ligands were not derived from the open ocean.
We also investigated the possible ligand production by phytoplankton in Otsuchi Bay using forward titration method. However, no increase in αCuL values was observed during the incubation experiments (Fig. 7), suggesting that phytoplankton growth did not result in the production of Cu-binding organic ligands in seawater. A previous study reported the production of Cu-binding organic ligands with log KʹCuL between 12.5 and 13.0 by conducting incubation experiments using natural estuarine seawater samples (Dryden et al. 2004). They concluded that cyanobacteria could be a major biological source of these ligands, similar to observations made by Moffett and Brand (1996). The release of Cu-binding organic ligands was in response to the addition of Cu into the incubation samples (Moffett and Brand 1996). In Otsuchi Bay, water properties are influenced by the North Pacific Ocean, where cyanobacteria exist (Tsuda et al. 1994; Kim et al. 2017), but its organic ligand production might not be so active as to be detectable. Furthermore, ambient Cu concentrations in the seawater of Otsuchi Bay did not suppress phytoplankton growth (Table 3) because the Cu2+ concentrations in the incubation samples were below the toxicity threshold for most of the phytoplankton, as reported by Brand et al. (1986). In a previous field observation study, no correlation was found between bulk chlorophyll and ligand concentrations (Croot 2003). Another culture study also reported that phytoplankton blooms in San Francisco Bay did not influence the concentration and organic complexation of Cu (Beck et al. 2002). In Otsuchi Bay, these very strong ligands were probably not produced in situ by phytoplankton.
Very strong ligands with a log KʹCuL1 of approximately 16.2 have been detected in the Southern Ocean (Buck et al. 2010). Buck et al. (2010) detected these very strong ligands using a forward titration method with a high detection window of 25 µM SA during incubation experiments. In this region, the sediments over the Antarctic Peninsula shelf may be a source of Cu-binding organic ligands (Buck et al. 2010; Nolting and de Baar 1994). Sediments are an important source of strong ligands with log KʹCuL > 11 in the estuary along the North Carolina coast (Shank et al. 2004) and also in Chesapeake Bay (Skrabal et al. 1997). A higher concentration of strong ligand at the bottom layer was observed at Stn. 18, suggesting that the bottom sediment might be the source of the strong ligand. However, more detailed investigations are needed to identify the source of the strong ligand.
5.3 The possible source of L2
The reverse titration method detected much higher concentrations of weak ligands (L2) with the same range of log KʹCuL2 (11.6–12.1) in the river waters (339–354 nM) than in the seawater samples in the bay (90–121 nM). Previous studies have reported that some marine microorganisms can release thiols as Cu-binding ligands (Leal et al. 1999; Dupont and Ahner 2005). Thiols have log KʹCuL2 values between 12 and 14 (Laglera and van den Berg 2003; Whitby et al. 2017). However, we did not detect newly produced organic ligands during the incubation experiments. Therefore, thiols are unlikely to be the main component of the L2 ligand in Otsuchi Bay. Another possible source of this weak Cu-binding organic ligand originating from freshwater is fulvic and humic acids with log KʹCuL2 values in the range of 10–12 in the estuarine (Kogut and Voelker 2001). Humic substances were also detected in Otsuchi Bay with log KʹCuL2 values of approximately 12.5 (Wong et al. 2018). Other previous studies have also found humic substances in other estuarine areas with log KʹCuL2 of ~ 12 (Whitby and van den Berg 2015; Dryden et al. 2007). Therefore, humic substances may be the dominant ligand classes responsible for controlling the weak Cu-binding organic ligands in Otsuchi Bay.
The concentrations of weak ligand L2 decreased from the three rivers in Otsuchi Bay to the central bay stations (Stn. 16 and Stn. 02) and increased outside the bay. L2 concentration at Stn. 18 was similar to that of the open-ocean samples (Stn. CV1003_S). This suggests that marine humic substances in Otsuchi Bay are also sources of L2 ligands.
6 Conclusion
In this study, we detected very strong ligands with high conditional stability constants (log KʹCuL1 > 15) and high concentrations of weak ligands L2 (with log KʹCuL2 < 13) using reverse titration CLE-AdCSV. Our findings showed that very strong ligands were neither derived from rivers nor from the open ocean. Results from the incubation experiments revealed that ligand production did not occur in Otsuchi Bay and was not the source of these ligands. The vertical profile of strong Cu-binding organic ligands in Otsuchi Bay showed enrichment in the bottom layer, suggesting that sediments are the possible sources of the ligands. Further, the concentrations of L2 decreased from river to bay, suggesting that the possible sources of the weak Cu-binding organic ligands in Otsuchi Bay were humic substances. The reverse titration method enabled the detection of previously “unseen” ligand classes that may control the toxicity and bioavailability of Cu in coastal regions. However, more investigations are needed to further elucidate the sources and roles of these ligands in seawater.
References
Achterberg EP, Colombo C, van den Berg CMG (1999) The distribution of dissolved Cu, Zn, Ni, Co and Cr in English coastal surface waters. Cont Shelf Res 19(4):537–558. https://doi.org/10.1016/S0278-4343(98)00093-4
Beck NG, Bruland KW, Rue EL (2002) Short-term biogeochemical influence of a diatom bloom on the nutrient and trace metal concentrations in South San Francisco Bay microcosm experiments. Estuaries 25:1063–1076. https://doi.org/10.1007/BF02692204
Brand LE, Sunda WG, Guillard RRL (1986) Reduction of marine phytoplankton reproduction rates by copper and cadmium. J Exp Mar Biol Ecol 96(3):225–250. https://doi.org/10.1016/0022-0981(86)90205-4
Bruland KW, Rue EL, Donat JR, Skrabal SA, Moffett JW (2000) Intercomparison of voltammetric techniques to determine the chemical speciation of dissolved copper in a coastal seawater sample. Anal Chim Acta 405(1–2):99–113. https://doi.org/10.1016/S0003-2670(99)00675-3
Buck KN, Bruland KW (2005) Copper speciation in San Francisco Bay: a novel approach using multiple analytical windows. Mar Chem 96(1–2):185–198. https://doi.org/10.1016/j.marchem.2005.01.001
Buck KN, Selph KE, Barbeau KA (2010) Iron-binding ligand production and copper speciation in an incubation experiment of Antarctic Peninsula shelf waters from the Bransfield Strait, Southern Ocean. Mar Chem 122(1–4):148–159. https://doi.org/10.1016/j.marchem.2010.06.002
Buck KN, Moffett J, Barbeau KA, Bundy RM, Kondo Y, Wu J (2012) The organic complexation of iron and copper: an intercomparison of competitive ligand exchange-adsorptive cathodic stripping voltammetry (CLE-ACSV) techniques. Limnol Oceanogr Methods 10(7):496–515. https://doi.org/10.4319/lom.2012.10.496
Campos MLA, van den Berg CMG (1994) Determination of copper complexation in sea water by cathodic stripping voltammetry and ligand competition with salicylaldoxime. Anal Chim Acta 284:481–496. https://doi.org/10.1016/0003-2670(94)85055-0
Croot PL (2003) Seasonal cycle of copper speciation in Gullmar Fjord, Sweden. Limnol Oceanogr 48(2):764–776. https://doi.org/10.4319/lo.2003.48.2.0764
Croot PL, Moffett JW, Brand LE (2000) Production of extracellular Cu complexing ligands by eucaryotic phytoplankton in response to Cu stress. Limnol Oceanogr 45(3):619–627. https://doi.org/10.4319/lo.2000.45.3.0619
Dryden CL, Gordon AS, Donat JR (2004) Interactive regulation of dissolved copper toxicity by an estuarine microbial community. Limnol Oceanogr 49(4):1115–1122. https://doi.org/10.4319/lo.2004.49.4.1115
Dryden CL, Gordon AS, Donat JR (2007) Seasonal survey of copper-complexing ligands and thiol compounds in a heavily utilized, urban estuary: Elizabeth River, Virginia. Mar Chem 103(3–4):276–288. https://doi.org/10.1016/j.marchem.2006.09.003
Dupont CL, Ahner BA (2005) Effects of copper, cadmium, and zinc on the production and exudation of thiols by Emiliania huxleyi. Limnol Ocenogr 50(2):508–515. https://doi.org/10.4319/lo.2005.50.2.0508
Fukuda H, Katayama R, Yang Y, Takasu H, Nishibe Y, Tsuda A, Nagata T (2016) Nutrient status of Otsuchi Bay (northeastern Japan) following the 2011 off the Pacific coast of Tohoku earthquake. J Oceanogr 72:39–52. https://doi.org/10.1007/s10872-015-0296-2
Hanawa K, Mitsudera H (1986) Variation of water system distribution in the Sanriku coastal area. J Oceanogr Soc Jpn 42(6):435–446. https://doi.org/10.1007/BF02110194
Holm-Hansen O, Lorenzen CJ, Holmes RW, Strickland JDH (1965) Fluorometric determination of chlorophyll. ICES J Mar Sci 30:3–15
Ishizu M, Itoh S, Tanaka K, Komatsu K (2017) Influence of the Oyashio current and Tsugaru warm current on the circulation and water properties of Otsuchi Bay, Japan. J Oceanogr 73:115–131
Jacquot JE, Kondo Y, Knapp AN, Moffett JW (2013) The speciation of copper across active gradients in nitrogen-cycle processes in the eastern tropical south Pacific. Limnol Oceanogr 58(4):1387–1394. https://doi.org/10.4319/lo.2013.58.4.1387
Kim T, Obata H, Gamo T, Nishioka J (2015) Sampling and onboard analytical methods for determining subnanomolar concentrations of zinc in seawater. Limnol Oceanogr Methods 13(1):30–39. https://doi.org/10.1002/lom3.10004
Kim D, Jeong JH, Kim TW, Noh JH, Kim HJ, Choi DH, Kim E, Jeon D (2017) The reduction in the biomass of cyanobacterial N2 fixer and the biological pump in the Northwestern Pacific Ocean. Sci Rep. https://doi.org/10.1038/srep41810
Kogut MB, Voelker BM (2001) Strong copper-binding behavior of terrestrial humic substances in seawater. Environ Sci Technol 35(6):1149–1156
Kremling K, Hydes D (1988) Summer distribution of dissolved Al, Cd Co, Cu, Mn and Ni in surface waters around the British Isles. Cont Shelf Res 8(1):89–105. https://doi.org/10.1016/0278-4343(88)90026-X
Laglera LM, van den Berg CMG (2003) Copper complexation by thiol compounds in estuarine waters. Mar Chem 82(1–2):71–89. https://doi.org/10.1016/S0304-4203(03)00053-7
Leal M, Vasconcelos M, van den Berg CMG (1999) Copper- induced release of complexing ligands similar to thiols by Emiliania huxleyi in seawater cultures. Limnol Oceanogr 44:1750–1762. https://doi.org/10.4319/lo.1999.44.7.1750
Li L, Liu J, Wang X, Shi X (2015) Dissolved trace metal distributions and Cu speciation in the southern Bohai Sea, China. Mar Chem 172:34–45. https://doi.org/10.1016/j.marchem.2015.03.002
Lohan MC, Statham PJ, Crawford DW (2002) Total dissolved zinc in the upper water column of the subarctic NorthEast Pacific. Deep-Sea Res Part II: Top Stud Oceanogr 49(24–25):5793–5808. https://doi.org/10.1016/S0967-0645(02)00215-1
Mashio AS, Obata H, Shimazaki T, Fukuda H, Ogawa H (2020) Spatiotemporal variations of platinum in seawater in Otsuchi Bay, Japan after the 2011 tsunami. Sci Total Environ 708:134659. https://doi.org/10.1016/j.scitotenv.2019.134659
Moffett JW, Brand LE (1996) Production of strong, extracellular Cu chelators by marine cyanobacteria in response to Cu stress. Limnol Oceanogr 41(3):388–395. https://doi.org/10.4319/lo.1996.41.3.0388
Moffett JW, Dupont C (2007) Cu complexation by organic ligands in the subarctic NW Pacific and Bering Sea. Deep-Sea Res Pt I 54:586–595. https://doi.org/10.1016/j.dsr.2006.12.013
Muller FLL, Batchelli S (2013) Copper binding by terrestrial versus marine organic ligands in the coastal plume of River Thurso, North Scotland. Estuar Coast Shelf Sci 133:137–146. https://doi.org/10.1016/j.ecss.2013.08.024
Nolting RF, De Baar HJW (1994) Behaviour of nickel, copper, zinc and cadmium in the upper 300 m of a transect in the Southern Ocean (57°–62oS, 49oW). Mar Chem 45:225–242
Nuester J, van den Berg CMG (2005) Determination of metal speciation by reverse titrations. Anal Chem 77(1):11–19. https://doi.org/10.1021/ac049078e
Omanović D, Garnier C, Pižeta I (2015) ProMCC: an all-in-one tool for trace metal complexation studies. Mar Chem 173:25–39. https://doi.org/10.1016/j.marchem.2014.10.011
Ružić I (1982) Theoretical aspects of the direct titration of natural waters and its information yield for trace metal speciation. Anal Chim Acta 140(1):99–113. https://doi.org/10.1016/S0003-2670(01)95456-X
Sander SG, Koschinsky A, Massoth G, Stott M, Hunter KA (2007) Organic complexation of copper in deep-sea hydrothermal vent systems. Environ Chem 4(2):81–89. https://doi.org/10.1071/EN06086
Santos-Echeandía J, Laglera LM, Prego R, van den Berg CMG (2008) Copper speciation in estuarine waters by forward and reverse titrations. Mar Chem 108(3–4):148–158. https://doi.org/10.1016/j.marchem.2007.11.004
Semeniuk DM, Cullen JT, Johnson WK, Gagnon K, Ruth TJ, Maldonado MT (2009) Plankton copper requirements and uptake in the subarctic Northeast Pacific Ocean. Deep-Sea Res Part I 56(7):1130–1142. https://doi.org/10.1016/j.dsr.2009.03.003
Shank GC, Skrabal SA, Whitehead RF, Kieber RJ (2004) Fluxes of strong Cu-complexing ligands from sediments of an organic-rich estuary. Estuar Coast Shelf Sci 60(2):349–358. https://doi.org/10.1016/j.ecss.2004.01.010
Skrabal SA, Donat JR, Burdige DJ (1997) Fluxes of copper-complexing ligands from estuarine sediments. Limnol Oceanogr 42(5I):992–996. https://doi.org/10.4319/lo.1997.42.5.0992
Sunda WG, Lewis JAM (1978) Effect of complexation by natural organic ligands on the toxicity of copper to a unicellular alga, Monochrysis lutheri. Limnol Oceanogr 23(5):870–876. https://doi.org/10.4319/lo.1978.23.5.0870
Suzuki R, Ishimaru T (1990) An improved method for the determination of phytoplankton chlorophyll using N, N-dimethylformamid. J Oceanogr Soc Jpn 46:190–194
Tsuda A, Sugisaki H, Takahashi K, Furuya K (1994) Succession of pelagic organisms in the size range 0.5-200 μm during a diatom bloom in Otsuchi Bay, Japan. J Estuarine, Coast Shelf Sci 39:173–184
van den Berg CMG, Donat JR (1992) Determination and data evaluation of copper complexation by organic ligands in sea water using cathodic stripping voltammetry at varying detection windows. Anal Chim Acta 257(2):281–291. https://doi.org/10.1016/0003-2670(92)85181-5
Whitby H, van den Berg CMG (2015) Evidence for copper-binding humic substances in seawater. Mar Chem 173:282–290. https://doi.org/10.1016/j.marchem.2014.09.011
Whitby H, Hollibaugh JT, van den Berg CMG (2017) Chemical speciation of copper in a salt marsh estuary and bioavailability to thaumarchaeota. Front Mar Sci 4:1–15. https://doi.org/10.3389/fmars.2017.00178
Whitby H, Posacka AM, Maldonado MT, van den Berg CMG (2018) Copper-binding ligands in the NE Pacific. Mar Chem 204:36–48. https://doi.org/10.1016/j.marchem.2018.05.008
Wiwit WKH, Fukuda H, Ogawa H, Mashio AS, Kondo Y, Nishioka J, Obata H (2021) Wide-range detection of Cu-binding organic ligands in seawater using reverse titration. Mar Chem 230:103927. https://doi.org/10.1016/j.marchem.2021.103927
Wong KH, Obata H, Kim T, Mashio AS, Fukuda H, Ogawa H (2018) Organic complexation of copper in estuarine waters: an assessment of the multi-detection window approach. Mar Chem 204:144–151. https://doi.org/10.1016/j.marchem.2018.07.001
Wong KH, Obata H, Kim T, Wakuta Y, Takeda S (2019) Distribution and speciation of copper and its relationship with FDOM in the East China Sea. Mar Chem 212:96–107. https://doi.org/10.1016/j.marchem.2019.04.005
Wong KH, Obata H, Kim T, Kondo Y, Nishioka J (2021) New insights into the biogeochemical cycling of copper in the subarctic Pacific: distributions, size fractionation, and organic complexation. Limnol Oceanogr. https://doi.org/10.1002/lno.11695
Schlitzer R (2020) Ocean data view. http://odv.awi.de
Acknowledgements
The authors are grateful to the staff of the International Coastal Research Center, Atmosphere and Ocean Research Institute, The University of Tokyo, for their help with collecting water samples from Otsuchi Bay. The authors would also like to thank the captain, crew, technicians, and scientists, especially Drs. Jun Nishioka, Yoshiko Kondo, and Taejin Kim, onboard R/V Hakuho-Maru during the KH-17-5 cruise for their assistance with sample collection. This research was supported by the Tohoku Ecosystem-Associated Marine Science (TEAMS) Project funded by the MEXT. This study was also supported by the University of Bengkulu and financially supported by the Ministry of Research, Technology and Higher Education of the Republic of Indonesia (KEMRISTEKDIKTI), the Indonesia Endowment Fund for Education (LPDP) [grant number PRJ-3672/LPDP.3/2016], and the Sasakawa Scientific Research Grant from the Japan Science Society [grant number 2020-3021].
Author information
Authors and Affiliations
Contributions
W: conceptualization, formal analysis, writing-original draft preparation, investigation, methodology, and funding acquisition. KHW: conceptualization, formal analysis, writing—original draft preparation, reviewing, and funding acquisition. HF: resources, writing, reviewing, and editing. HO: resources, writing, reviewing, and editing. ASM: resources, writing, reviewing, and editing. HO: conceptualization, investigation, writing—original draft preparation, resources, funding acquisition, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wiwit, Wong, K.H., Fukuda, H. et al. Organic complexation of copper in Japanese estuarine waters using reverse titration method. J Oceanogr 79, 335–348 (2023). https://doi.org/10.1007/s10872-022-00674-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-022-00674-1