Abstract
The large amounts of phosphate fertilizers which are used in agriculture activities in Egypt lead to the contamination of large quantities of soil surrounding the agricultural fields. Also, the use of traditional flood irrigation method makes the problem more worse. An extensive laboratory testing program is carried out to determine the Atterberg limits, compaction characteristics, hydraulic conductivity, and shear strength parameters of clean and contaminated soils. Three types of soils are used including coarse sand, fine to medium sand, and silty clay. Batch tests are used to study the short-term effect of di-ammonium phosphate (DAP) contamination on the soil properties. Contaminated specimens are prepared by mixing the dried soil samples with different di-ammonium phosphate concentrations of 5, 10, and 20 %. The results indicate a significant reduction in Atterberg limits and increase in hydraulic conductivity for silty clay soil with the increase of phosphate concentration. The change that is induced by chemical reaction in the micro-structure of the soil is studied by laser scanning microscope before and after soil contamination. For sand soil, γ dmax is significantly increased with the increase of DAP concentrations up to 5 % and slightly increased with concentrations from 5 to 20 % which indicates excess phosphate in the soil. However, the general trend of hydraulic conductivity is towards reduction. In addition, the friction angle of two sand samples decreases about 10 % due to the increase of DAP concentration up to 20 %. For silty clay, the reduction in cohesion is about 20 % and the increase of friction angle is about 10 % with the increase of DAP up to 20 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Egypt, the soil formation in the Nile River and Delta is silt-clay mixtures of good quality, deposited during thousands of years of Nile flooding. After the construction of the High Aswan Dam, the soil fertility has decreased leading to the increase in consumption of fertilizers which has tripled between the periods from 1970 to 2000. Egypt has a long history of using mineral fertilizers; its first use of Chilean nitrates is from 1902. For over 30 years, all mineral fertilizers were imported, until the local production of phosphate fertilizers started in 1936. As published by Food and Agriculture Organization (FAO) report in 2005, there are four agro-ecological zones which can be identified based on soil characteristics and water resources. These zones are old land which is located in the Nile valley and Delta Regions, new land which is located mainly on both the east and west sides of the Delta and scattered over various areas in the country, oases, and rain-fed areas in the north coastal areas. The estimated fertilizer requirements on new and old lands are plotted in Fig. 1.
Estimated fertilizer requirements on new and old lands. Source: Ministry of Agricultural and Land Reclamation (MALR), Egypt 2003
Phosphorus is applied to soil in inorganic fertilizers or in organic manures in the form of plant and animal residues. Of all forms of phosphorus occurring in soils, the monovalent anion H2PO4 − is the only one available to the growing of the plants. Phosphorus fertilizers have different solubilities in irrigation water. Fertilizers as di-ammonium phosphate (DAP), mono-ammonium phosphate (MAP), mono-potassium phosphate (MKP), urea phosphate (UP), and phosphoric acid (PA) represent suitable options for completely soluble phosphate fertilizers.
(DAP) is the most widely phosphorus (P) fertilizer used in the world. It is made from two common constituents in the fertilizer industry, and it is popular because it contains high nutrient content which equals 18 % and has excellent physical properties. DAP fertilizer is an excellent source of P and nitrogen (N) with chemical formula (NH4)2HPO4 with density 1.62 g/cm3 and melting point 155 °C. It has a high solubility and thus it dissolves quickly in soil to release plant-available phosphate and ammonium. Its solubility in water at 20 °C equals 588 g/L. Molecular weight is 132.06 and DAP is alkaline with solution pH 7.5 to 8.
The residual amount of phosphate may cause soil and groundwater pollution. The degree of impact to the subsurface environment and groundwater systems depends on the concentration of phosphate, seepage potential, hydraulic conductivity of the soils, direction and migration rate of phosphate, site hydrogeology, and soil/phosphate interaction. Other factors such as temperature, rainfall, wind, and topography also affect the susceptibility of phosphate in a particular environment (Taha 2000).
In connection with any possible applications, knowledge of the geotechnical properties and behavior of contaminated soil is required. The main focus of this study is to determine the effect of contamination with di-ammonium phosphate on hydraulic, compaction characteristics, and shear strength parameters of sand and silty clay soil.
Other researchers (Lutz and Haque 1975; Sridharan and Sivapullaiah 1987; Hamissa 2000; Soule and Burns 2001; Roque and Didier 2006; Sunil et al. 2009; Rajeev and Kassim 2011) have shown that contaminants change the properties of their host soils, and this behavior has been shown to be dependent on the concentration of the contaminant solution.
Khoraminezhad and Alavi (2009) investigated the influence of phosphorus ions on the geotechnical properties of bentonite. Bentonite specimens were artificially contaminated by mixing the soil with di-ammonium phosphate (DAP) in concentrations ranging from 0.01 to 0.1 mol/l, and the geotechnical experiments such as compaction, unconfined compressive strength, and direct shear box were performed. The results indicate that increasing phosphate ion concentration in soil electrolyte may lead to an increase in cohesion and unconfined compressive strength of contaminated bentonite.
Nayak et al. (2007) performed an extensive laboratory testing program to study the immediate effect of leachate contamination on the properties of lateritic soil (the compaction characteristics and hydraulic conductivity). Contaminated specimens were prepared by mixing the lateritic soil with leachate in the amount of 5, 10, and 20 % by weight to vary the degree of contamination. The results indicated a small reduction in maximum dry density and an increase in hydraulic conductivity due to leachate contamination.
Saussaye et al. (2014) studied the influence of sulfate, chloride, nitrate, and phosphate ions, introduced as CaSO4.2H2O, NaCl, NH4NO3, and KH2PO4, on the physical and mechanical properties of a treated soil under accelerated curing conditions. The results showed that only phosphate ions have a deleterious effect on the stabilization. The combination of one anion with sulfate ion induces important disturbances on both volumetric swelling and indirect tensile strength.
Fernandez and Quigley (1985) studied the effect of simple hydrocarbon liquids on the hydraulic conductivity of natural clay. The natural soils used for their study were obtained from the brown, oxidized, surface crust of the thick clay deposits at Sarnia, Ontario. The test fluids were selected to present a range of dielectric constants from 80 (polar water) to 2 (nonpolar benzene). The hydraulic conductivity testing equipment generates a constant flow rate, q, which could be varied from 6 × 10−6 to 1 × 10−2 mL/s. Two different soil placement procedures have been used depending on whether water or hydrocarbon is presented in the soil samples.
The results were presented after the equilibrium condition had occurred and showed that when clay was mixed with pure liquids which dielectric constant decreased from 80 to 2 at a void ratio of 0.8 yields to an increase in hydraulic conductivity values from 5 × 10−9 to 1 × 10−4 cm/s. Also for water-wet samples (k = 10−8 cm/s), there is no change in hydraulic conductivity while permeated with water-insoluble hydrocarbons of low dielectric constant as benzene, cyclohexane, and xylene.
Fernandez and Quigley (1988) described hydraulic conductivity studies which were carried out on water-compacted natural clayey soil permeated with aqueous leachate solutions containing high amounts of organic liquids (ethanol and dioxane) that may increase the hydraulic conductivity, k, of water-saturated clay soil leading to destroying its effectiveness as barriers for contaminants. The two main objectives of the testing program were to determine the threshold concentrations of these water-soluble organics necessary to produce a significant increase in k and to test the dry soil mixed with pure organics. The permeant viscosity plays an important role leading to the decrease in k for dilute to moderate concentrations of organics soluble in water.
The permeation of the soil samples was carried out using the constant volumetric flow rate hydraulic conductivity equipment described by Fernandez and Quigley (1985). Static effective stresses of about 160 KPa were applied on two control test specimens by means of a spring-loaded device acting on the soil surface. The kinematic viscosities of the permeant mixtures were measured using a Cannon-Fenske routine viscometer. The viscosity of the solutions increases with the increase of the content of organics, reaching maximum values at 40 % ethanol and 60 % dioxane. All test results were carried out on clay samples molded with water and compacted by kneading to simulate the real compacted liner conditions. Results indicate that aqueous solutions of water-soluble ethanol and dioxane appear to have a little adverse effect on hydraulic conductivity at concentrations up to 70 %. Also, the decrease in k occurs in the dilute concentration range and explained by the increase in permeant viscosity.
Because petrochemicals and transport fuel pollutants can alter the consolidation characteristics of clayey soil causing an increase in the total or differential settlements of the foundations of engineering structures resting on it, Di Matteo et al. (2011) investigate the consolidation characteristics of commercial kaolinite clay which artificially contaminated with ethanol-gasoline blends. The clay which was used in this study was extracted from the Vimianzo quarry (Galicia, northwest Spain). The mineral composition of the clay was determined by X-ray powder diffraction, and the settlement properties of contaminated kaolinite clay were studied by a standard tecnotest fixed-ring odometer, following the ASTM D 2435 procedure. Test results indicated that, as the fraction of ethanol in blends increases, the equivalent liquid limit of soil decreases, showing a dividing point for blends containing about 85 % of ethanol. Also, results indicated that a minimum of compression index for kaolinite is reached when water is used as pore fluid (the structure of the kaolinite leads to parallel-arrayed clay).
Spagnoli et al. (2012) performed several laboratory tests (Atterberg limits, vane shear test, and sedimentation test) on kaolinite and Na-smectite mixed with pore fluids with different pH values. For Atterberg limits, the liquid limit for Kaolinite has not changed with the change of pH values. For Na-smectite, there is a variation of the liquid limit as a function of pore water composition and pH. The results also show an increase in undrained shear strength for kaolinite at high and low pH; this effect depends on consistency values (IC). In contrast, the Na-smectite seems not to be affected by the pH variations. For sedimentation test for both clays, the increase of electrolyte concentration accelerates the sedimentation processes.
This paper presents a framework of a laboratory testing program carried out to determine the short-term effect of phosphate contamination on the hydraulic conductivity, compaction characteristics, and shear strength parameters of three types of soils. These types include coarse sand, fine to medium sand, and silty clay. To select a representative fertilizer used in agricultural lands, a database is prepared from published literature in the FAO report (2005) of the production, imports, exports, and consumption of fertilizers in the period between 1998 and 2002 in Egypt as shown in Fig. 2. The fertilizer which is used in the laboratory tests is di-ammonium phosphate fertilizer (NH4)2HPO4.
Production, imports, exports, and consumption of nitrogen, phosphate, and potash. Source: Ministry of Agricultural and Land Reclamation (MALR), Egypt 2003
Scope of the problem
The extensive use of phosphate fertilizers in agriculture followed by the massive amount of irrigation water leads to deep percolation of the residual amount which has not been taken by the plants’ roots. This residual amount of fertilizers causes hazards to the surrounding soil. From geotechnical engineers’ point of view, any contaminants affect the geotechnical properties of the soil.
Methodology
The main focus of this study is to determine the effect of agricultural contamination especially from phosphate fertilizers on Atterberg limits; liquid limits, plastic limits and plasticity index, hydraulic conductivity, and compaction characteristics; max dry density and the optimum moisture content, and shear strength parameters; and cohesion and angle of shearing resistance for the selected three soil samples. To attain the study objectives, the following steps are followed:
-
a)
Selecting soil samples from three sources and studying their geotechnical characteristics which are affected by the presence of phosphate contaminant which is applied to the land as a type of a fertilizer
-
b)
Preparing phosphate solutions with different concentrations which are 5, 10, and 20 % to represent the observed concentrations in real case in the Egyptian agricultural lands
Soil
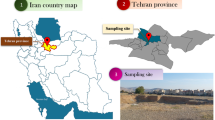
Representative soil samples from the three sources are obtained from test pits of 0.25 m, two samples are coarse sand and fine to medium sand from Bir al-Abd area near El-Arish city in Sinai in Egypt, and the third one is silty clay from Sahl El-Tena area near Qantarah city in Egypt. Figures 3 and 4 shows the locations of these sites. The soil samples are dried in the oven at 105 °C before using for the laboratory tests. Table 1 shows the main physical characteristics of uncontaminated soil samples. To vary the degree of phosphate contamination, the dry soil samples are mixed with 20 % water content with phosphate solution concentrations varying between 5, 10, and 20 %. Compaction, Atterberg limits, hydraulic conductivity, and shear strength tests are carried out on soil samples after 24 h to investigate the short-term effect of di-ammonium phosphate.
Sieve analysis
This method is applicable for soils that are mostly granular. It was done on the coarse sand and fine to medium sand soils. The sample is about 500 g. A stake of sieves was prepared in which their numbers are 4, 8, 16, 30, 50, 100, and 200. The sieve no. 200 was at the bottom and the pan was placed under it.
Hydrometer analysis for silty clay
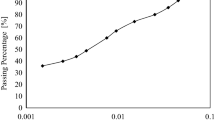
It is a procedure adopted for determination of the particle-size distribution in silty clay soil for the fraction that is finer than 0.075 mm. Fifty grams of oven-dried soil was prepared for the test. The deflocculating agent was 4 % solution of sodium hexametaphosphate. Then, take 125 cm3 of the mixture and add it to the soil and allow to soak for about 16 h. The soil preparation is shown in Fig. 5. The hydrometer readings were taken at time t = 0.5, 1, 2, 4, 6, 8, 15, and 30 min. and 1, 2, 4, 8, and 24 h. For each reading, the hydrometer was inserted into the cylinder containing the soil-water suspension about 30 s before the reading, Fig. 6b shows the settle of soil particles at different times.
X-ray diffraction analysis for silty clay
The measurement conditions for the test were voltage = 40.0 (kV), current = 30.0 (mA), divergence slit = 1.000 (°), scatter slit = 1.000 (°), receiving slit = 0.300 (mm), drive axis = Theta-2Theta, scan range = 10.000–80.000 (°), scan mode = continuous scan, scan speed = 12.0000 (°/min), sampling pitch = 0.0200 (°), and preset time = 0.10 (s). The X-ray analyses of the uncontaminated and contaminated silty clay soil with 20 % di-ammonium phosphate are presented in Fig. 7a, b.
Di-ammonium hydrogen phosphate
With the purpose of selecting representative fertilizer used in agricultural activities in Egypt, DAP is selected as the most common commercial type available. When applied as plant food, it temporarily increases the soil pH because its average pH in solution is 7.5–8. The typical formulation is 18–46–0 (18 % N, 46 % P2O5, 0 % K2O).
Compaction characteristics
The compaction characteristics of coarse sand and fine to medium sand soils were performed in the laboratory using a standard proctor test to determine the maximum dry unit weight (γ dmax) and the optimum water content. The equipment used in the test consists of cylindrical mold which is 101.6 mm in diameter and 116.4 mm in height, as shown in Fig. 8. There is an extension that can be attached to the top of the mold. The volume of the mold is 943.9 cm3. Soil sample was put on three layers, each one compacted 25 times with a hummer which is 2.5 kg in weight and 51 mm in diameter and falling from a height of 30.5 cm.
Hydraulic conductivity
Falling head permeability tests were carried out on uncontaminated and contaminated soil samples to study the hydraulic conductivity for the three types of soil. After compaction of the soil, the collar was removed and the soil specimen was carefully trimmed. Before the beginning of the seepage stage, a filter paper was placed on each face of the soil specimen so as to prevent the clogging of the perforated disks by the soil fines. After placing the bottom and top plates, the nuts were fastened. Figure 9 shows the falling head permeability test setup. The soil is saturated by allowing distilled water to flow continuously through the sample from the stand pipe. Saturation of the soil sample is ensured under steady-state flow conditions which are
-
a)
Flow rate of water proportional to hydraulic gradient
-
b)
Volume of water proportional to time
The hydraulic conductivity reported in Table 2 is calculated by using the following equation:
where k is the coefficient of permeability in centimeter per second, a is cross-sectional area of the stand pipe in centimeter squared, A is cross-sectional area of the sample in centimeter squared, t is time taken for the drop from height h 1 to h 2 in seconds; h 1 is initial height of the fluid in stand pipe in centimeters, h 2 is final height of the fluid in stand pipe in centimeters after time t.
Results
Compaction characteristics change due to phosphate
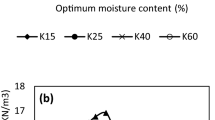
Standard proctor compaction tests are carried on sand samples after 24 h after mixing with 20 % constant water content with varying phosphate concentrations in the solution to be 5, 10, and 20 %. The results are plotted in Figs. 10 and 11 in the form of dry density versus water content curves. The maximum dry densities for the two soils are 16.91 and 18.63 kN/m3 at the optimum moisture content of 8 and 10 %, respectively, at phosphate concentration 0 %. With the presence of phosphate up to 5 %, the compaction characteristics significantly increased with γ dmax equals 17.51 and 19.62 kN/m3 at the optimum moisture content of 10 and 8 %, respectively. Further increase of phosphate content to 10 and 20 %, the compaction characteristics do not change much; this indicates that too much phosphate is already presented in the soil. Table 3 presents the compaction characteristics of uncontaminated and contaminated soil samples. Also, Fig. 12 shows the max dry densities of sand samples.
Hydraulic conductivity change due to phosphate
The phosphate used in the present study has an alkaline characteristic (pH = 7.5). Figure 13a–c shows the variation of the hydraulic conductivity with percentage of phosphate added to the three soil samples. With the increase of phosphate concentration, the hydraulic conductivities of coarse sand and fine to medium sand decrease when compared to their base values (uncontaminated). In contrast, the hydraulic conductivity of silty clay soil increases with the increase of phosphate concentration. This is due to attribution of chemical reaction between the alkaline contaminant and the clay minerals. It is reported that strongly alkaline liquids can dissolve clay minerals (Uppot and Stephenson 1989).The dissolution of clay mineral particles by alkaline liquids increases the effective pore space and the hydraulic conductivity increases.
Photomicrographs (Fig. 14a, b) of silty clay soil are performed for clean and contaminated samples. The main elements of the micro-structure are voids and aggregates of particles. The structure of the contaminated soil sample is appeared as aggregated in laser scanning microscope analysis. The aggregated structure increases the effective pore space and thus increases the hydraulic conductivity. Sunil et al. (2006) indicate that strongly alkaline solutions lead to the destruction of soil structure as the particles clog the pore space. When the dissolution progress in the zones of clogging has occurred, the particles will be removed and the hydraulic conductivity will be increased.
Effect of phosphate on Atterberg limits of silty clay
It is widely accepted that liquid and plastic limit tests are considered very important geotechnical tests which can give valuable information about the engineering behavior of fine grained soils. The liquid limit is also an indicator of the quantity of water held as double-layer water. Sridharan et al. (1986, 1988) reported that the change in the liquid limit is related to the change in the double-layer thickness.
The Atterberg limits of soil are determined using the standard liquid limit apparatus as shown in Fig. 15 according to the Egyptian standard. Figure 16 illustrates the variation of liquid limit (W L), plastic limit (W P), and plasticity index (P.I) of silty clay soil. It is observed that the phosphate has a significant effect on the Atterberg limits of silty clay. The liquid and plastic limits of silty clay decrease with the increase of phosphate added. As shown in Table 4, the liquid limit (W L) of the soil has decreased from 30.53 to 21.11 % (when phosphate concentration in the solution increased from 0 to 20 %). Similar trend is observed for plastic limit (W P). It has decreased from 15.34 to 11.15 % in addition to the reduction of the plasticity index (I.p) from 15.19 to 9.96 %.
Effect of phosphate on shear strength characteristics
The shear test is performed for the three soil samples according to the Egyptian code of soil mechanics and foundation design to calculate the shear strength parameters and how the phosphate contaminant affects them. Table 5 shows the shear strength parameters for clean and contaminated soil. For silty clay soil, there is a slight decrease in cohesion and increase in friction angle because of the reduction in clay content after the interaction with the phosphate solution.
Direct shear test on sand
Figure 17 shows a direct shear test machine. It consists mainly of a direct shear box, which is split into two valves (top and bottom) and holds the soil specimen, a proving ring to measure the horizontal load applied to the specimen, and a yoke which the vertical load can be applied to the soil specimen. A horizontal load to the top half of the shear box is applied by a motor. The rate of the movement of the top half of the shear box has been adjusted to be 1.0 mm/min.
Sample preparation
Firstly, all sand samples were dried in the oven at 105 °C before using for the test. Three phosphate solutions were prepared with different concentrations varying between 5, 10, and 20 %. Then, add the constant amount of solution (20 % of the dry weight of the soil). Finally, mix the soil with the solution to be completely homogeneous.
Test procedure
Remove the shear box assembly and the loading head. Insert the two vertical pins to keep the two halves of the shear box together. Fill known volume with sand in layers and use a tamper to compact the sand layers then weight the sand to calculate the dry density. Determine the dimensions of the soil specimen (length L, width B, and height). By knowing the volume of soil specimen, the required weight, W, can be calculated according to the dry. Slip the loading head down from the top of the shear box to rest on the soil specimen. Put the shear box assembly in place in the direct shear machine. Apply the desired normal load, N, on the specimen by hanging dead weights to the vertical load yoke then remove the two pins to release the two halves of the shear box. Apply the horizontal load, S, to the top half of the shear box and record the reading of the proving ring gauge till; the proving ring dial gauge reaches the maximum and then falls, or remains constant. The test was repeated three times with different loads with constant dry unit weight of compaction of the sand specimen.
Calculation
-
Calculate the void ratio of the specimen
where G s is the specific gravity of the soil and γ w is the unit weight of water.
-
Calculate the normal stress, σ, on the specimen
-
Calculate the shear stress τ, as:
-
Plot the graph of shear strength, S, vs. normal stress σ. This graph will be straight line passing through the origin, and the angle of friction, φ, of the soil can be determined from the slope of the line.
Triaxial test on silty clay
The consolidated-undrained (C-U) test was performed for the silty clay sample in order to calculate cohesion and angle of friction of silty clay soil. In this test, firstly, the soil is compacted under the surrounding pressure allowing the excess water to flow then the test performed without allowing excess water to drain from the soil sample. The triaxial specimen used is about 71.1 mm diameter × 165.1 mm length. The initial length (L 0) was measured four times and the average of these four values taken; also, the diameter (D 0) was measured twelve times four at top, four at middle, and four at bottom of the specimen and the average taken.
The triaxial cell was placed on the compression machine and the adjustment made so that the piston of the cell made contact with the top platen of the specimen. The chamber of the triaxial cell was filled with water. Apply the hydrostatic pressure σ 3 with strain rate of compression machine about 0.5 %.
The experimental results for the friction angle of coarse sand, fine to medium sand, and silty clay are plotted in Fig. 18. The change in the friction angle values are also tabulated in Table 6.
Discussion
The preceding results present the short-term effect of (DAP) on the hydraulic conductivity, compaction characteristics, and shear strength parameters of sand soils and on the hydraulic conductivity, Atterberg limits, and shear strength parameters of silty clay soil by using batch tests. The results indicate that about 70 % reduction in hydraulic conductivity for sand soil samples and the values for hydraulic conductivity of silty clay are quintuple with increasing the phosphate contaminant up to 20 %. In addition, the phosphate contaminant significantly affects Atterberg limits for silty clay soil to decrease about 30 %. However, the effect is not significant on the compaction characteristics for both coarse sand and fine to medium sand.
Compaction characteristics for sand samples
The increase in the maximum dry density reflects the effect caused by chemical reaction (due to change in the nature of the pore fluid) between the solution and the soil. Hence, it is anticipated that in the present study, the increase in maximum dry density is due to the chemical reaction between the alkaline solution and the soil. At 20 % concentration, too much phosphate is already presented in soil which can cause more chemical reaction between the alkaline solution and soil particles.
Hydraulic conductivity for sand
The solution used in the present study has an alkaline characteristic (pH = 7.5). Figure 13a, b shows the hydraulic conductivity of the soil decreased when compared to its base value (without contamination). With the increase of phosphate concentration, the hydraulic conductivity of the sand soil decreased. Because the alkaline solutions affect the soil particles to be finer, thus, the decrease in the effective pore space leads to reduction in the hydraulic conductivity.
Shear test analysis for sand
The friction angle of two sand samples was measured in direct shear test, and the results show that the friction angle is decreased while increasing the concentration of phosphate in the solutions as shown in Fig. 18. The friction angle of soil is affected by particle roundness and particle size (lower roundness or larger median particle size results in larger φ′). Because of the added minerals (ammonium and phosphate) to the sand, it has a lower sliding frictional resistance compared to that of sand particle formations; the friction angle is decreased especially at the higher concentration of ammonium and phosphate.
Atterberg limits for silty clay
The liquid limit is an indicator of the quantity of water held as double-layer water; Sridharan et al. (1986, 1988) reported that the change in the liquid limit is related to the change in the double-layer thickness. While the LL is 30.53 % at 0 % phosphate, it drops to 21.11 % at 20 % phosphate. The (HPO4)−2 and the (NH4)+1 ions narrow the double diffusion layer (DDL), causing clay particles to aggregate and hence leading to more compact structure for the clay particles and reduces the amount of pore fluid necessary to induce particle mobilization.
Hydraulic conductivity for silty clay
On the basis of Gouy theory and the observed fabric of the soils under the laser scanning microscope, it is concluded that for a given void ratio, the high hydraulic conductivity values are directly related to low dielectric constant Є values. That causes double-layer contraction, during mixing and formation of floccs or peds with microchannels and macropores between them leading the silty clay soil to agglomerates into sand-sized peds causing an increase in the measured hydraulic conductivity.
Shear strength parameters for silty clay
The results in Table 5 show that the decrease in undrained shear strength (cohesion) occurs for silty clay while increasing the concentration of the di-ammonium phosphate in the solution. A possible explanation for this behavior could be the face-to-face (FF) flocculation which occurs at pH values higher than 7 (in case of di-ammonium phosphate) (Brandenburg and Lagaly 1988), and due to the increase of H+ concentration, the material becomes slightly stiffer than when the pores were occupied with water only. For lower values of pH for pH = 4, the material flocculates in the edge-to-face (EF) mode (Brandenburg and Lagaly 1988).
Conclusions
To study the effect of di-ammonium phosphate fertilizer contamination on the properties of sand and clay soils, an extensive laboratory testing program is carried out. To vary the degree of contamination, the amount of di-ammonium phosphate mixed varied from 5 to 20 % to change the concentration of the solution. The following conclusions can be made based on test results:
-
The compaction characteristics and hydraulic conductivity of soil are likely to get altered due to chemical reaction.
-
With the increase of di-ammonium phosphate contaminants, the compaction characteristics of coarse sand and fine to medium sand are increased but insignificantly.
-
The hydraulic conductivities of sand samples are decreased with the increase of the di-ammonium phosphate concentrations, in contrast with silty clay as the hydraulic conductivity increases with the increase of phosphate contamination.
-
For coarse sand, the hydraulic conductivity significantly decreased at 5 % phosphate concentration and then reduced slightly with phosphate concentration up to 20 %, the same for fine to medium sand;with 10 % di-ammonium phosphate added, there is a sudden drop in the hydraulic conductivity values.
-
For silty clay soil, the increase of hydraulic conductivity after 10 % phosphate was not significant; this indicates the excess of phosphate in the soil.
-
The low di-ammonium phosphate concentration at 5 % affects the liquid and plastic limits of silty clay more than the higher values at 10 and 20 %.
-
Generally, shear strength parameters of tested soils are affected by di-ammonium phosphate contamination. Especially for sand samples tested, there is a reduction in friction angle with the increase of di-ammonium phosphate contaminants. In contrast with silty clay soil, the friction angle increases and cohesion decreases with the increase of di-ammonium phosphate concentration in the solution.
References
Brandenburg U, Lagaly G (1988) Rheological properties of sodium montmorillonite dispersions. Appl Clay Sci 3:263–279
Di Matteo L, Bigotti F, Ricco R (2011) Compressibility of kaolinitic clay contaminated by ethanol-gasoline blends. J Geotech Geoenviron Eng ASCE 137(9):846–849
FAO (2005) Fertilizer use by crop in Egypt. First Version, Rome, Italy
Fernandez F, Quigley RM (1985) Hydraulic conductivity of natural clays permeated with simple liquid hydrocarbons. Can Geotech J 22(2):205–214
Fernandez F, Quigley RM (1988) Viscosity and dielectric constant controls on the hydraulic conductivity of clayey soils permeated with water-soluble organics. Can Geotech J 25(3):582–589
Hamissa MR (2000) Fertilizer use in Egypt. Egyptian Soil Science Society (ESSS), Golden Jubilee Congress 1950–2000, on soil and sustainable agriculture in the New Century, Cairo
Khoraminezhad B, Alavi Moghaddam MR, Bayat HO (2009) Investigating effects of phosphorus ions on geotechnical properties of bentonite. 8th International Congress on Civil Engineering, Shiraz, Iran
Lutz JF, Haque I (1975) Effects of phosphates on some physical and chemical properties of clays. Soil Sci Soc Am J 39(1):33–36
MALR (2003) Bulletin of agriculture economics, central administration of agriculture economics, ministry of agriculture and land reclamation, published data
Nayak S, Sunil BM, Shrihari S (2007) Hydraulic and compaction characteristics of leachate-contaminated lateritic soil. J Eng Geol UK 94:137–144
Rajeev Kumar P, Kassim KAB (2011) Effect of chemical byproducts on the plasticity and permeability characteristics of a silty soil. 14th Asian regional conference on soil mechanics and geotechnical engineering, Hong Kong, China
Roque AJ, Didier G (2006) Calculating hydraulic conductivity of fine grained soils to leachates using linear expressions. J Eng Geol UK 85(1):147–157
Saussaye L, Boutouil M, Baraud F, Leleyter L (2014) Influence of anions on the geotechnical properties of soils treated with hydraulic binders: individual and coupling effects. J Constr Build Mater 65(29):303–309
Soule NM, Burns SE (2001) Effects of organic cation structure on behavior of organobentonites. J Geotech Geoenviron Eng ASCE 127(4):363–370
Spagnoli G, Rubinos D, Stanjek H, Fernandez-Steeger T, Feinendegen M, Azzam R (2012) Undrained shear strength of clays as modified by pH variations. Bull Eng Geol Environ 71(1):135–148
Sridharan A, Sivapullaiah PV (1987) Engineering behavior of soils contaminated with different pollutants. In: 3rd international symposium environmental geotechniques and problematic soils and rocks. AIT, Bangkok
Sridharan A, Rao SM, Murthy NS (1986) Liquid limit of montmorillonite soils. Geotech Test J 9(3):156–159
Sridharan A, Rao SM, Murthy NS (1988) Liquid limit of kaolinitic soils. Geotechnique 38(2):191–198
Sunil BM, Sitaram N, Shrihari S (2006) Effect of pH on the geotechnical properties of laterite. J Eng Geol UK 85(1):197–203
Sunil BM, Shrihari S, Sitaram N (2009) Shear strength characteristics and chemical characteristics of leachate-contaminated lateritic soil. J Eng Geol 106:20–25
Taha MH (2000) Soil fertility management in Egypt. Regional workshop on soil fertility management through farmer field schools in the Near East, Amman, Jordan
Uppot JO, Stephenson RW (1989) Permeability of clays under organic permeants. J Geotech Eng ASCE 115(1):115–131
Acknowledgments
The first author would like to thank the Egyptian Ministry of Higher Education (MoHE) for providing him the financial support (PhD scholarship) for this research as well as the Egypt-Japan University of Science and Technology (E-JUST) and JICA for offering the facilities and tools needed to conduct this work and also the faculty of Engineering, Alexandria University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eltarabily, M.G.A., Negm, A.M., Saavedra Valeriano, O.C. et al. Effects of di-ammonium phosphate on hydraulic, compaction, and shear strength characteristic of sand and clay soils. Arab J Geosci 8, 10419–10432 (2015). https://doi.org/10.1007/s12517-015-1959-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-015-1959-4