Abstract
Purpose of Review
In hypertrophic cardiomyopathy (HCM), the presence of vessel abnormalities with microvascular dysfunction is a well-known feature. The aim of this review is to present the evidences that support this notion and to describe the physiopathologic and clinical consequences of microvascular dysfunction.
Recent Findings
After having demonstrated the presence and significance of microvascular dysfunction in HCM, the myocardial blood flow (MBF) measurement by positron emission tomography (PET) is ready to develop into a clinical tool for disease evaluation, in particular for patient prognostication. Alternative methods are becoming available, but they are not yet equally reliable.
Summary
By means of MBF measurement using quantitative PET, the importance of microvascular dysfunction in HCM has been clearly demonstrated, explaining the chest pain and the ischemic abnormalities frequently registered in this disease. The physiopathologic implications of microvascular dysfunction have been established, and an increasing number of reports indicate that the assessment of microvascular dysfunction is as well important for patient characterization and prognostication. Most recently, perfusion measurements using magnetic resonance imaging have been used, but their role is still under debate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most frequent genetic myocardial disease and its prevalence ranges approximately between 0.16 and 0.29% of the adult population [1,2,3]. The diagnosis is based on the detection of left ventricular (LV) hypertrophy, defined as a diastolic wall thickness > 13 mm on echocardiography or cardiac magnetic resonance (CMR) in the absence of other recognizable causes of secondary hypertrophy [1, 4, 5, 6•]. More stringent criteria, as the ≥ 15-mm thickness proposed by the ESC, could reduce the number of hypertrophic patients identified as HCM [7]. Most probably, however, the rate of HCM diagnosis within the population is going to increase, because of the more extensive familiar screening, and the wider use of genetic testing and more sensitive diagnostic modalities, so that prevalence as high as 0.6% has been foreseen [8]. HCM is an autosomal, single-gene disease, with dominant inheritance pattern, but with a very wide range of phenotypic expression, caused by the multiple possible mutations of the causal genes, and by several nongenetic influences [9,10,11,12,13].
The phenotypic expression of HCM is LV hypertrophy, with its histologic pattern of myocyte disarray, interstitial fibrosis, and thickening and narrowing of the intramural coronary arteries [14,15,16,17]. The consequent impairment of diastolic function and the possible obstruction of the LV outflow tract (OT) have been considered the main physiopathologic features [18–19]. The clinical expression of the disease can be absent or minimal for many years. Hypertrophy eventually causes symptoms, related to the diastolic dysfunction, the LVOT obstruction, and the imbalance between oxygen supply and demand [20,21,22]. In particular, effort dyspnea and chest pain are frequent initial complaints [3,4,5, 6•, 7, 22, 23]. Left atrial dilatation and atrial fibrillation are also possible [24,25,26]. Furthermore, all these factors (hypertrophy, LVOT obstruction, cellular disarray, fibrosis, and microvascular impairment) concur in causing various arrhythmias, syncope, and (arrhythmic) sudden death, which is the most feared clinical manifestation of HCM, particularly menacing in young people [27,28,29,30,31]. A possible late evolution in a minority of cases is systolic heart failure [32].
Indirect Evidences of Microvascular Involvement in HCM
In this quite heterogeneous scenario of anatomical, physiopathologic, and clinical manifestations, the importance of vascular abnormalities and myocardial ischemia in HCM has constantly increased since the time of their first detection [16, 33]. According to the different modalities and criteria used to define the presence of ischemia in HCM, its incidence ranges from approximately 20% to over 60% of patients, with an average just below 50% [34••]. The structural abnormalities in the small vessels of HCM patients, such as vessel wall thickening, with prevalent media participation and lumen restriction, and reduction in capillary density, have been convincingly demonstrated [16, 17, 35,36,37,38]. Together with the direct vessel involvement, other tissutal disease-related abnormalities, such as fibrosis, may contribute to ischemia in HCM [15, 39, 40]. Autoptic studies had identified signs of prior necrosis in HCM patients [33, 41, 42]. The relatively frequent occurrence of chest pain is well known, and several reports demonstrated the presence of myocardial perfusion abnormalities [23, 43,44,45,46,47,48]. These abnormalities were initially related to the presence of epicardial coronary artery disease, and the current guidelines still include the use of myocardial perfusion imaging for ruling out coronary artery disease in HCM [5]. However, the negligible role of epicardial vessel stenosis in causing the ischemic changes and symptoms in HCM patients was clear very early. Already in 1979, Rubin et al. had tested the usefulness of thallium-201 for ruling out coronary artery disease as the cause of anginal symptoms in HCM: in their study, 9/10 patients with normal coronary angiograms had indeed normal stress perfusion, but one showed a significant exercise-induced defect [43]. One year later, Pitcher et al. identified various patterns of stress and rest perfusion abnormalities in thallium-201 scans performed in HCM patients, who had normal vessels in coronary angiography in the large majority of cases [44]. In a larger patient cohort, thallium-201 single-photon emission computed tomography (SPECT) registered perfusion abnormalities, mainly stress-induced, in 41 of 72 patients, and the authors interpreted the data as the demonstration of a dynamic ischemic process that contributed to the clinical symptoms [45]. The concept was reinforced by another study that showed the reduction of stress-induced perfusion defects in HCM patients after verapamil therapy [46]. Cannon et al. demonstrated that the stress-induced perfusion defects were related to the occurrence of true ischemia, as shown by lactate extraction, in the majority of cases, and for the first time suggested a more severe impairment of the subendocardium, because of the several patients with transient ischemic dilation [47]. The importance of subendocardial ischemia in HCM was also emphasized by a study by Yoshida et al., who identified a close relation between the occurrence of transient ischemic dilatation and an abnormal blood pressure response to exercise [48].
Demonstration of Microvascular Disease in HCM
Taken together, all these studies converged in indicating microvascular disease as the main determinant of perfusion abnormalities in HCM. However, a direct evidence of abnormal myocardial blood flow (MBF) without epicardial vessel disease in these patients could be achieved only by performing the quantitative measurement of MBF by means of perfusion positron emission tomography (PET) [49] (Figs. 1 and 2). This imaging modality is nowadays getting a growing role in the clinical arena, also because of the wider diffusion of PET facilities and the major technical advances, but for many years, its main contribution was to help expanding our knowledge of disease physiopathology, and in this regard, HCM was a favorite field of utilization [50]. Camici et al. published the first demonstration that patients with HCM had an abnormal response to coronary vasodilation in 1991 [51]. While the finding of impaired maximal MBF in hypertrophied walls could be expected on the basis of the abovementioned anatomical abnormalities, already in this very early report, the complexity of microvascular involvement in HCM was apparent, because the authors found some degree of impairment even in the normal, nonhypertrophied walls [51]. Therefore, the study supported the concept that microvascular dysfunction is an early phenomenon in HCM and not just a late consequence of the anatomical changes, in particular fibrotic substitution [51]. The same group published various other studies in HCM patients aimed to confirm and better delineate the role of microvascular dysfunction in the disease. Gistri et al. demonstrated that verapamil did not significantly improved maximal MBF in HCM as compared to placebo; however, it favorably affected subendocardial perfusion, in partial agreement with the visual changes detected by thallium-201 SPECT [46, 52]. This finding was confirmed by a later study that showed an improvement in the subendocardium/subepicardium MBF ratio after verapamil treatment, although the average wall value was not significantly modified [53]. Lorenzoni et al. showed that there was no significant difference in resting and maximal MBF, and in myocardial flow reserve (MFR) in HCM patients with versus those without history of syncope or of nonsustained ventricular tachycardia [54]. Conversely, the same authors demonstrated that HCM patients with systolic dysfunction had a lower maximal MBF and MFR, and that there was an inverse relation between these parameters and the severity of the New York Heart Association class [55]. These reports suggested a potential clinical role of the assessment of microvascular dysfunction using quantitative PET for evaluating HCM severity and for patient prognostication [55]. Indeed, just few years later, the first demonstration of the clinical value of microvascular dysfunction assessment was published, as reported below, contributing to establishing the role of ischemia in HCM [22]. The large majority of the studies devoted to the definition of microvascular involvement, however, were still focused to improve the physiopathologic characterization of HCM.
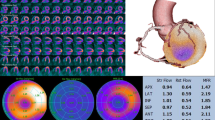
Images of an asymptomatic male patient with HCM (septal thickness 22 mm) and dynamic LVOT obstruction, without demonstrated genetic abnormalities. Left panel: CMR imaging findings of limited patchy LGE at the anterior septal junction (yellow arrow). Middle panel: stress (regadenoson) 13NH3 perfusion PET showing slightly reduced uptake in the same location (white arrows). Right panel: stress parametric perfusion images with demonstration of an extensive maximal MBF reduction, involving the antero-septal wall, with prevalent impairment of the subendocardial layer (in the color scale the maximum—yellow—is set at 2.5 mL/min/g of tissue) (arrowheads)
Images of a female patient with history of effort chest pain and previous normal perfusion scintigraphy, with positive genetics for HCM. CMR imaging (left and middle upper panels) shows borderline septal and apical thickness. Coronary CT angiography demonstrates completely normal epicardial coronary vessels (right upper panel). Because of persistent symptoms, 13NH3 perfusion PET has been performed showing normal resting perfusion (left lower panel), but a severe apical perfusion defect during dipyridamole stress (middle panel, arrows), and a remarkable impairment of maximal MBF in the apex and the septal subendocardium in the stress parametric perfusion images (in the color scale the maximum—yellow—is set at 2.5 mL/min/g of tissue) (right lower panel, arrowheads)
Features of Microvascular Dysfunction in HCM
After that the causative role of microvascular dysfunction for ischemic symptoms and signs in HCM was demonstrated, other studies aimed at defining its features and meaning within the disease spectrum. Tadamura et al. published a paper showing that abnormalities in maximal MBF and MFR can be registered early in HCM pediatric patients (mean age 13 years), therefore confirming the initial role of microvascular abnormalities in the disease [56]. The importance of microvascular dysfunction in HCM was later confirmed at the other end of the disease spectrum: Ohba et al. demonstrated that the MFR is more impaired in HCM patients in the end-stage dilated phase than in patients with dilated cardiomyopathy [57]. Other studies were devoted to better understand the spatial distribution of MVD in HCM. For this aim, CMR imaging, and in particular the detection of late gadolinium enhancement (LGE) after gadolinium injection became a most useful tool for studying the site and the extent of tissutal abnormalities in HCM, besides being the reference standard for HCM diagnosis and prognostication (Fig. 1) [58]. The detection of LGE was considered as a sign of fibrotic scarring and almost immediately translated in an adverse prognostic indicator for HCM [59, 60]. Knaapen et al. demonstrated that also resting MBF can be abnormal in HCM patients, and that these abnormalities are related to the presence of LGE, but not to detectable reductions in systolic function [61]. Although the early involvement of the interventricular septum is an established feature of the disease, Sotgia et al. showed that there is a progressive impairment of the microvascular circulation that is minimal in the farther segments and intermediate in those adjacent to the septum, apparently preceding the insurgence of LGE [62]. More recently, the possibility that other causes together with microvascular dysfunction contribute to the occurrence of LGE has been suggested, because of the demonstration of patients with LGE in the absence of MBF abnormalities [63]. Knaapen et al. registered a more severe impairment of the subendocardial layers, which they also connected to the presence of LVOT obstruction [64]. Others have most recently confirmed this observation in a cohort without significant LVOT obstruction, therefore supporting a central role of microvascular dysfunction as compared to compressive forces in producing this effect [65]. This interpretation is in agreement with the observation that maximal wall thickness is more important than LVOT obstruction for determining the severity of maximal MBF impairment [66]. Abnormalities in microvascular circulation are possible causes of the decreased metabolic efficiency in HCM patients, and probably contribute to the increase the contractile dysfunction beyond the effect of myocardial injuries [67, 68]. On the other hand, there is a relationship between the presence of decreased maximal MBF and the occurrence of atrial fibrillation [69]. Olivotto et al. demonstrated that some genotypes present with a more severe MBF impairment [70]. Another study indicated the importance of the interaction between genotype and phenotype by identifying carriers of a specific mutation, who do not show microvascular impairment, but have already signs of reduced metabolic efficiency [71].
Recent reports using both quantitative and gated PET demonstrate that microvascular dysfunction, and in particular the more severe impairment of the subendocardium, can play a role in the development of an abnormal and likely ischemic functional response. Bravo et al. were the first to show a relationship between the degree of maximal MBF impairment and the presence of abnormal LV ejection fraction (EF) response [66]. The same authors registered a high proportion of HCM patients with transient ischemic dilation, and a more severe MBF abnormality than in those with normal volumetric response [72]. Our group detected a higher prevalence of subendocardial MBF impairment in the HCM patients with significant LVEF drop after vasodilator stress [73]. Yalcin et al. confirmed these findings, indicating subendocardial impaired MBF as the cause of transient ischemic dilation in a percentage of HCM patients and showed a lower LVEF in these subjects [74]. Another field in which the assessment of microvascular dysfunction has been connected to clinics is the evaluation of changes produced by therapeutic interventions in HCM. As already mentioned, very limited results have been reached using calcium channel blockers in terms of improved microvascular reactivity, although they are indicated in patients who do not tolerate beta-blockers [46, 52, 53]. A significant objective increase in maximal MBF has been on the other hand demonstrated as an additional favorable effect in HCM patients with LVOT obstruction submitted to myectomy or septal ablation [75,76,77].
Clinical Meaning of Microvascular Dysfunction in HCM
Together with their pathophysiologic implications, these last studies show that the assessment of microvascular dysfunction could have relevance for the clinical characterization of HCM patients and that microvascular dysfunction severity could be an important predictive parameter. This concept is supported by the adverse prognostic meaning of perfusion defects detected by SPECT myocardial perfusion imaging, mostly in the absence of epicardial coronary artery disease, in HCM patients [78]. The prognostic value of a blunted vasodilator response as demonstrated by quantitative PET was clearly proved in the most important paper by Cecchi et al., who showed that the outcome of HCM patients after a long-term follow-up was significantly related to their level of maximal MBF at the moment of enrolment, with a 9.6 hazard ratio for death from cardiovascular causes and a 20.1 hazard ratio for any unfavorable event in the lowest tertile as compared to the patients in the two other tertiles [79]. In the same cohort, the authors also confirmed the tight relationship between severely blunted maximal MBF and the occurrence of end-stage systolic heart failure in HCM patients [80]. Although these papers have been extensively cited as the demonstration that maximal MBF impairment is a valuable prognostic indicator, the clinical scenario of HCM has remarkably changed since the late 1990s, and nowadays, most HCM patients have a relatively uneventful course [30, 31, 81]. However, a latest paper has confirmed the value of maximal MBF as a prognostic indicator even in the presence of an extremely small number of events during a more than 4-year follow-up [82•]. Interestingly, higher overall values of maximal MBF were registered and there was a clear increase in the upper threshold of the highest risk group; accordingly, patients who would have been classified as low risk in the Cecchi’s study would be now in the lowest, highest risk tertile [82•]. Another new result was the demonstration that the presence of more diffuse microvascular involvement, showed by lower values of maximal MBF in the nonhypertrophied lateral wall, was the most powerful predictor of cardiovascular death in this series [82•]. Finally, it is of note that young patients with extreme septal hypertrophy, so far regarded as a high-risk group, present with a very wide variability of maximal MBF values, so that the predictive value of septal thickness alone could be questioned [82•]. With regard to the prediction of death in HCM, attention has been given to the capability of identifying patients with increased risk of severe ventricular arrhythmias. Old studies using myocardial perfusion imaging with thallium-201 had suggested that there was a higher prevalence of signs of ischemia in HCM patients with severe arrhythmic episodes, and that verapamil and beta-blockers had a favorable effect [83]. On the other hand, PET data had suggested that the maximal MBF in absolute terms was unable to differentiate them [54]. Most recently, however, a more refined parameter, the maximal MBF heterogeneity, seems able to help identifying the patients at higher risk of sustained and nonsustained ventricular tachycardia [84]. Unfortunately, clinical studies dealing with the capability of microvascular dysfunction assessment by quantitative PET to identify HCM patients with high-risk profile for menacing arrhythmias are lacking.
Another field for potential clinical applications of microvascular dysfunction evaluation is the assessment of treatment effectiveness, in particular since various drugs have been proposed to improve myocardial flow in HCM, with the purpose to reverse the progression of microvascular dysfunction itself [85,86,87]. Certainly, the degree of maximal MBF impairment could be a helpful surrogate end point to test the efficacy of these therapies, and maybe it could be superior to the so far adopted measurement of fibrosis extent [88, 89].
All the abovementioned studies converge in indicating that the evaluation of microvascular dysfunction could be useful in at least a proportion of HCM patients, also because the other prognostic markers do not perfectly identify the high-risk subjects. In particular, it could be useful for a more refined stratification in patients with other unfavorable conditions, such as left atrial dilatation, significant diastolic dysfunction, or myocardial fibrosis [90]. The current guidelines for HCM prognostication report a grade III (no benefit) recommendation for the use of quantitative PET in disease prognostication [5]. However, in more recent guidelines about multimodality imaging in HCM, it is recognized that quantitative assessment of MBF could play an interesting role in patient stratification [6].
Alternative Approaches to Microvascular Dysfunction Assessment in HCM
Among the imaging modalities used for HCM diagnosis, characterization and prognostication, CMR is the sole that offers the possibility of performing perfusion studies from which derive MBF measurements [91••, 92•, 93]. In particular, there are several recent reports that deal with direct or indirect flow parameters obtained from perfusion sequences in CMR and describe their role in HCM characterization. Ismail et al., using a pixelwise approach to CMR perfusion data, showed that it is possible to measure both average wall and layer (subendocardial and subepicardial) MBF at rest and during adenosine stimulation, with results that allow differentiating patients with more severe MBF impairment and more severe morphologic abnormalities, in particular wall thickness [94]. Other groups proposed indirect parameters of MBF, with a fair capability to identify differences in segments with versus without LGE [95, 96]. However, methodological problems in CMR measurements of MBF are still not completely resolved, and differences in the results’ reliability are reported, according to the interference of LGE and to the MBF measurement approach [97, 98]. The use of MFR as the reference parameter to overcome the limitations of the MBF measurements has been as well proposed [99]. Although the central role of CMR for the characterization of HCM cannot be overemphasized, the use of MBF measurements is still under evaluation, and it is worth mentioning that two most recent reviews on CMR in HCM do not include them among the multiple parameters to be considered [92•, 100]. As for computed tomography (CT), there are just reports on the use of CT coronary angiography to rule out epicardial coronary disease in HCM patients with chest pain (Fig. 2) [101].
Conclusion
Coronary microvascular dysfunction is a pivotal feature of HCM, and probably the most important cause of ischemia in these patients. The significance of ischemia is not just to explain one HCM symptom, i.e., chest pain, but most importantly to be one of the causes of other pathophysiologic changes, such as fibrosis, electrical instability, and functional evolution. Therefore, the assessment of microvascular dysfunction can be very useful to characterize the disease severity of the single patient and it is a mainstay of HCM prognostication. Unfortunately, the lack of a simple and easy available method for MBF measurement precludes the widespread evaluation of microvascular dysfunction in all HCM patients, and has so far as well prevented to explore the occurrence of MBF abnormalities in the relatives of patients, or in the subjects with genetic changes only. With regard to these limitations, however, the constantly increasing diffusion of PET facilities and the major technical advances in this field could make possible to include this modality in the work up of at least selected groups of HCM patients.
Another major obstacle to the definitive establishment of microvascular dysfunction evaluation in HCM is the lack of large clinical trials aimed at eventually demonstrating its capability to positively influence the patient outcome in terms of risk assessment [102]. Similarly, studies are needed that explore the response of microvascular dysfunction to the various possible therapeutic options already in use for HCM, or that test the potential usefulness of newly proposed treatments. This is particularly important for those therapies aimed at correcting physiopathogic mechanisms of the disease before they reach a phenotypic expression of clinical significance [91••]. Also with regard to this point, however, the wider accessibility of PET, the availability of new perfusion tracers, and maybe the progress of alternative approaches to MBF quantification, for instance using CMR, are expected to help overcoming the current difficulties.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785–9.
Zou Y, Song L, Wang Z, Ma A, Liu T, Gu H, et al. Prevalence of idiopathic hypertrophic cardiomyopathy in China: a population-based echocardiographic analysis of 8080 adults. Am J Med. 2004;116:14–8.
Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242–55.
Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, et al. ACC/ESC clinical expert consensus document on hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the European Society of Cardiology Committee for practice guidelines (committee to develop an expert consensus document on hypertrophic cardiomyopathy). J Am Coll Cardiol. 2003;42:1687–713.
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA guidelines for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212–60.
• Cardim N, Galderisi M, Edvardsen T, Plein S, Popescu BA, D'Andrea A, et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging. 2015;16:280 The most recent guideline about imaging in HCM.
Authors/Task Force Members, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–79. https://doi.org/10.1093/eurheartj/ehu284.
Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–54. https://doi.org/10.1016/j.jacc.2015.01.019.
Erdmann J, Daehmlow S, Wischke S, Senyuva M, Werner U, Raible J, et al. Mutation spectrum in a large cohort of unrelated consecutive patients with hypertrophic cardiomyopathy. Clin Genet. 2003;64:339–49.
Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, et al; EUROGENE Heart Failure Project. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 2003;107:2227–2232. https://doi.org/10.1161/01.CIR.0000066323.15244.54.
Millat G, Bouvagnet P, Chevalier P, Dauphin C, Jouk PS, Da Costa A, et al. Prevalence and spectrum of mutations in a cohort of 192 unrelated patients with hypertrophic cardiomyopathy. Eur J Med Genet. 2010;53:261–7. https://doi.org/10.1016/j.ejmg.2010.07.007.
Walsh R, Buchan R, Wilk A, John S, Felkin LE, Thomson KL, et al. Defining the genetic architecture of hypertrophic cardiomyopathy: re-evaluating the role of non-sarcomeric genes. Eur Heart J. 2017;38:3461–8. https://doi.org/10.1093/eurheartj/ehw603.
Walsh R, Thomson KL, Ware JS, Funke BH, Woodley J, McGuire KJ, et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet Med. 2017;19:192–203. https://doi.org/10.1038/gim.2016.90.
Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552–63. https://doi.org/10.1056/NEJMoa1002659.
Briasoulis A, Mallikethi-Reddy S, Palla M, Alesh I, Afonso L. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: a meta-analysis. Heart. 2015;101:1406–11. https://doi.org/10.1136/heartjnl-2015-307682.
Maron BJ, Wolfson JK, Epstein SE, Roberts WC. Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1986;8:545–57.
Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart. 2000;84:476–82.
Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–9. https://doi.org/10.1161/CIRCULATIONAHA.106.644682.
Shah JS, Esteban MT, Thaman R, Sharma R, Mist B, Pantazis A, et al. Prevalence of exercise-induced left ventricular outflow tract obstruction in symptomatic patients with non-obstructive hypertrophic cardiomyopathy. Heart. 2008;94:1288–94. https://doi.org/10.1136/hrt.2007.126003.
Autore C, Bernabò P, Barillà CS, Bruzzi P, Spirito P. The prognostic importance of left ventricular outflow obstruction in hypertrophic cardiomyopathy varies in relation to the severity of symptoms. J Am Coll Cardiol. 2005;45:1076–80.
Biagini E, Spirito P, Rocchi G, Ferlito M, Rosmini S, Lai F, et al. Prognostic implications of the Doppler restrictive filling pattern in hypertrophic cardiomyopathy. Am J Cardiol. 2009;104:1727–31. https://doi.org/10.1016/j.amjcard.2009.07.057.
Maron MS, Olivotto I, Maron BJ, Prasad SK, Cecchi F, Udelson JE, et al. The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:866–75.
Elliott PM, Kaski JC, Prasad K, Seo H, Slade AK, Goldman JH, et al. Chest pain during daily life in patients with hypertrophic cardiomyopathy: an ambulatory electrocardiographic study. Eur Heart J. 1996;17:1056–64.
Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104:2517–24.
Siontis KC, Geske JB, Ong K, Nishimura RA, Ommen SR, Gersh BJ. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical correlations, and mortality in a large high-risk population. J Am Heart Assoc. 2014;3:e001002. https://doi.org/10.1161/JAHA.114.001002.
Guttmann OP, Pavlou M, O’Mahony C, Monserrat L, Anastasakis A, Rapezzi C, et al; Hypertrophic Cardiomyopathy Outcomes Investigators. Prediction of thrombo-embolic risk in patients with hypertrophic cardiomyopathy (HCM Risk-CVA). Eur J Heart Fail 2015;17:837–845. https://doi.org/10.1002/ejhf.316.
Elliott PM, Poloniecki J, Dickie S, Malik M, McKenna WJ. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–8.
Spirito P, Autore C, Rapezzi C, Bernabò P, BAdagliacca R, Maron MS, et al. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation. 2009;119:1703–10.
O’Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, et al; Hypertrophic Cardiomyopathy Outcomes Investigators. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 2014;35:2010–2020. https://doi.org/10.1093/eurheartj/eht439.
Maron BJ, Rowin EJ, Casey SA, Link MS, Lesser JR, Chan RH, et al. Hypertrophic cardiomyopathy in adulthood associated with low cardiovascular mortality with contemporary management strategies. J Am Coll Cardiol. 2015;65:1915–28. https://doi.org/10.1016/j.jacc.2015.02.061.
Maron MS, Rowin EJ, Olivotto I, Casey SA, Arretini A, Tomberli B, et al. Contemporary natural history and management of nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2016;67:1399–409.
Melacini P, Basso C, Angelini A, Calore C, Bobbo F, Tokajuk B, et al. Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur Heart J. 2010;31:2111–23.
Maron BJ, Epstein SE, Roberts WC. Hypertrophic cardiomyopathy and transmural myocardial infarction without significant atherosclerosis of the extramural coronary arteries. Am J Cardiol. 1979;43:1086–102.
•• Bravo PE. Is there a role for cardiac positron emission tomography in hypertrophic cardiomyopathy? J Nucl Cardiol 2018. https://doi.org/10.1007/s12350-018-1298-4. A most recent and comprehensive review about PET and HCM.
Roberts WC, Ferrans VJ. Pathologic anatomy of the cardiomyopathies. Idiopathic dilated and hypertrophic types, infiltrative types, and endomyocardial disease with and without eosinophilia. Hum Pathol. 1975;6:287–342.
Krams R, Kofflard MJ, Duncker DJ, Von Birgelen C, Carlier S, Kliffen M, et al. Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation. 1998;97:230–3.
Schwartzkopff B, Mundhenke M, Strauer BE. Alterations of the architecture of subendocardial arterioles in patients with hypertrophic cardiomyopathy and impaired coronary vasodilator reserve: a possible cause for myocardial ischemia. J Am Coll Cardiol. 1998;31:1089–96.
Johansson B, Mörner S, Waldenström A, Stål P. Myocardial capillary supply is limited in hypertrophic cardiomyopathy: a morphological analysis. Int J Cardiol. 2008;126:252–7.
Lombardi R, Betocchi S, Losi MA, Tocchetti CG, Aversa M, Miranda M, et al. Myocardial collagen turnover in hypertrophic cardiomyopathy. Circulation. 2003;108:1455–60.
Shirani J, Pick R, Roberts WC, Maron BJ. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol. 2000;35:36–44.
Basso C, Thiene G, Corrado D, Buja G, Melacini P, Nava A. Hypertrophic cardiomyopathy and sudden death in the young: pathologic evidence of myocardial ischemia. Hum Pathol. 2000;31:988–98.
Gori F, Basso C, Thiene G. Myocardial infarction in a patient with hypertrophic cardiomyopathy. N Engl J Med. 2000;342:593–4.
Rubin KA, Morrison J, Padnick MB, Binder AJ, Chiaramida S, Margouleff D, et al. Idiopathic hypertrophic subaortic stenosis: evaluation of anginal symptoms with thallium-201 myocardial imaging. Am J Cardiol. 1979;44:1040–5.
Pitcher D, Wainwright R, Maisey M, Curry P, Sowton E. Assessment of chest pain in hypertrophic cardiomyopathy using exercise thallium-201 myocardial scintigraphy. Br Heart J. 1980;44:650–6.
O’Gara PT, Bonow RO, Maron BJ, Damske BA, Van Lingen A, Bacharach SL, et al. Myocardial perfusion abnormalities in patients with hypertrophic cardiomyopathy: assessment with thallium-201 emission computed tomography. Circulation. 1987;76:1214–23.
Udelson JE, Bonow RO, O’Gara PT, Maron BJ, Van Lingen A, Bacharach SL, et al. Verapamil prevents silent myocardial perfusion abnormalities during exercise in asymptomatic patients with hypertrophic cardiomyopathy. Circulation. 1989;79:1052–60.
Cannon RO 3rd, Dilsizian V, O’Gara PT, Udelson JE, Schenke WH, Quyyumi A, et al. Myocardial metabolic, hemodynamic, and electrocardiographic significance of reversible thallium-201 abnormalities in hypertrophic cardiomyopathy. Circulation. 1991;83:1660–7.
Yoshida N, Ikeda H, Wada T, Matsumoto A, Maki S, Muro A, et al. Exercise-induced abnormal blood pressure responses are related to subendocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1998;32:1938–42.
Sciagrà R, Passeri A, Bucerius J, Verberne HJ, Slart RH, Lindner O, et al. Cardiovascular Committee of the European Association of Nuclear Medicine (EANM). Clinical use of quantitative cardiac perfusion PET: rationale, modalities and possible indications. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM). Eur J Nucl Med Mol Imaging. 2016;43:1530–45.
Camici PG, Gropler RJ, Jones T, L'Abbate A, Maseri A, Melin JA, et al. The impact of myocardial blood flow quantitation with PET on the understanding of cardiac diseases. Eur Heart J. 1996;17:25–34.
Camici P, Chiriatti G, Lorenzoni R, Bellina RC, Gistri R, Italiani G, et al. Coronary vasodilation is impaired in both hypertrophied and nonhypertrophied myocardium of patients with hypertrophic cardiomyopathy: a study with nitrogen-13 ammonia and positron emission tomography. J Am Coll Cardiol. 1991;17:879–86.
Gistri R, Cecchi F, Choudhury L, Montereggi A, Sorace O, Salvadori PA, et al. Effect of verapamil on absolute myocardial blood flow in hypertrophic cardiomyopathy. Am J Cardiol. 1994;74:363–8.
Choudhury L, Elliott P, Rimoldi O, Ryan M, Lammertsma AA, Boyd H, et al. Transmural myocardial blood flow distribution in hypertrophic cardiomyopathy and effect of treatment. Basic Res Cardiol. 1999;94:49–59.
Lorenzoni R, Gistri R, Cecchi F, Olivotto I, Chiriatti G, Elliott P, et al. Syncope and ventricular arrhythmias in hypertrophic cardiomyopathy are not related to the derangement of coronary microvascular function. Eur Heart J. 1997;18:1946–50.
Lorenzoni R, Gistri R, Cecchi F, Olivotto I, Chiriatti G, Elliott P, et al. Coronary vasodilator reserve is impaired in patients with hypertrophic cardiomyopathy and left ventricular dysfunction. Am Heart J. 1998;136:972–81.
Tadamura E, Yoshibayashi M, Yonemura T, Kudoh T, Kubo S, Motooka M, et al. Significant regional heterogeneity of coronary flow reserve in paediatric hypertrophic cardiomyopathy. Eur J Nucl Med. 2000;27:1340–8.
Ohba M, Hosokawa R, Kambara N, Tadamura E, Mamede M, Kubo S, et al. Difference in myocardial flow reserve between patients with dilated cardiomyopathy and those with dilated phase of hypertrophic cardiomyopathy: evaluation by 15O-water PET. Circ J. 2007;71:884–90.
Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–95.
Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156–64.
Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiolì. 2003;41:1561–7.
Knaapen P, van Dockum WG, Götte MJ, Broeze KA, Kuijer JP, Zwanenburg JJ, et al. Regional heterogeneity of resting perfusion in hypertrophic cardiomyopathy is related to delayed contrast enhancement but not to systolic function: a PET and MRI study. J Nucl Cardiol. 2006;13:660–7.
Sotgia B, Sciagrà R, Olivotto I, Casolo G, Rega L, Betti I, et al. Spatial relationship between coronary microvascular dysfunction and delayed contrast enhancement in patients with hypertrophic cardiomyopathy. J Nucl Med. 2008;49:1090–6.
Bravo PE, Zimmerman SL, Luo HC, Pozios I, Rajaram M, Pinheiro A, et al. Relationship of delayed enhancement by magnetic resonance to myocardial perfusion by positron emission tomography in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2013;6:210–7.
Knaapen P, Germans T, Camici PG, Rimoldi OE, ten Cate FJ, ten Berg JM, et al. Determinants of coronary microvascular dysfunction in symptomatic hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;294:H986–93.
Sciagrà R, Passeri A, Cipollini F, Castagnoli H, Olivotto I, Burger C, et al. Validation of pixel-wise parametric mapping of myocardial blood flow with 13NH3 PET in patients with hypertrophic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2015;42:1581–8.
Bravo PE, Pinheiro A, Higuchi T, Rischpler C, Merrill J, Santaularia-Tomas M, et al. PET/CT assessment of symptomatic individuals with obstructive and nonobstructive hypertrophic cardiomyopathy. J Nucl Med. 2012;53:407–14.
Timmer SAJ, Germans T, Götte MJW, Rüssel IK, Dijkmans PA, Lubberink M, et al. Determinants of myocardial energetics and efficiency in symptomatic hypertrophic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2010;37:779–88.
Timmer SAJ, Germans T, Götte MJW, Rüssel IK, Lubberink M, Ten Berg JM, et al. Relation of coronary microvascular dysfunction in hypertrophic cardiomyopathy to contractile dysfunction independent from myocardial injury. Am J Cardiol. 2011;107:1522–8.
Sciagrà R, Sotgia B, Olivotto I, Cecchi F, Nistri S, Camici PG, et al. Relationship between atrial fibrillation and blunted hyperemic myocardial blood flow in patients with hypertrophic cardiomyopathy. J Nucl Cardiol. 2009;16:92–6.
Olivotto IW, Girolami FMK, Sciagrà R, Ackerman MJ, Sotgia B, Bos JM, et al. Microvascular function is selectively impaired in hypertrophic cardiomyopathy patients with sarcomere myofilament gene mutations. J Am Coll Cardiol. 2011;58:839–48.
Timmer SA, Germans T, Brouwer WP, Lubberink M, van der Velden J, Wilde AA, et al. Carriers of the hypertrophic cardiomyopathy MYBPC3 mutation are characterized by reduced myocardial efficiency in the absence of hypertrophy and microvascular dysfunction. Eur J Heart Fail. 2011;13:1283–9.
Bravo PE, Tahari A, Pozios I, Luo HC, Bengel FM, Wahl RL, et al. Apparent left ventricular cavity dilatation during PET/CT in hypertrophic cardiomyopathy: clinical predictors and potential mechanisms. J Nucl Cardiol. 2016;23:1304–14.
Sciagrà R, Calabretta R, Cipollini F, Passeri A, Castello A, Cecchi F, et al. Myocardial blood flow and left ventricular functional reserve in hypertrophic cardiomyopathy: a 13NH3 gated PET study. Eur J Nucl Med Mol Imaging. 2017;44:866–75.
Yalçin H, Valenta I, Yalçin F, Corona-Villalobos C, Vasquez N, Ra J, et al. Effect of diffuse subendocardial hypoperfusion on left ventricular cavity size by 13N-Ammonia perfusion PET in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2016;118:1908–15.
Jörg-Ciopor M, Namdar M, Turina J, Jenni R, Schwitter J, Turina M, et al. Regional myocardial ischemia in hypertrophic cardiomyopathy: impact of myectomy. J Thorac Cardiovasc Surg. 2004;128:163–9.
Soliman OI, Geleijnse ML, Michels M, Dijkmans PA, Nemes A, van Dalen BM, et al. Effect of successful alcohol septal ablation on microvascular function in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2008;101:1321–7.
Timmer SA, Knaapen P, Germans T, Dijkmans PA, Lubberink M, Ten Berg JM, et al. Effects of alcohol septal ablation on coronary microvascular function and myocardial energetics in hypertrophic obstructive cardiomyopathy. Am J Physiol Heart Circ Physiol. 2011;301:H129–37.
Sorajja P, Chareonthaitawee P, Ommen SR, Miller TD, Hodge DO, Gibbons RJ. Prognostic utility of single-photon emission computed tomography in adult patients with hypertrophic cardiomyopathy. Am Heart J. 2006;151:426–35.
Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349:1027–35.
Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, et al. Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47:1043–8.
Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64:83–99.
• Castagnoli H, Ferrantini C, Coppini R, Passeri A, Baldini K, Berti V, et al. Role of quantitative myocardial positron emission tomography for risk stratification in patients with hypertrophic cardiomyopathy: a 2016 reappraisal. Eur J Nucl med Mol imaging. 2016;43:2413–22 The most recent paper about the prognostic implications of microvascular dysfunction.
Dilsizian V, Bonow RO, Epstein SE, Fananapazir L. Myocardial ischemia detected by thallium scintigraphy is frequently related to cardiac arrest and syncope in young patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1993;22:796–804.
Lu DY, Yalçin H, Yalçin F, Zhao M, Sivalokanathan S, Valenta I, et al. Stress myocardial blood flow heterogeneity is a positron emission tomography biomarker of ventricular arrhythmias in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2018;121:1081–9.
Neglia D, Fommei E, Varela-Carver A, Mancini M, Ghione S, Lombardi M, et al. Perindopril and indapamide reverse coronary microvascular remodelling and improve flow in arterial hypertension. J Hypertens. 2011;29:364–72.
Spoladore R, Maron MS, D'Amato R, Camici PG, Olivotto I. Pharmacological treatment options for hypertrophic cardiomyopathy: high time for evidence. Eur Heart J. 2012;33:1724–33.
Coppini R, Ferrantini C, Mazzoni L, Sartiani L, Olivotto I, Poggesi C, et al. Regulation of intracellular Na(+) in health and disease: pathophysiological mechanisms and implications for treatment. Glob Cardiol Sci Pract. 2013;2013:222–42.
Shimada YJ, Passeri JJ, Baggish AL, O’Callaghan C, Lowry PA, Yannekis G, et al. Effects of losartan on left ventricular hypertrophy and fibrosis in patients with nonobstructive hypertrophic cardiomyopathy. JACC Heart Fail. 2013;1:480–7.
Axelsson A, Iversen K, Vejlstrup N, Ho C, Norsk J, Langhoff L, et al. Efficacy and safety of the angiotensin II receptor blocker losartan for hypertrophic cardiomyopathy: the INHERIT randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2015;3:123–31.
Bogaert J, Olivotto I. MR imaging in hypertrophic cardiomyopathy: from magnet to bedside. Radiology. 2014;273:329–48.
•• Marian AJ, Braunwald E. Hypertrophic cardiomyopathy. Genetics, pathogenesis, clinical manifestations, diagnosis and therapy. Circ Res. 2017;121:749–70 Outstanding recent review about HCM, its genetics and clinics.
• Amano Y, Kitamura M, Takano H, Yanagisawa F, Tachi M, Suzuki Y, et al. Cardiac MR imaging of hypertrophic cardiomyopathy: techniques, findings, and clinical relevance. Magn Reson Med Sci. 2018;17:120–31 The most recent review about the role of CMR imaging in HCM.
Jan MF, Tajik AJ. Modern imaging techniques in cardiomyopathies. Circ Res. 2017;121:874–91.
Ismail TF, Hsu LY, Greve AM, Gonçalves C, Jabbour A, Gulati A, et al. Coronary microvascular ischemia in hypertrophic cardiomyopathy - a pixel-wise quantitative cardiovascular magnetic resonance perfusion study. J Cardiovasc Magn Reson. 2014;16:49. https://doi.org/10.1186/s12968-014-0049-1.
Zhang YD, Li M, Qi L, Wu CJ, Wang X. Hypertrophic cardiomyopathy: cardiac structural and microvascular abnormalities as evaluated with multi-parametric MRI. Eur J Radiol. 2015;84:1480–6.
Yin L, Xu HY, Zheng SS, Zhu Y, Xiao JX, Zhou W, et al. 3.0 T magnetic resonance myocardial perfusion imaging for semi-quantitative evaluation of coronary microvascular dysfunction in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2017;33:1949–59.
Villa AD, Sammut E, Zarinabad N, Carr-White G, Lee J, Bettencourt N, et al. Microvascular ischemia in hypertrophic cardiomyopathy: new insights from high-resolution combined quantification of perfusion and late gadolinium enhancement. J Cardiovasc Magn Reson. 2016;18:4. https://doi.org/10.1186/s12968-016-0223-8.
Bietenbeck M, Florian A, Shomanova Z, Meier C, Yilmaz A. Reduced global myocardial perfusion reserve in DCM and HCM patients assessed by CMR-based velocity-encoded coronary sinus flow measurements and first-pass perfusion imaging. Clin Res Cardiol. 2018 May 17;107:1062–70. https://doi.org/10.1007/s00392-018-1279-2.
Tezuka D, Kosuge H, Terashima M, Koyama N, Kishida T, Tada Y, et al. Myocardial perfusion reserve quantified by cardiac magnetic resonance imaging is associated with late gadolinium enhancement in hypertrophic cardiomyopathy. Heart Vessel. 2018;33:513–20.
Kamal MU, Riaz IB, Janardhanan R. Cardiovascular magnetic resonance imaging in hypertrophic cardiomyopathy: current state of the art. Cardiol J. 2016;23:250–63.
Chaikriangkrai K, Chebrolu L, Bhatti S, Chang SM. Diagnosis of ischemia in hypertrophic cardiomyopathy: role of computed tomography and nuclear stress testing. Curr Opin Cardiol. 2015;30:483–92.
Olivotto I, Tomberli B, Spoladore R, Mugelli A, Cecchi F, Camici PG. Hypertrophic cardiomyopathy: the need for randomized trials. Glob Cardiol Sci Pract. 2013;2013:243–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Roberto Sciagrà declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiac Nuclear Imaging
Rights and permissions
About this article
Cite this article
Sciagrà, R. Microvascular Dysfunction in Hypertrophic Cardiomyopathy. Curr Cardiovasc Imaging Rep 12, 3 (2019). https://doi.org/10.1007/s12410-019-9478-4
Published:
DOI: https://doi.org/10.1007/s12410-019-9478-4