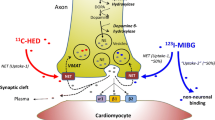
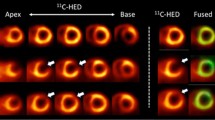
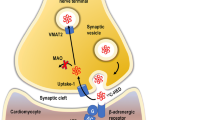
Abstract
Heart failure (HF) is associated with reduced cardiac sympathetic neuronal uptake of norepinephrine (NE), which can be assessed noninvasively using different radiotracers and planar or single-photon emission computed tomography (SPECT)/positron emission tomography (PET) imaging. Such sympathetic derangement in HF patients has shown to be an indicator of unfavorable prognosis. Therefore, cardiac sympathetic imaging might be useful as an indicator of the effectiveness of the medical therapy and consequently for risk stratification of patients with HF to more effectively guide specific therapies. This article reviews the current status on the subject and evaluates the literature published over recent years.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) is a significant contributor to morbidity and mortality in the western world. Improvement of treatment and outcome of HF is a major worry because of the progressive increase in prevalence and incidence of HF.
There is evidence showing that as clinical condition and/or left ventricular (LV) function improves as consequence of standard HF medical treatment, there is a parallel improvement in cardiac sympathetic nerve function as assessed by Iodine-123 meta-iodobenzylguanidine (123I-mIBG), supporting the notion that restitution of cardiac neuronal uptake of norepinephrine (NE) is one of the favorable effects of treatment and that cardiac sympathetic imaging is important for risk stratification in HF patients [1, 2]. AdreView Myocardial Imaging for Risk Evaluation in Heart Failure (ADMIRE-HF) is the largest prospective multicenter study that has validated the prognostic role of cardiac 123I-mIBG imaging in HF patients thus far. The study included 961 HF patients in New York Heart Association (NYHA) functional class II–III with ischemic or non-ischemic cardiomyopathy with a site-reported left ventricular ejection fraction (LVEF) ≤35 %, who were on guideline-recommended medical therapy and followed up for a median of 17 months (Table 1). Late 123I-mIBG heart-to-mediastinum ratio (HMR) demonstrated its value in predicting the primary composite endpoint of cardiac death, potentially life-threatening arrhythmias, or HF progression [3]. Preserved HMR showed an extreme negative predictive value in relation to cardiac death or cumulative arrhythmic events, in agreement with results from other studies with less numerous populations. Furthermore, HMR confirmed an independent prognostic capability, complementary to other commonly used risk markers including LVEF and B-type natriuretic peptide (BNP), potentially helping to differentiate patients likely to benefit from medical treatment from those that are likely to show unfavorable response and thus might be better treated non-medically (e.g., device treatment and cardiac transplantation). This article reviews the current status on the subject and evaluates the literature published over recent years.
Cardiac Sympathetic Imaging for Risk Stratification in Patients with HF: ADMIRE-HF Sub-Studies
Regardless of the auspicious results of the ADMIRE-HF trial, questions related with how 123I-mIBG imaging and associated risk estimates could be incorporated into patient prediction of HF progression and patient management decision-making persist. In addition, it is still debatable which is the best response variable for the evaluation of treatment efficacy in HF patients [4]. All these concerns have led to a series of sub-studies of the original trial with additional analyses considering different endpoints (Table 1).
The ADMIRE-HF extension (ADMIRE-HFX) study analyzed survival data from all patients of the ADMIRE-HF trial to complete a follow-up of two full years, with additional surveillance to 470 of the surviving patients at the closure of the primary trial [5]. The study explored the extra value of 123I-mIBG cardiac imaging when added to a modified version of Seattle Heart Failure Model (SHFM-D) [6], which includes several demographic and clinical risk markers for multivariate predictor of risk and mode of death in HF, together with the differential benefit of implantable cardioverter defibrillator (ICD) placement obtained in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) [6]. During follow-up (median 2 years, mean 21 months), 101 deaths occurred among the 961 patients, with 63 % of all deaths of cardiac origin. The addition of late HMR (considered as a continuous variable rather than categorized at 1.60 as in the ADMIRE-HF) to the SHFM-D significantly improved all-cause mortality risk prediction, in contrast to many similar analysis in which newer risk markers have not shown an improvement in conjunction with an established risk model. The addition of HMR to the SHFM-D was statistically and clinically significant as assessed by different methods including the Cox proportional hazards model, 1- and 2-year area under the receiver-operator characteristic (ROC) curve (AUC) change, net reclassification improvement (NRI) and integrated discrimination improvement (IDI) change, and discrimination slope methodology. However, HMR did not improve risk stratification in low-risk patients, which advocates for greater prognostic utility in higher-risk SHFM-D patients. There was a low correlation of SHFM-D and HMR (0.144, P < .0001), which suggests that the SHFM-D and HMR measure different components of risk in HF patients and may also explain the increased prognostic value of the combined model. A further and more recent sub-analysis of the ADMIRE-HFX study has demonstrated that late HMR provides independent prognostic information that is additive to currently available risk stratification procedures, showing its consistent significance when added to standard risk models for mortality and mortality-equivalent events (resuscitated arrest, successful defibrillation for ventricular tachycardia, or fibrillation) using two different co-primary analysis methods that included multivariate Cox proportional hazards and logistic regression [7••]. During follow-up, in addition to 101 deaths, there were 136 patients with mortality-equivalent events. HMR was significant in all multivariate proportional hazards and logistic regression models for the two mortality endpoints, both models developed with only clinical variables (standard demographic and medical history variables) and with those including LVEF and BNP. For standard models including BNP, the addition of HMR did not significantly increase AUC. However, there was significant NRI with the addition of HMR to a proportional hazards model containing BNP and LVEF. Although BNP was a powerful predictor of mortality, there was only a weak significant negative correlation between this measurement and HMR, indicating that both parameters reflect different attributes of the heart. Interestingly, increases in AUC were greater for 1-year than 2-year regression analyses, suggesting that the supplementary information provided by HMR at a single point in time has greater potential utility for early decision-making, given that interval changes in therapy and disease progression can affect myocardial neuronal status and, therefore, the prognostic relevance of previous 123I-mIBG imaging results. Future development of nomograms or computer programs using the models developed in this study could facilitate use of 123I-mIBG imaging results in the clinical arena.
In another sub-analysis of the ADMIRE-HF trial, Parker et al. [8] showed that late HMR may also risk-stratify HF patients for cardiac-related hospitalization, particularly when used together with BNP. During follow-up (median 1.4 years), 304 of 961 (31.6 %) patients were hospitalized at least once. Time to hospitalization was analyzed with the Kaplan-Meier method and compared to HMR using multiple-failure Cox regression. Among patients hospitalized for any cause, 88 % had HMR <1.6, and patients with HMR ratio <1.6 experienced hospitalization significantly earlier than patients with higher HMR. A low HMR was significantly associated with cardiac-related hospitalizations (hazard ratio [HR] 1.48, 95 % confidence interval (CI) 1.05 to 2.0) after correcting for elevated BNP and time since HF. Additional studies of hospitalization or re-hospitalization among recently hospitalized HF patients might explore the incremental benefit and clinical role of cardiac sympathetic imaging to risk-stratify patients for hospitalization.
The prognostic significance of cardiac nuclear imaging in HF patients has recently been assessed by means of quantitative analysis of 123I-mIBG single-photon emission computed tomography (SPECT) imaging, alone and in combination with 99mTc-tetrofosmin SPECT imaging in 938 patients from the ADMIRE-HFX study (those who had both SPECT studies processable), with a focus on differences between patients with ischemic (I, n = 619) and non-ischemic (NI, n = 319) HF [9]. During follow-up, there were 99 deaths (62 cardiac), and 2-year all-cause and cardiac mortality was not significantly different between IHF and NIHF patients. Three 123I-mIBG measures were significant predictors of mortality: total dysinnervation extent, summed segmental dysinnervation score (cardiac mortality), and the number of segments with dysinnervation. The extent, summed defect score, or the number of abnormal segments on 99mTc-tetrofosmin SPECT did not predict death. 123I-mIBG/99mTc-tetrofosmin mismatch extent was also not a significant predictor. While efforts have been expended in previous attempts to quantify the severity of diminished myocardial 123I-mIBG uptake, these data suggest that accurately defining the amount of dysinnervated myocardium is more substantial than obtaining precise estimates of the severity of dysinnervation, which could potentially simplify the clinical use of 123I-mIBG imaging, as it may be only necessary to identify the extent of segmental dysinnervation above a certain threshold to establish a high-risk status in HF patients. Interestingly, mortality was higher in patients with dysinnervation involving >50 % of the myocardium in both IHF and NIHF patients. However, the prognostic significance of patterns of 123I-mIBG and 99mTc-tetrofosmin uptake differed between IHF and NIHF patients. Highest cardiac mortality risk for IHF patients was seen with perfusion defects involving 20–40 % of the myocardium. By comparison, NIHF patients with smaller perfusion abnormalities (<20 % of myocardium), but with a large discrepancy between 123I-mIBG and 99mTc-tetrofosmin defect sizes, were at highest risk of cardiac death. These findings extend the observations previously made from HMR measurements on planar scintigraphy to quantitative results obtained from automated analyses of 123I-mIBG SPECT images [10] and illustrate not only the importance of both regional and global dysinnervations as a predictor of outcomes for ischemic and NI HF patients but also the importance of the severity of perfusion abnormalities in the interpretation of innervation SPECT results, in contrast with some previous studies using different methods to quantify innervation/perfusion mismatch. SPECT imaging allows precise demonstration of the myocardial regions affected by dysinnervation and theoretically better discrimination among levels of risk. Moreover, the relationship between myocardial blood flow, myocyte function, and sympathetic neuronal status can only be effectively investigated using SPECT.
Sudden death is common among HF patients with significant LV dysfunction, but it is also the most frequent cause of cardiovascular death among HF patients with normal or slightly reduced systolic function. However, the ability to effectively risk-stratify these patients is limited. Although LVEF is a potent predictor of mortality and sudden death when significantly reduced, it is less useful in risk stratifying those patients with normal or only slightly reduced LVEF (45 %). Shah et al. [11] retrospectively assessed the influence of the different range of LVEFs on the prognostic value of cardiac 123I-mIBG imaging among patients of the ADMIRE-HF trial. In that trial, there were 901 patients who had site-reported LVEF ≤35 %, but core laboratory-determined LVEFs ranged from 20 to 58 %, with 386 patients (43 %) having an LVEF >35 %. At all levels of LVEF, the late HMR of <1.6 was associated with a higher risk of death or potentially lethal arrhythmia and of the composite of cardiovascular death, arrhythmic event, and HF progression. Comparing patients with LVEF ≤35 and >35 %, there was no evidence of effect modification of LVEF on the risk associated with low HMR for death or arrhythmic event and for the composite. For the outcome of death or arrhythmic event in particular, the HMR appeared to improve the risk stratification beyond clinical and biomarker data among both LVEF groups. Therefore, cardiac 123I-mIBG imaging appears to have prognostic value across a spectrum of LVEFs although further studies prospectively assessing its prognostic value in HF patients with LVEF >35 % are warranted.
The likelihood of arrhythmic events is related to both myocardial scar burden and the status of cardiac sympathetic innervation. Sood el al. [12] showed that myocardial perfusion defects at rest (representing scar) on SPECT imaging further stratified the risk of developing ventricular arrhythmic events beyond late HMR in HF patients. In this sub-study, all patients from the ADMIRE-HF trial with complete myocardial perfusion and innervation data (n = 929; NI cardiomyopathy, NICM, n = 317 –34 %– ; ischemic cardiomyopathy, ICM, n = 612 –66 %–) were stratified by HMR (<1.6/≥1.6) and by visual estimation of summed rest score (SRS) (≤8/>8) and subsequently categorized as high risk (HMR <1.6, SRS >8) and low risk (HMR ≥1.6, SRS ≤8). During follow-up (median 17 months), 63 arrhythmic events occurred. Patients with NICM with a high SRS >8 and a low HMR <1.6 resulted to be at a greater risk for ventricular arrhythmia. Contrariwise, patients with ICM or NICM and a relatively preserved late HMR (≥1.6) and a low SRS (≤8) resulted to have a trend toward an inferior risk of ventricular arrhythmia. These results suggest that the arrangement of HMR and scar quantification by myocardial perfusion SPECT imaging at rest provide risk classification into both high- and low-risk groups for ventricular arrhythmia.
The ADMIRE-HF sub-analysis of Al Badarin et al. [13], focusing specifically on the prediction of potentially life-threatening arrhythmic events, is also interesting. They prospectively followed up (median 17 months) 778 patients from the ADMIRE-HF trial who did not have prior history of ventricular arrhythmia or an ICD at the time of trial enrollment. Serious arrhythmic events (a composite of sudden cardiac death (SCD), appropriate ICD therapy including defibrillation or anti-tachycardia pacing, resuscitated cardiac arrest or sustained LV tachycardia of >30 s) occurred in 54 (6.9 %) unique patients with an event rate of 6.9 and 9.4 % at 1 and 2 years, respectively. Multivariable survival regression was used to determine independent predictors of arrhythmias and develop a predictive risk score. Independent predictors were late HMR <1.6 (HR 3.48, 95 %CI 1.52 to 8), LVEF <25 % (HR 1.97, 95 %CI 1.28 to 3.05), and systolic blood pressure (SBP) <120 mm Hg (HR 1.19, 95 %CI 1.03 to 1.39). The risk score using these three simple variables effectively stratified the study population, with primary prevention indications for ICD implantation, according to the risk of clinically relevant arrhythmias. External validation of this score in other HF cohorts as well as cost-effectiveness analysis may further support the role of 123I-mIBG imaging in device implantation decisions in HF patients. However, these data should be interpreted carefully since HRs were not obtained with standardized coefficients; i.e., HMR, LVEF, and SBP had different units of measurement, so that the predictors are not comparable to each other. Furthermore, even if standardized coefficients had been used, HRs may still not be appropriate as they do not consider potential correlation among the predictors [14].
Cardiac Sympathetic Imaging for Risk Stratification in Patients with HF: Japanese Pooled Cohort Studies
Japan has a robust clinical experience with cardiac 123I-mIBG imaging [2]. This led to recommend its use for the management of HF patients, particularly for the evaluation of severity and prognosis of HF (class IB indication) by the nuclear cardiology guidelines of the Japanese Circulation Society [15]. A series of recent multiple-cohort database analyses reinforces the amount of evidence and clinical use of cardiac 123I-mIBG imaging for risk stratification in HF (Table 2).
Nakata et al. [16] sought to define quantitative thresholds for late HMR that would allow classification of high- and low-risk HF patients as an aid to therapeutic decision-making. Six prospective HF Japanese cohort studies including 1322 patients were updated, and the individual datasets were combined for a patient-level analysis. All-cause mortality was the primary outcome analyzed. During follow-up (mean 78 months), 326 deaths occurred, and the population mortality rate was 5.6, 11.3, and 19.7 % at 1, 2, and 5 years, respectively. Multivariate Cox proportional hazard model analysis for all-cause mortality identified age, NYHA class, HMR, and LVEF as significant independent predictors. Sub-analysis of the 512-patient sub-population with BNP results identified BNP, NYHA class, and HMR as significant predictors. ROC analysis identified an HMR of 1.68 as the optimal threshold for categorizing the population into high-risk and low-risk of mortality for any LVEF value. Survival rates declined gradually with diminishing HMR, with 5-year annual all-cause mortality rates >7 % for HMR <1.25 and <2 % for HMR ≥1.95. Addition of HMR to clinical information resulted in a significant NRI. These pooled analyses confirmed the long-term prognostic value of HMR in HF patients independently of other risk markers and showed that patient survival rate declines linearly depending on the reduction of HMR and that categorical evaluations could be used to define meaningful thresholds for lethal event risk (in this case for the Japanese population). However, due to the known existence of large variations in HMR values depending on the scintillation camera, collimator, administration dose and specific activity of 123I-mIBG, and imaging protocol, the dichotomous risk stratification in low-risk vs. high-risk patients based on an HMR threshold may be questionable [4]. Nakajima et al. [17] have recently proposed a calibration phantom method to cross-calibrate HMRs among institutions. The coefficient of conversion of this calibration method was measured in 225 experiments in 84 hospitals in Japan. The measured HMR was successfully converted to the standardized HMR among institutions. The use of such standardized HMR comparable to that obtained with the medium-energy collimator improved risk classification in patients with HF.
Nakajima et al. [18], based on the same pooled Japanese database [16], selected those HF patients with known 5-year outcome (n = 933) and developed a prediction model for 5-year cardiac mortality, a time frame appropriate to the use of most current therapeutic approaches in HF. They used multivariate Cox proportional hazard analysis and logistic regression analysis for single late HMR and other multiple variables and calculated AUC. Logistic models with single or multiple variables were used to create a prediction model with calculation and generation of nomograms of cardiac mortality. Inverse prediction of HMR was also performed on the basis of the prediction models to assess the threshold for low risk of mortality. NRI analysis was also implemented on the basis of the prediction models with and without HMR. The 5-year risk levels were established as low (<5 %, corresponding to the accepted low-risk threshold of 1 % mortality per year), intermediate (5–25 %), and high (>25 %, corresponding to >5 % mortality per year). HMR showed a significant additive value for predicting 5-year cardiac mortality. As expected, NYHA class showed the highest odds ratio due to the worse prognosis in HF patients with NYHA classes III and IV. Addition of HMR reduced the prognostic influence of LVEF to the model. This is interesting since a non-trivial fraction of high-risk HF patients has preserved LVEF, which could be identified by 123I-mIBG imaging. The study produced two models of significant predictive variables: model 1 included NYHA class, age, gender, and LVEF, and model 2 included additionally HMR to model 1. Model 2 had a significant NRI for all subjects of 13.8 % and significantly improved the identification of patients at low risk of cardiac death when compared to model 1. The apparently better efficacy of HMR for reclassifying downward HF patients into low-risk groups contrasts with the finding in the ADMIRE-HFX sub-analysis using the SHFM-D model [5], but patients in the ADMIRE-HFX trial probably had worse functional status and higher prevalence of ischemic HF. Considering the results of both studies, 123I-mIBG imaging may be useful in reclassifying either upward to a higher-risk cohort [5] or downward to a lower risk population [18].
Again, based on the same long-term pooled Japanese database [16] and using multivariate logistic regression model, Nakajima et al. [19••] have recently generated risk charts with three commonly used clinical variables (age, NYHA class and LVEF) together with late HMR, in a comprehensible way for the use of cardiac 123I-mIBG imaging in the clinical practice for classification of low-risk and high-risk HF patients, even when BNP is available. Risk charts using short (2-year) and long-term (5-year) cardiac mortality in patients with HF (n = 1280 and 933, respectively) were created. Cardiac mortality was 10 and 22 % for the sub-population of 2- and 5-year analyses, respectively. The annualized mortality rate was <1 % in patients with NYHA class I–II and HMR ≥2.0, irrespective of age and LVEF, and it was four to six times higher in patients with NYHA class III–IV and HMR <1.40 as compared to HMR ≥2.0, in all LVEF classes. In a sub-group of patients with BNP results (n = 491 and 359 for 2- and 5-year models, respectively), the 5-year model showed incremental value of HMR in addition to BNP.
Cardiac Sympathetic Imaging for Risk Stratification in Patients with HF: Other Studies (Table 3)
The Prediction of ARrhythmic Events with Positron Emission Tomography (PAREPET) trial prospectively assessed whether imaging hibernating and/or denervated myocardium could forecast arrhythmic death in ischemic cardiomyopathy [20]. PET imaging was performed with 13N-ammonia (perfusion), 18F-deoxyglucose (metabolism), and 11C-hydroxyephedrine (innervation). The study included 204 patients with stable ischemic heart disease and HF (LVEF ≤35 %) on optimal medical treatment, who were not considered for coronary revascularization and who had no prior cardiac arrest/ICD discharge, or recent myocardial infarction (<30 days), or revascularization (PCI <3 months; bypass grafting <1 year) but were eligible for primary prevention ICD. The primary endpoint was sudden cardiac arrest (SCA), defined as arrhythmic death or ICD discharge for ventricular fibrillation or tachycardia >240 beats/min. During follow-up (median 4.1 years), SCA occurred in 33 patients, who had significantly larger amount of denervated and viable denervated myocardium, while the amount of infarct and hibernating myocardium was comparable to those patients with no SCA. The amount of denervated myocardium had the strongest significant correlation with SCA. Lower tertiles of denervation showed SCA rates of 1.2 and 2.2 %/year, whereas the highest tertile showed a rate of 6.7 %/year. Each 1 % increase in the volume of denervated myocardium was associated with a 5.7 % increase in the risk of SCA. Multivariate predictors of SCA were the amount of denervation, LV end-diastolic volume index, serum creatinine, and no angiotensin inhibition therapy. With adequate thresholds, the absence of all four risk factors identified low risk (44 % of patients; SCA <1 %/year), whereas ≥2 factors identified high risk (20 % of patients; SCA approximately 12 %/year), improving the classification of patients most likely to benefit from an ICD. Further prospective validation of the model is needed, but because it is independent of LVEF among patients with moderate-to severe LV dysfunction, it may be useful to expand the risk stratification to a larger number of patients at risk of SCA.
Recently, Verschure et al. [21••] have reported a meta-analysis using the individual data of 636 HF patients retrieved from six published studies, five from Europe (n = 564) and one from the USA (n = 37), plus 35 additional patients added to the aggregated database, showing the intermediate- to long-term prognostic value of late HMR. All-cause mortality, cardiac mortality, arrhythmic events, and heart transplantation were assessed to determine which provided the strongest prognostic significance for cardiac 123I-mIBG imaging. The majority of the patients were male, had NI HF and a decreased LVEF, and were on conventional HF medical treatment. During follow-up (mean 36.9 months), 83 deaths occurred (67 cardiac), and there were 33 arrhythmic events and 56 heart transplants. In univariate regression analysis, HMR was a significant predictor of all event categories, but lowest HRs were for the composite endpoint of any event (HR 0.30, 95 %CI 0.19 to 0.46), all cause (HR 0.29, 95 %CI 0.16 to 0.53), and cardiac mortality (HR 0.28, 95 %CI 0.14 to 0.55). In multivariate analysis, HMR was an independent predictor for all event categories, except for arrhythmias, which indicates that HMR not only is useful as a dichotomous predictor of events (high vs. low risk), but also has prognostic implication over the full range of the outcome value for all event categories except arrhythmias. The less robust performance of HMR for prediction of arrhythmic events than for mortality has been observed in other studies. It is possible that the use of planar rather than SPECT assessment of myocardial innervation might have contributed to the lower performance of the imaging results, in agreement with other reports about the relationship between regional 123I-mIBG defect extent as assessed by SPECT imaging and the susceptibility of arrhythmic events [9, 22, 23].
Conclusions
Cardiac sympathetic derangement in HF patients is an indicator of unfavorable prognosis. Consequently, the use of cardiac sympathetic imaging in these patients might be useful as indicator of the effectiveness of the medical therapy and as guide-specific therapies. Although cardiac sympathetic imaging will not be the single determining factor for a treatment decision (same as other current risk markers), more complete information on the patient’s condition and future prognosis can only improve the ability to select the most effective management approach. However, it remains to be proven whether cardiac sympathetic improvement in response to effective HF medication translates to variations in response to drug dose changes in individual HF patients and whether patients with HF and LVEF ≤40 % randomized to cardiac sympathetic imaging-guided medical treatment would have better outcome than patients treated following current guidelines [24].
Despite the abundance of data on the value of many of current risk markers in arrhythmic risk stratification, most of them have failed thus far to be considered in clinical guidelines that define appropriateness for ICD therapy, being LVEF the single criterion that dictates eligibility for ICD therapy for the primary prevention of SCD. This is mainly due to the reluctance to accept the tradeoff between sensitivity and specificity, i.e., to accept few incremental arrhythmic events in patients who are not protected by an ICD in return for higher rates of appropriate device utilization in ICD recipients. In the future, the clinical use of cardiac sympathetic imaging for individual patient-risk stratification and therapeutic decision-making will depend on further development of the technique and further prospective documentation of its benefits in clinically relevant situations.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Jacobson AF, Narula J. Introduction to cardiac neuronal imaging: a clinical perspective. J Nucl Med. 2015;56:3S–6.
Nakajima K, Nakata T. Cardiac 123I-MIBG imaging for clinical decision making: 22-year experience in Japan. J Nucl Med. 2015;56:11S–9.
Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212–21.
Petretta M, Pellegrino T, Cuocolo A. Cardiac neuronal imaging with 123I-meta-iodobenzylguanidine in heart failure: implications of endpoint selection and quantitative analysis on clinical decisions. Eur J Nucl Med Mol Imaging. 2014;41:1663–5.
Ketchum ES, Jacobson AF, Caldwell JH, Senior R, Cerqueira MD, Thomas GS, et al. Selective improvement in Seattle Heart Failure Model risk stratification using iodine-123 meta-iodobenzylguanidine imaging. J Nucl Cardiol. 2012;19:1007–16.
Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120:835–42.
Narula J, Gerson M, Thomas GS, Cerqueira MD, Jacobson AF. 123I-MIBG imaging for prediction of mortality and potentially fatal events in heart failure: the ADMIRE-HFX study. J Nucl Med. 2015;56:1011–8. Demonstration of 123 I-mIBG late HMR as independent prognostic marker, additive to currently available risk stratification procedures.
Parker MW, Sood N, Ahlberg AW, Jacobson AF, Heller GV, et al. Relationship between quantitative cardiac neuronal imaging with 123I-meta-iodobenzylguanidine and hospitalization in patients with heart failure. Eur J Nucl Med Mol Imaging. 2014;41:1666–72.
Clements IP, Kelkar AA, Garcia EV, Butler J, Chen J, Folks R, et al. Prognostic significance of 123I-mIBG SPECT myocardial imaging in heart failure: differences between patients with ischaemic and non-ischaemic heart failure. Eur Heart J Cardiovasc Imaging 2015. doi:10.1093/ehjci/jev295.
Clements IP, Chen J, Folks R, Butler J, Jacobson AF. Quantitative iodine 123 meta-iodobenzylguanidine (123I mIBG) SPECT imaging in heart failure with left ventricular systolic dysfunction: development and validation of automated procedures in conjunction with technetium-99 m tetrofosmin myocardial perfusion SPECT. J Nucl Cardiol 2015. doi:10.1007/s12350-015-0097-4.
Shah AM, Bourgoun M, Narula J, Jacobson AF, Solomon SD. Influence of ejection fraction on the prognostic value of sympathetic innervation imaging with Iodine-123 MIBG in heart failure. J Am Coll Cardiol Img. 2012;5:1139–46.
Sood N, Al Badarin F, Parker M, Pullatt R, Jacobson AF, Bateman TM, et al. Resting perfusion MPI-SPECT combined with cardiac 123I-mIBG sympathetic innervation imaging improves prediction of arrhythmic events in non-ischemic cardiomyopathy patients: sub-study from the ADMIRE-HF trial. J Nucl Cardiol. 2013;20:813–20.
Al Badarin FJ, Wimmer AP, Kennedy KF, Jacobson AF, Bateman TM. The utility of ADMIRE-HF risk score in predicting serious arrhythmic events in heart failure patients: incremental prognostic benefit of cardiac 123I-mIBG scintigraphy. J Nucl Cardiol. 2014;21:756–62.
Saba S, Aban I, Soman P. 123I-mIBG scintigraphy: yet another risk stratifier for the heart failure toolbox! J Nucl Cardiol. 2014;21:909–12.
JCS Joint Working Group. Guidelines for clinical use of cardiac nuclear medicine (JCS 2010) – digest version –. Circ J. 2012;76:761–7.
Nakata T, Nakajima K, Yamashina S, Yamada T, Momose M, Kasama S, et al. A pooled analysis of multicenter cohort studies of 123I-MIBG-imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging. 2013;6:772–84.
Nakajima K, Okuda K, Yoshimura M, Matsuo S, Wakabayashi H, Imanishi Y, et al. Multicenter cross-calibration of I-123 metaiodobenzylguanidine heart-to-mediastinum ratios to overcome camera-collimator variations. J Nucl Cardiol. 2014;21:970–8.
Nakajima K, Nakata T, Yamada T, Yamashina S, Momose M, Kasama S, et al. A prediction model for 5-year cardiac mortality in patients with chronic heart failure using 123I-metaiodobenzylguanidine imaging. Eur J Nucl Med Mol Imaging. 2014;41:1673–82.
Nakajima K, Nakata T, Matsuo T, Jacobson AF. Creation of mortality risk charts using 123I meta-iodobenzylguanidine heart-to-mediastinum ratio in patients with heart failure: two- and five year risk models. Eur Heart J Cardiovasc Imaging 2015. doi:10.1093/ehjci/jev322. Creation of clinically useful mortality risk charts with three common clinical variables including age, NYHA class and LVEF, together with 123 I-mIBG late HMR for prognostic stratification in heart failure patients.
Fallavollita JA, Heavey BM, Luisi Jr AJ, Michalek SM, Baldwa S, Mashtare Jr TL, et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63:141–9.
Verschure DO, Veltman CE, Manrique A, Somsen GA, Koutelou M, Katsikis A, et al. For what endpoint does myocardial 123I-MIBG scintigraphy have the greatest prognostic value in patients with chronic heart failure? Results of a pooled individual patient data meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15:996–1003. A meta-analysis showing the intermediate to long-term prognostic value of 123 I-mIBG late HMR.
Boogers MJ, Borleffs CJ, Henneman MM, van Bommel RJ, Boersma E, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter defibrillator patients. J Am Coll Cardiol. 2010;55:2769–77.
Bax JJ, Kraft O, Buxton AE, Fjeld JG, Parizek P, Agostini D, et al. 123 I-MIBG scintigraphy to predict inducibility of ventricular arrhythmias on cardiac electrophysiology testing: a prospective multicenter pilot study. Circ Cardiovasc Imaging. 2008;1:131–40.
Wessler BS, Udelson JE. Neuronal dysfunction and medical therapy in heart failure: can an imaging biomarker help to “personalize” therapy? J Nucl Med. 2015;56:20S–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Albert Flotats and Ignasi Carrió declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
With regard to the authors’ research cited in this paper, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. In addition, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
This article is part of the Topical Collection on Cardiac Nuclear Imaging
Rights and permissions
About this article
Cite this article
Flotats, A., Carrió, I. Cardiac Innervation Imaging: Implications for Risk Stratification and Therapeutic Decision-Making. Curr Cardiovasc Imaging Rep 9, 6 (2016). https://doi.org/10.1007/s12410-015-9368-3
Published:
DOI: https://doi.org/10.1007/s12410-015-9368-3