Abstract
Background
The Seattle Heart Failure Model (SHFM) is a multivariable model that uses demographic and clinical markers to predict survival in patients with heart failure. Inappropriate activation of the sympathetic nervous system, which contributes to the progression of heart failure and increased mortality, can be assessed using iodine-123 meta-iodobenzylguanidine (MIBG) cardiac imaging. This study investigated the incremental value of MIBG cardiac imaging when added to the SHFM for prediction of all-cause mortality.
Methods
Survival data from 961 NYHA II-III subjects in the ADMIRE-HFX trial were included in this analysis. The predictive value of the SHFM alone and in combination with MIBG heart-to-mediastinum ratio (H/M) was compared for all-cause mortality (101 deaths during a median follow-up of 2 years).
Results
The addition of H/M to the SHFM in a Cox model significantly improved risk prediction (P < .0001), with a greater utility in higher risk SHFM patients. The observed 2-year mortality in the highest-risk SHFM subjects (rounded SHFM score of 1) was 24%, but varied from 46% with H/M <1.2 to 0% with H/M >1.8. Net reclassification improvement was 22.7% (P < .001), with 14.9% of subjects who died reclassified into a higher risk category than suggested by SHFM score alone (P = .01) and 7.9% of subjects who survived reclassified into a lower risk category (P < .0001). The 2-year integrated discrimination improvement (+4.14%, P < .0001) and the 1-year area under the receiver-operator characteristic curve (+0.04, P = .026) both showed significant improvement for the combined model with H/M compared to the SHFM alone.
Conclusion
The addition of MIBG imaging to the SHFM improves risk stratification, especially in higher risk patients. MIBG may have clinical utility in higher risk patients who are being considered for devices such as ICD, CRT-D, LVAD, and cardiac transplantation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heart failure is an important contributor to morbidity and mortality, with one in nine death certificates in the United States mentioning heart failure and approximately 50% of patients with the condition dying within 5 years of their diagnosis.1 The Seattle Heart Failure Model (SHFM) is a multivariable predictor of risk and mode of death in heart failure that has been validated in tens of thousands of subjects, ranging from ambulatory outpatients to ventricular assist device (VAD) recipients.2-6 SHFM includes a variety of demographic and clinical markers to predict 1- to 5-year mortality in heart failure patients. It also shows changes in life expectancy with alterations in heart failure medications and by placement of implantable cardioverter-defibrillators (ICD), biventricular cardiac resynchronization therapy (CRT), and left VADs (LVADs). A modified version of the SHFM (SHFM-D) was developed using a separate derivation cohort of 10,038 HF patients for analysis of the differential benefit of ICD placement in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT).7 The SHFM-D was validated in the placebo arm of SCD-HeFT with a 4-year predicted and observed survival of 71% and a 1-year c-statistic of 0.71 in the derivation cohort and in the validation cohorts. Subsequent application of the SHFM-D has resulted in an area under the receiver-operator characteristic curve (AUC) as high as 0.81.8
The reduced cardiac output of the failing heart leads to many compensatory neuroendocrine changes, one of which is a sustained activation of the sympathetic nervous system and subsequent changes in expression of autonomic receptors in the myocardium.9,10 Direct measurement of serum levels of norepinephrine has shown promise in smaller11,12 studies, although its predictive power has been less impressive in larger trials when compared against other markers.13 This is due in part to the complex relationship between local myocardial and systemic levels of norepinephrine. Indirect measures of autonomic nervous system function and altered sympathetic/parasympathetic balance, such as heart rate variability and turbulence, have also been evaluated but are currently only infrequently used for clinical risk assessment of heart failure patients.14 Myocardial imaging using an analogue of norepinephrine, iodine-123 meta-iodobenzylguanidine (MIBG), was first reported in the early 1980s as a potential marker of sympathetic dysregulation in the human heart.15,16 Numerous small studies have since demonstrated that sympathetic neuronal imaging provides prognostic information in heart failure patients.17,18
None of the variables included in the SHFM provides a direct measure of cardiac sympathetic neuronal status. An indication that addition of such a measure could improve performance of the SHFM was provided by a recent analysis of data from a small HF trial in the pre-β-blocker era.19 This study was undertaken to expand upon these observations to better assess the incremental value of MIBG imaging when added to the SHFM. We hypothesized that a model combining the SHFM-D risk score and cardiac MIBG imaging results would be additive to both for all-cause and cardiac mortality prediction.
Methods
Patient Population
The primary efficacy population from the AdreView Myocardial Imaging for Risk Evaluation in Heart Failure (ADMIRE-HF) trial was examined in this study. The population consisted of 961 NYHA II-III heart failure patients with impaired systolic function (ejection fraction ≤35%) who were on guideline-recommended medical therapy.20 Subjects were excluded at enrollment if they had a functioning cardiac pacemaker (including for resynchronization) or had ever received electrical therapy (defibrillation or pacing, including appropriate ICD shock) for a ventricular arrhythmia. Subjects were not withdrawn from the trial if they received a device (ICD with or without CRT) after enrollment in the trial or as a result of any device activation (pacing, shock, etc). Subjects were followed for a maximum of 2 years after cardiac MIBG imaging. The original trial was closed and the primary data analyzed after a pre-specified number of cardiac end points occurred. 470 of the surviving patients who did not reach 2 years of follow-up were subsequently enrolled in ADMIRE-HFX and underwent additional surveillance to reach two full years of follow-up. The extension study was approved by the Institutional Review Board or Ethics Committee at each participating center, and all subjects signed informed consent for the additional data collection. The combined dataset from the original and extension trials was used for the present analysis.
Baseline clinical data recorded prior to administration of MIBG were used to calculate the SHFM-D. The SHFM-D was calculated as previously described, with variables including age, gender, systolic blood pressure, ischemic origin, NYHA class, ejection fraction, serum sodium and creatinine, the use of medications [angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), β-blocker, carvedilol, statin, digoxin, and furosemide equivalent daily dose in milligrams per kilogram], and a variable ICD benefit as shown in SCD-HeFT.7 Rare missing values were assigned the cohort mean of the variable.
Statistical Methods
SHFM-D and the late heart-to-mediastinum ratio (H/M) on cardiac MIBG imaging were compared independently and in combination against the end points of all-cause mortality and cardiac mortality, both of which were independently adjudicated by a panel of 5 cardiologists.21 Statistical analyses were conducted using commercial statistics packages (SPSS 19.0, Chicago, IL; Stata 11.2, College Station, TX). Baseline comparisons were completed using Student’s t test. Cox proportional hazards modeling was employed with the use of Chi-squared calculations for comparison of factors. The Hosmer-Lemeshow goodness-of-fit statistic was used to assess model calibration.22 To facilitate graphical comparisons of risk profiles, H/M and SHFM-D scores were clustered into risk groups: <1.2, 1.2-1.39, 1.4-1.59, 1.6-1.79, ≥1.8 for H/M and rounded-to-the-nearest integer (−2, −1, 0, 1, 2) for SHFM-D.
To allow a comparison of risk estimates with disparate variables, SHFM-D score and H/M risk were expressed as one standard deviation changes in risk score (0.688 and 0.202, respectively). The H/M variable was added to the SHFM-D and a formal interaction term was tested (SHFM-D * H/M) to determine if the risk of H/M varied with the SHFM-D score. H/M Kaplan-Meier survival curves were compared via log rank testing among absolute strata of SHFM-D score. SHFM-D scores for this comparison were rounded to the nearest integer (−1, 0, or 1) to cluster patients into risk groupings. The small number of patients (n = 31) with rounded scores of −2 or +2 was included in the −1 or 1 risk strata, respectively, for ease of depiction.
The SHFM-D and H/M were combined in a Cox proportional hazards model.23 The net reclassification improvement (NRI) and integrated discrimination improvement (IDI),24 discrimination slope method,25 and area under the receiver operator characteristic curve (AUC)26 were calculated using published methods in SPSS 19. Comparison of AUCs was done with the Hanley-McNeil method in Stata 11.2. For all analyses, P ≤ .05 was considered statistically significant.
Results
SHFM-D and H/M as Single Variable Predictors
101 deaths occurred among 961 subjects, with a median of 24 months and a mean of 21 months (25th to 75th percentile range 21-24 months) of follow-up. There were 64 cardiac deaths (63% of all deaths). Baseline characteristics of all subjects and comparison between the 860 survivors and the 101 who died are shown in Table 1. Subjects who died were older with lower EF, greater QRS width, higher daily diuretic dose, and increased serum creatinine. The average SHFM-D risk score was −0.05 in the survivors and 0.44 in the patients who died (P < .0001). Figure 1 depicts Kaplan-Meier survival curves generated for rounded SHFM-D risk scores (five categories of grouped risk), which varied from 0% to 30% 2-year mortality. The mean H/M was 1.45 in survivors and 1.35 in those who died (P < .0001). Figure 2 depicts Kaplan-Meier survival curves based on H/M scores divided into five risk groups, which varied from 0% to 24% 2-year mortality.
The SHFM-D was highly predictive of all-cause mortality with an 85.6% increase in risk for each one standard deviation increase in the risk score (95% CI 1.568-2.197, P < .0001) and of cardiac death specifically with an 89.1% increase in risk for each one standard deviation increase in the score (95% CI 1.534-2.332, P < .0001). The SHFM-D predicted versus Kaplan-Meier observed survival was similar at 1 year (95.1% ± 0.1% vs 94.6% ± 0.7%), and 2 years, (90.1% ± 0.2% vs 88.3% ± 1.1%). The Hosmer-Lemeshow goodness-of-fit statistic showed appropriate calibration at 1 year (P = .46) and 2 years (P = .17).
H/M was predictive of all-cause mortality with a 71.5% increase in risk for each one standard deviation decrease in H/M (95% CI 1.366-2.154, P < .0001) and of cardiac death specifically with an 69.0% increase in risk for each one standard deviation decrease in the score (95% CI 1.271-2.249, P = .0003). There was a low correlation of SHFM-D and H/M (0.144, P < .0001).
The SHFM-D and H/M Multivariable Model
Kaplan-Meier survival curves were generated by stratification across categories of SHFM-D and H/M (Figure 3A-C). H/M did not improve risk stratification in low-risk patients (SHFM-D of −1, P = .59). Clinically relevant and statistically significant differences were seen for H/M in the KM curves for SHFM-D scores of 0 and 1. The observed 2-year survival in the high-risk group with a SHFM-D rounded score of 1 (mean survival of 76%) varied by H/M value: 54%, 72%, 82%, 88%, and 100% for, respective, H/M groups of <1.2, 1.2-1.39, 1.40-1.59, 1.6-1.79, and ≥1.80.
Each panel depicts one stratum of rounded-to-the-nearest-integer SHFM-D risk score. Within each panel, Kaplan-Meier curves are presented for different ranges of H/M. In parentheses, the number of deaths/number of subjects for each group are shown. A H/M <1.2, 1.6 to <1.8, and ≥1.8 each had 100% survival and the survival curves are overlapping
In a combined Cox model, SHFM-D and H/M were independent predictors of survival with an 82.1% (P < .0001) and 60.3% (P < .0001) increase in risk, respectively, for a one standard deviation change. For the endpoint of cardiac mortality, each one standard deviation increase in SHFM-D was associated with an 86.1% increase in risk (P < .0001) and each one standard deviation decrease in H/M was associated with a 57.9% increase in risk (P = .002).
An interaction term was introduced into the model (SHFM-D * H/M) to determine if the predictive power of H/M varied with the SHFM-D score for all-cause mortality, which was significant (P = .039). After dividing the dataset into two cohorts by the median value of SHFM-D, each one standard deviation change in H/M was associated with a 10.9% (P = .61) increase in risk below the median and an 88.5% increase in risk (P < .0001) above the median. The impact of addition of H/M to the SHFM-D score with the continuous interaction term is depicted in Figure 4. A subject with an SHFM-D score of 1 had an estimated annual mortality of 10.5%. The estimated 1-year mortality with addition of H/M varied from 2.3% with a H/M of 1.8 to 30% with a H/M of 1, corresponding to a 12.8-fold difference in risk for a 4 standard deviation change in H/M ratio.
The impact of H/M added to the SHFM-D score with the interaction term within ADMIRE-HFX. Each line represents approximately one standard deviation change in H/M (1SD = 0.202). The black line is the estimate by the SHFM-D alone. Estimated 1-year mortality changes with addition of H/M from 2.3% with H/M of 1.8 to 30% for H/M of 1. This corresponds to a 12.8-fold difference in risk for a 4 standard deviation change in H/M ratio
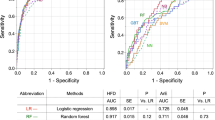
The 1-year ROC curves for SHFM-D alone, H/M alone, and the combined model in prediction of all-cause mortality are shown graphically in Figure 5. The combined model significantly increased the 1-year AUC by 0.039 (P = .026) and 2-year AUC by 0.028 (P < .05). The change in 1-year AUC increment was greater above the median SHFM-D (+0.069) than below the median SHFM-D (+0.01), concordant with the Cox model results.
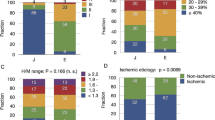
The NRI is depicted in Table 2. Cut-points for 2-year mortality of <10%, 10-20%, 20-40%, and >40% demonstrated improvement of risk characterization (P < .0001) for the combined model compared to the SHFM-D alone, with a NRI of 22.7%. 14.9% of patients who died were reclassified into a higher risk category by the combined model as compared to their risk category by SHFM-D alone, while 7.9% of patients who survived were reclassified into a lower risk category. A graphical depiction of reclassification for the 761 subjects not censored before 2 years of follow-up, with the 20-40% and >40% risk subjects grouped together into a >20% risk bucket is shown in Figure 6. The IDI at 2 years corresponded to a 4.14% absolute change (P < .0001) in estimated mortality when H/M was added to the SHFM-D. The IDI for the combined model was especially significant in those with an SHFM-D score above the all-subject SHFM-D median (5.92% improvement in absolute risk prediction, P < .0001). There was an average 30% increase in the discrimination slope in those who died versus an 11% decrease in those censored alive (between group comparison, P = .0002).
Graphical depiction showing how subject risk classification (<10%, 10%-20%, >20% 2-year mortality) changed from starting SHFM-D risk groups to SHFM-D + H/M risk groups in terms of both percentage of subjects falling within each reclassified group (pie charts) and observed mortality for the reclassified risk groups (bar charts) in the 761 subjects who died or reached two full years of follow-up
Discussion
Additive Value of MIBG Cardiac Imaging and Risk Stratification
A large number of clinical, laboratory, and ECG variables have been shown to have prognostic significance, individually and in combination, in HF patients.27 The challenge for each new prognostic marker is to demonstrate significant added value in risk prediction. SHFM combines many aspects of baseline risk—from a patient’s ejection fraction and NYHA class to lab markers and medications—into one quantitative number that clinicians can integrate together in a holistic way to estimate patient risk.
This analysis shows that cardiac MIBG, with H/M treated as a continuous variable for risk assessment rather than dichotomized at 1.60 as in the primary study analyses,20 adds meaningful prognostic information when added to the SHFM-D. This is in contrast to many similar analyses in which newer risk markers did not show an improvement in AUC in conjunction with an established multivariate risk model. For example, the addition of 10 biomarkers to the Framingham risk score failed to produce a statistically significant increase in the AUC,28 while the addition of either peak oxygen consumption in HF-ACTION29 or the biomarkers NT-ProBNP and ST230 failed to significantly increase the 1-year c-statistic when added to the SHFM. In the Framingham population, age and gender alone provided an AUC of 0.75 for death and the addition of the conventional risk factors increased the AUC by 0.05 to 0.80. Coronary calcification by CT scan increased the ROC by 0.042 when added to age alone, and likely even less when added to a clinical risk model that includes age.31 The incremental value of H/M was 0.04 when added to the robust SHFM-D model, similar to adding coronary artery calcification to age or conventional risk factors to age and gender. For comparison, age and gender in this cohort provided an AUC of only 0.585, which is typical in heart failure populations, while the SHFM increased the AUC by 0.11 and H/M by an additional 0.04 for the entire cohort and by 0.069 for the cohort with above-median SHFM risk score.
The addition of H/M to the SHFM was statistically and clinically significant by the Cox proportional hazards model, 1- and 2-year AUC change, NRI and IDI change, and the discrimination slope methodology. The AUCs demonstrated in this relatively low event rate population (annual mortality of ~6%, with 37% non-cardiac deaths) are comparable to previous validation cohorts of the SHFM seen in study populations with significantly higher annual mortality.2 The greatest improvement in risk discrimination was seen in those patients above the median SHFM score (>4% annual mortality) with an AUC change of 0.069. The small and not clinically significant correlation between SHFM and H/M risk scores potentially explains the increased prognostic value of the combined model: the low correlation coefficient suggests that the SHFM and H/M are measuring different components of risk in the heart failure population.
Potential Applications to Heart Failure Therapy
Numerous studies have demonstrated the prognostic significance of MIBG cardiac imaging results in HF patient populations,17,32-34 but there remains uncertainty regarding how best to use this procedure. While dysfunction of the cardiac adrenergic nervous system clearly contributes to adverse outcomes in heart failure patients, identifying those most likely to benefit from addition of a direct assessment of this system to the many other accepted tests routinely used in the initial evaluation and monitoring of therapy is critical. By starting with a model that already incorporates most of the important prognostic factors (SHFM-D), the potential value of the additional insight provided by cardiac MIBG imaging becomes clearer. In clinical practice, the combined power of the SHFM-D plus H/M model offers an improved estimate of the absolute mortality risk, which might alter clinical decisions such as timing of listing for cardiac transplantation or implantation of an ICD, CRT-D, or LVAD.
The SHFM is used to identify patients who meet annual mortality thresholds to qualify for cardiac transplantation and placement of LVAD.35 Consideration of LVAD placement is recommended by the latest AHA/ACC guidelines in patients with a 1-year mortality rate of >50%. Retrospective application of the SHFM to patients who have received bridge to transplant or destination LVADs suggests most centers are using >25%-30% annual mortality as a threshold, consistent with Center for Medicare and Medicaid Services guidelines of mean survival of <2 years.5,36,37 Recent studies have advocated placement in a less critically ill patient population,38 with an SHFM annual mortality ≥16.5% being used as a threshold in an ongoing National Heart, Lung, and Blood Institute trial of early versus usual VAD placement in ambulatory NYHA 3 heart failure patients.39 Adding H/M to the SHFM alone allowed significant risk reclassification. The number of subjects who died during the study period and were classified in the highest risk category of >40% 2-year mortality increased from 1 subject based on SHFM score alone to 13 subjects with the added information from H/M. Identification of higher risk patients by MIBG with SHFM may allow earlier consideration and implementation of LVAD than possible by SHFM alone.
Multiple studies have suggested a differential benefit to ICD placement, with a higher risk patient population not showing mortality benefit after implantation.40,41 Despite the bulk of this study population being lower risk, the 10% with the most unfavorable risk scores by the combined model had 2-year Kaplan-Meier mortality of 37%, 4.5 times that of the remainder of the cohort. This is the population that appears not to benefit from an ICD, possibly because subjects die of progressive heart failure rather than sudden cardiac death after placement of an ICD. The SHFM has been demonstrated to allow prediction of a high-risk (>20% annual mortality) patient population that does not benefit from a primary prevention ICD.7 It is possible that this high-risk population can be identified even more accurately via the combined model.
Study Limitations
The study population was predominantly ambulatory and in NYHA class II, with lower cardiac mortality than seen in most therapeutic trials of symptomatic HF patients. Despite the low event rate, the present analyses successfully applied a well-validated risk model to expand on the limited multivariate analyses performed in the original ADMIRE-HF trial.20,21 It is likely that inclusion of other variables in the original SHFM such as hemoglobin, percent lymphocytes, uric acid, and total cholesterol would have increased the AUC, but it is unlikely that the incremental value of MIBG would have been altered. It would have been helpful to have a longer time of observation to increase the number of events. This cohort had a relatively high rate of non-cardiac deaths (37%), which likely decreased the AUC of both SHFM and MIBG. It would be helpful to validate the prognostic utility of MIBG in a higher risk HF population (>10% annual mortality), where it appears MIBG may have the most clinical utility. The present analyses utilized only planar MIBG H/M data, even though several clinical trials examining arrhythmic event risk stratification have shown that MIBG SPECT (but not planar) results were able to identify patients with increased likelihood of inducible ventricular arrhythmias42 or appropriate ICD shocks.43 As this study was not a randomized trial to any particular intervention based on MIBG results, caution should be applied to use of the findings as a basis for selection of patients for device therapy.
Conclusions
MIBG provides a meaningful increase in risk stratification when added to the SHFM in ambulatory NYHA 2-3 heart failure patients. The additional prognostic information may assist in assessing higher risk patients for clinical interventions such as CRT-D, ICD, transplant, and LVAD.
References
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012;125:e2-220.
Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 2006;113:1424-33.
May HT, Horne BD, Levy WC, Kfoury AG, Rasmusson KD, Linker DT, et al. Validation of the Seattle Heart Failure Model in a community-based heart failure population and enhancement by adding B-type natriuretic peptide. Am J Cardiol 2007;100:697-700.
Kalogeropoulos AP, Georgiopoulou VV, Giamouzis G, Smith AL, Agha SA, Waheed S, et al. Utility of the Seattle Heart Failure Model in patients with advanced heart failure. J Am Coll Cardiol 2009;53:334-42.
Ketchum ES, Moorman AJ, Fishbein DP, Mokadam NA, Verrier ED, Aldea GS, et al. Predictive value of the Seattle Heart Failure Model in patients undergoing left ventricular assist device placement. J Heart Lung Transplant 2010;29:1021-5.
Mozaffarian D, Anker SD, Anand I, Linker DT, Sullivan MD, Cleland JG, et al. Prediction of mode of death in heart failure: The Seattle Heart Failure Model. Circulation 2007;116:392-8.
Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation 2009;120:835-42.
Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail 2011;4:180-7.
Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med 1982;307:205-11.
Francis GS. Neurohumoral mechanisms involved in congestive heart failure. Am J Cardiol 1985;55:A15-21.
Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984;311:819-23.
Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: Evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation 1986;73:615-21.
Latini R, Masson S, Anand I, Salio M, Hester A, Judd D, et al. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val-HeFT. Eur Heart J 2004;25:292-9.
Lorvidhaya P, Addo K, Chodosh A, Iyer V, Lum J, Buxton AE. Sudden cardiac death risk stratification in patients with heart failure. Heart Fail Clin 2011;7:157-74. vii.
Kline RC, Swanson DP, Wieland DM, Thrall JH, Gross MD, Pitt B, et al. Myocardial imaging in man with I-123 meta-iodobenzylguanidine. J Nucl Med 1981;22:129-32.
Wieland DM, Brown LE, Rogers WL, Worthington KC, Wu JL, Clinthorne NH, et al. Myocardial imaging with a radioiodinated norepinephrine storage analog. J Nucl Med 1981;22:22-31.
Verberne HJ, Brewster LM, Somsen GA, van Eck-Smit BL. Prognostic value of myocardial 123I-metaiodobenzylguanidine (MIBG) parameters in patients with heart failure: A systematic review. Eur Heart J 2008;29:1147-59.
Momose M, Okayama D, Nagamatsu H, Kondo C, Hagiwara N, Sakai S. Long-term prognostic stratification by a combination of (123)I-metaiodobenzylguanidine scintigraphy and ejection fraction in dilated cardiomyopathy. Ann Nucl Med 2011;25:419-24.
Kuramoto Y, Yamada T, Tamaki S, Okuyama Y, Morita T, Furukawa Y. Usefulness of cardiac iodine-123 meta-iodobenzylguanidine imaging to improve prognostic power of Seattle Heart Failure Model in patients with chronic heart failure. Am J Cardiol 2011;107:1185-90.
Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol 2010;55:2212-21.
Jacobson AF, Lombard J, Banerjee G, Camici PG. 123I-mIBG scintigraphy to predict risk for adverse cardiac outcomes in heart failure patients: Design of two prospective multicenter international trials. J Nucl Cardiol 2009;16:113-21.
Lemeshow S, Hosmer DW Jr. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol 1982;115:92-106.
van Houwelingen HC. Validation, calibration, revision and combination of prognostic survival models. Stat Med 2000;19:3401-15.
Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157-72. (discussion 207-212).
Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010;21:128-38.
Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 2007;115:928-35.
Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s heart disease: A textbook of cardiovascular medicine. Philadelphia: Elsevier; 2011.
Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med 2006;355:2631-9.
Levy WC, Aaronson KD, Dardas TF, Williams P, Haythe J, Mancini D. Prognostic impact of the addition of peak oxygen consumption to the Seattle Heart Failure Model in a transplant referral population. J Heart Lung Transplant 2012;31:817-24.
Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail 2011;4:180-7.
Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J Am Coll Cardiol 2007;49:1860-70.
Kasama S, Toyama T, Sumino H, Kumakura H, Takayama Y, Minami K, et al. Prognostic value of cardiac sympathetic nerve activity evaluated by [123I]m-iodobenzylguanidine imaging in patients with ST-segment elevation myocardial infarction. Heart 2011;97:20-6.
Akutsu Y, Kaneko K, Kodama Y, Li HL, Suyama J, Shinozuka A, et al. Iodine-123 mIBG imaging for predicting the development of atrial fibrillation. JACC Cardiovasc Imaging 2011;4:78-86.
Horiguchi Y, Morita Y, Tsurikisawa N, Akiyama K. 123I-MIBG imaging detects cardiac involvement and predicts cardiac events in Churg-Strauss syndrome. Eur J Nucl Med Mol Imaging 2011;38:221-9.
Mancini D, Lietz K. Selection of cardiac transplantation candidates in 2010. Circulation 2010;122:173-83.
Strueber M, O’Driscoll G, Jansz P, Khaghani A, Levy WC, Wieselthaler GM. Multicenter evaluation of an intrapericardial left ventricular assist system. J Am Coll Cardiol 2011;57:1375-82.
Pamboukian SV, Tallaj JA, Brown RN, Nielsen T, George JF, Kirklin JK, et al. Comparison of observed survival after ventricular assist device placement versus predicted survival without assist device using the Seattle Heart Failure Model. ASAIO J 2012;58:93-7.
Meyns BP, Simon A, Klotz S, Wittwer T, Schlensak C, Rega F, et al. Clinical benefits of partial circulatory support in New York Heart Association Class IIIB and Early Class IV patients. Eur J Cardiothorac Surg 2010;58:S173-8.
Klotz S, Meyns B, Simon A, Wittwer T, Rahmanian P, Schlensak C, et al. Partial mechanical long-term support with the CircuLite Synergy pump as bridge-to-transplant in congestive heart failure. Thorac Cardiovasc Surg 2010;58:S173-8.
Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 2008;51:288-96.
Frankenstein L, Zugck C, Nelles M, Schellberg D, Remppis A, Katus H. Primary ICD-therapy in patients with advanced heart failure: Selection strategies and future trials. Clin Res Cardiol 2008;97:594-600.
Bax JJ, Kraft O, Buxton AE, Fjeld JG, Parizek P, Agostini D, et al. 123 I-mIBG scintigraphy to predict inducibility of ventricular arrhythmias on cardiac electrophysiology testing: A prospective multicenter pilot study. Circ Cardiovasc Imaging 2008;1:131-40.
Boogers MJ, Borleffs CJ, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol 2010;55:2769-77.
Conflict of interest
Dr Jacobson is an employee of GE Healthcare and owns shares in the General Electric Company. Dr Caldwell has served as a consultant for GE Healthcare. Dr Cerqueira is a consultant for GE Healthcare and Astellas Pharma USA, is on the Speakers’ Bureau of Astellas Pharma USA, and has received research grants from Perceptive Informatics USA. Dr Agostini has received research support and honoraria from GE Healthcare. Dr Levy and the University of Washington Center for Commercialization have received a grant from GE Healthcare for the use of the SHFM in this study, research grants from HeartWare, Thoratec, and NIH, and licensing fees from Epocrates.
Author information
Authors and Affiliations
Corresponding author
Additional information
See related editorial, doi:10.1007/s12350-012-9615-9.
The study was supported financially by GE Healthcare.
Rights and permissions
About this article
Cite this article
Ketchum, E.S., Jacobson, A.F., Caldwell, J.H. et al. Selective improvement in Seattle Heart Failure Model risk stratification using iodine-123 meta-iodobenzylguanidine imaging. J. Nucl. Cardiol. 19, 1007–1016 (2012). https://doi.org/10.1007/s12350-012-9603-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-012-9603-0