Abstract
The systematic and comprehensive geochemical analysis of dissolved fluoride (F−) in thirty-nine groundwater samples were carried out to understand the spatial and temporal variability, areas of potential risk, and mechanism of fluoride precipitation/solubility in Central India. The dissolved ions showed weathering of carbonate minerals in the study area. Fluoride concentrations were beyond the permissible limit (>1.5 mg/l) in the pre-monsoon and monsoon seasons. The fluoride concentrations showed following spatial variations: urban area > village in agricultural area > village in forest area. The human population living in village, urban, and forest areas reported skin, mouth, and stomach problems due to high fluoride in the groundwater. Rainfall shifted the unsaturated to saturated values of SIC and SIF in the monsoon season. Strong indicators of alkaline condition were effective-CO2 pressure (\( { \log }P_{{{\text{CO}}_{ 2} }} \)) and HCO3 −/Ca+2 ratio. The dissolved fluoride showed positive relationship with pH, TDS, and HCO3 −/Ca+2 explaining the global variability observed in ground water fluoride.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoride is a common natural contaminant in groundwater supplies in both industrialized and developing countries. Globally, researchers have reported fluorosis due to intake of fluoride-rich water (Kim and Jeong 2005; Chae et al. 2007; Naseem et al. 2010). The problem of excessive fluoride in ground water in India was first reported in 1937 in the state of Andhra Pradesh (Short et al. 1937). At present, fluorosis is endemic in at least 20 states, affecting more than 65 million people, including 6 million children in Indian subcontinent (Godfrey et al. 2006; Ayoob and Gupta 2006; Khaiwal and Garg 2007). The Bureau of Indian Standards (BIS) and Indian Council of Medical Research (ICMR) prescribed fluoride concentration of 1.0 mg/l as the desirable limit, and 1.5 mg/l as the maximum permissible limit (WHO 2004) in drinking water, if there is no alternate source (Susheela 1999). According to census 2001, 48 % of the households in the state of Madhya Pradesh, Central India have hand pumps in which fluoride concentrations exceed 1.5 mg/l (CGWB 2010) and villagers who consumed such non-potable groundwaters suffered from yellow, cracked teeth, joint pains, crippled limbs and also aged rapidly.
Globally, many studies reported that the geological formations determine the water–rock interaction, responsible for the spatial variation in the groundwater fluoride (Handa 1975; Nordstrom and Jenne 1977; Edmunds et al. 1984; Robertson 1986; Hitchon 1995) and are commonly associated with rural areas, arid and semi-arid climate, granites, and gneisses, and advanced stage of groundwater development in the Indian subcontinent (Rao 2009). Chae et al. (2007) found following order of median fluoride concentration with respect to geology: metamorphic rocks > granitoides > complex rocks > volcanic rocks > sedimentary rocks. According to Pertti and Backman (1995) granite contains 0.05–0.14 % of fluorine, which is much higher than other types of rock (0.01–0.05 %). Igneous rocks (such as granites, gneisses, and basalts) and sedimentary rocks (such as shales, limestone, and sandstone) contain fluoride in the range of 300–1200 and 50–800 ppm, respectively (Kabata-Pendias and Pendias 2001). The major source of fluoride entering the hydrological system can be traced to volcanic activity associated with rift formation and chemical weathering of volcanic rocks (Gaciri and Davies 1993).
The groundwater percolation through the weathered rocks under arid to semi-arid conditions with relatively high alkalinity in the aquifer dissolves fluoride-bearing minerals (e.g., fluorspar, flourapatite, cryolite, and hydroxyl apatite) and releases fluoride into solution (Falvey 1999; Carrillo-Rivera et al. 2002; Farooqi et al. 2007). With respect to ground water chemistry, many studies reported high fluoride concentrations in Na–HCO3-type groundwater and lowest in Ca–HCO3-type ground water (Lee et al. 1997; Abu Rukah and Alsokhny 2004; Chae et al. 2007; Chidambaram et al. 2013). However, these studies lack information about the mechanism of seasonal variation in groundwater fluoride (Patel et al. 2014). In this scenario, the present work was carried out to understand the spatial and temporal variability, areas of potential risk, and mechanism of fluoride precipitation/solubility around Sidhi District, Central India.
Materials and Methods
Study Area
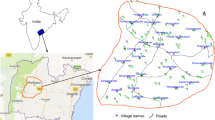
The Sidhi District is situated on the North-eastern boundary of the Madhya Pradesh State, India which lies over a transitional area between the Indo-Gangetic plain in the north and the Deccan plateau in the south. It is situated between 22°47.5′ and 24°42.10′ North latitude and 81°18.40′ and 82°48.30′ East latitude (Fig. 1). The land is largely undulating terrain, which often has steep gradients not ideal for cultivation; the valleys along the major rivers like the Son and the Gopad have fertile soils.
The total population of the area is 11, 26,515 spread with a density of 110/km2 (Census 2011). The total land covered in the Sidhi district is 10,536 km2 in which the forest area covers 40 %. The portion of the land used for agricultural purpose is 47 % but only 17 % of the land used for agricultural purpose has assured supply of irrigation and the rest of the agricultural land is dependent on rain fed irrigation and produces only one crop a year. The climate of the area is tropical monsoon type and the average annual rainfall varies from 1000 to 1200 mm, peaks in the months of July and August. The crops grown are rice, maize, barley, pigeon pea, and jute in Kharif season (monsoon). The natural vegetation comprises tropical dry deciduous forests.
Geology
The physiography is characterized by low hill, extensive plateaus, and river valleys such as Son river valley which is a depository of the Gondwana rocks. Topographically the district can be divided into three zones, Vindhyan hills or Kaimour range, Gondwana zone and Archean zone. Clay minerals like kaolinite, halloysite, diaspore, gibbsite, nacrite, dicktite, etc., were derived by the localized weathering of arkosic metasediments (Mehrotra et al. 1979). Sidhi area exposes the oldest rock units of tonalite trondjhemite series formed during proto-continental stage (3.7–2.9 Ga) representing the ancient crust (Roy and Bandyopadhyay 1990). The sand gravel, bauxite, china clay, fireclay, limestone, gold, and graphite are important minor minerals extracted in the district. Sporadic and some concentrated occurrence of granite veins were reported from the district. Similarly some low-grade copper and coal deposits were also recorded in various parts of Sidhi district.
Son catchment has a large amount of river-deposited sediments. The Son catchment slopes from North south and is interrupted locally by sandy regions and basins. A significant aspect of the catchment reflects various depositional surfaces having a succession of overlaying sediments. Highly productive and extensive, unconfined and semi-confined aquifers have formed due to presence of favorable factors such as highly fertile Neoarchaean to Palaeoproterozoic basement-cum-provenance, episodic mafic and acid magmatism and basement reactivation generated channels-ways for hydrothermal activity at suitable locales viz fracture/shear zones in the Sidhi district (Banerjee et al. 2010). Water level fluctuates with seasonal recharge and discharge and varied from 12.2 to 48.8 m below ground level, showed varying degree of hydraulic conductivity.
Field Sampling and Laboratory Analysis
To understand the spatial and temporal variation in fluoride enrichment, 39 groundwater samples were collected from hand pumps in such a way that they represent entire geological formations and land use patterns at varying topography of the Sidhi District in the pre-monsoon season (June, 2013) and monsoon season (August, 2013). Among 39 groundwater samples, 18 locations lie in the Vindhyan region, seven in the Dharwar region, seven in Archean range, four in Permean, and three in Jurassic. The information about the age of hand pumps were collected from local people and the range varied from less than 1–20 years, with an average of 7 years. Similarly, the depth information was collected from users (local people) and it varied from 12.2 to 48.8 m with an average of 25.9 m in the pre-monsoon season.
The groundwater samples were collected in clean polypropylene bottles without any air bubbles. Samples from each location were collected for anion and cation analysis. Groundwater samples were filtered by 0.45 m Millipore filter paper and acidified with 2 N HNO3 (Ultra pure Merck) for cation analysis and HBO3 acid was used as a preservative for nitrate analysis. In situ measurements for temperature, pH, EC, TDS, Salinity, and ORP were carried out in the field using multi-parameter analysis kit PCSTestr35. The bottles were properly labeled and brought to laboratory for determining the physico-chemical analysis.
Major cation analysis (Na+, K+, Ca+2, and Mg+2) was carried out by EEL Flame Photometer (APHA 1995). The concentration of HCO3 − was measured by acid titration, Cl− by AgNO3 titration, SO − 24 by Ba(ClO4)2 titration, NO3 − by the UV spectrophotometer screening method; the dissolved silica was determined by the molybdosilicate method, PO −34 by the ascorbic acid method, and fluoride (F−) by SPANDS method (APHA 1995). To maintain the purity and quality of the procedure, a known standard was used after every five samples. High purity reagents (Merck) and milli-Q water (Model Milli-Q, Biocel) were used for all the analyses. Analyses were carried out in duplicates to quantify the error. An overall precision was obtained below 5 % for the entire samples. Analytical precision for the measurement of ions was determined by calculating the Normalized Inorganic Charge Balance (NICB), which is defined as [Tz+−Tz−/Tz++Tz−] and represents the fractional difference between the total cations and total anions ((Edmond et al. 1995; Huh et al. 1998). The saturation index of calcite and fluorite was derived using PHREEQC version 2. The partial pressure of the CO2 at 25 °C was calculated from pH and HCO3 − content of the groundwater using equation \( { \log }P_{{{\text{CO}}_{ 2} }} \) = 7.9 + log aH2CO3 − pH (Panigrahy and Raymahashay 2005). Saturation of calcite and fluorite was computed with the help of the standard formulas (Hem 1970; Parkhurst and Appelo 1999):
where the prefix “a” represents the activity of the ion in moles/l.
To evaluate the potential relationship between the various physicochemical parameters, the factor analysis for groundwater samples was carried out by using “Statistical Package for Social Sciences (SPSS), version-16.0.″ The “Principal component analysis” and “Varimax Rotation” were used for extracting and deriving factors, respectively. The map of spatial distribution pattern of dissolved fluoride was produced by using the Arcview (9.3) software for ordinary kriging interpolation.
Results
The values of physico-chemical parameters of pre-monsoon and monsoon season are given in Table 1. The pH of groundwater was alkaline in the monsoon season (7.1–8.5) while acidic to alkaline variation in pH (6.2–7.8) of groundwater in the pre-monsoon season. Electrical conductivity ranged from 52 to 1244 and 60.8 to 1352 μS/cm in the pre-monsoon and monsoon season, respectively. The fluoride concentrations are higher than the maximum tolerance limit (1.5 mg/l) recommended by World Health Organization (WHO 2004). It varied from 1.4 to 2.8 and 2.1 to 3.5 mg/l in the pre-monsoon and monsoon, respectively. The ionic balances were generally within ± 5 %. Dominating cations were of the order: Ca+2 > Mg+2 > Na+ > K+ in both the seasons. The dominating anions were of the order: HCO3 − > SO −24 > Cl− > F− > NO3 − and HCO3 − > SO +24 > Cl− > NO3 − > F− in the pre-monsoon and monsoon seasons, respectively. The K+ showed high concentrations in the pre-monsoon season (1.8–8.8 mg/l). The NO -3 concentrations varied from 0 to 16.2 mg/l in the monsoon season. The SiO2 showed significant spatial and seasonal variations and varied from 13.8 to 62.1 mg/l in the pre-monsoon season and 13.1–71.6 mg/l in the monsoon season.
Discussion
Seasonal and Spatial Variation in Geochemistry
The groundwater samples showed an increment in all the ion concentrations in the monsoon season indicating rainwater infiltration through soil profile and bed-rock leading to desorption of ions. The positive linear relationship for (Ca+2 + Mg+2) versus Tz+ in both the seasons showed weathering of carbonate minerals as the major sources of Ca+2 + Mg+2 (70–80 %) in the total cationic concentrations (Fig. 2a, b). The linear relationship for HCO3 − versus Ca+2 + Mg+2 (Fig. 2c, d), revealed that the dissolution of carbonate minerals involve concomitant production of bicarbonate ions from dissolved carbon dioxide and release of cations from rock minerals (Raiswell and Thomos 1984). These relationships involve following reactions:
The above weathering reactions are supported by the Piper diagram (Fig. 2e, f); a trilinear representation of the ions found in the water and is used to classify water types or chemical facies. These water types show hydrochemical diversity among the ground water samples. The dominant facies are Ca–Mg–HCO3 and Ca–HCO3 indicating occurrence of temporary hardness as well as signifying the weathering of carbonate minerals in the groundwater strata.
Mechanism of Fluoride Enrichment
Qualitative assessment of groundwaters in Sidhi district reveals that the urban and village areas are experiencing greater seasonal fluctuations in groundwater levels compared to forest areas (Table 2). The fluoride concentrations showed following spatial variations: urban area > village in agricultural area > Village in forest area. In urban areas, dissolved fluoride varied from 1.5 to 2.8 and 2.4 to 3.5 mg/l in the pre-monsoon and monsoon, respectively; in village areas, the range of dissolved fluoride varied from 1.7 to 2.4 and 2.1 to 3.5 mg/l in the pre-monsoon and monsoon, respectively. However, in forest areas, the dissolved fluoride showed variation from 1.5 to 2.5 and 2.6 to 3.3 mg/l in the pre-monsoon and monsoon, respectively.
In monsoon, the dissolved fluoride significantly increased (>3.0 mg/l) in the groundwater locations around Dharwar and Archean regions, and few locations in Vindhyan and Permean regions (Fig. 3a, b). Geologically, the groundwater fluoride in the Archean and Dharwar regions are greater than Vindhyan region. Karunakaran (1974) reported sporadic and some concentrated occurrence of granite veins in the study area. Banerjee et al. 2010 studied the Sidhi crystalline comprise gneisses and ferruginous breccias and mylonitized hybrid rocks, profusely traversed by younger intrusives such as granites and quartzofeldspathic veins in north Sidhi (Vindhyan); syenites, granites, and quartz veins around eastern and central Sidhi (Dharwar and Archean) and basic dykes in south Sidhi (Permian). These geological signatures are signifying multiple reactivation episodes in terms of sedimentation, tectonic, and magmatic activities. These factors provided thermal gradient and facilitated hydrothermal solution movements for the remobilization of fluoride from the mineralized zones that leach into the groundwaters and contribute to perennial high fluoride concentrations.
The significant statistical relationship between pH and fluoride reveals that the alkaline conditions favor dissolution of F- bearing minerals (Fig. 4a, b). A strong correlation was observed between TDS and F− in the pre-monsoon and monsoon seasons (Fig. 4c, d), which explains that both were derived from the weathering. Rao and Devadas (2003) reported a high rate of evapotranspiration during the pre-monsoon temporarily precipitates the salts, including F−-rich salts in the top layers of the soil. The infiltrating water can leach these soils in the subsequent monsoon resulting in greater TDS, F−, and pH (Rao et al. 1993).
The effective-CO2 pressure (\( { \log }P_{{{\text{CO}}_{ 2} }} \)) is greater than atmospheric value of −3.5 (Table 3). This is a global trend indicating that naturally, water bodies are commonly out of equilibrium with the atmosphere. This is due to long standing water bodies having a fraction of CO2 groundwater and the rate of re-equilibration with the atmosphere by releasing excess CO2 is relatively slow (Stumn and Morgan 1970).
Strong indicators of alkaline condition in groundwater depend on the effective-CO2 pressure (\( { \log }P_{{{\text{CO}}_{ 2} }} \)) and HCO3 −/Ca2+ ratio. This is evident from an inverse relationship between pH and \( { \log }P_{{{\text{CO}}_{ 2} }} \) (Fig. 5a), and dissolved F− and \( { \log }P_{{{\text{CO}}_{ 2} }} \) (Fig. 5b), while the dissolved Ca2+ and HCO3 −concentrations decrease with \( { \log }P_{{{\text{CO}}_{ 2} }} \) (Fig. 5c, d). These relationships are responsible for an increase in the HCO3 −/Ca2+ratio and dissolved F− in alkaline conditions. The HCO3 −/Ca+2 ratios (based on epm values) are >1 in both seasons (Fig. 6a, b) demonstrating favorable chemical conditions during the fluoride dissolution process (Saxena and Ahmed 2003). The number of peaks and troughs indicates spatial variation in the weathering of carbonate minerals, which is complementing the precipitation/dissolution of fluoride. The high HCO3 −/Ca+2 ratios in the pre-monsoon season might be due to precipitation of carbonate minerals. The lesser HCO3 −/Ca+2 ratios may be due to the leaching of salts from the soils in the subsequent monsoon season.
In pre-monsoon, the SIC of 59 % groundwater locations are in equilibrium and remaining 41 % are undersaturated with respect to calcite while the 90 % groundwater locations are oversaturated and remaining 10 % are in equilibrium with calcite in the monsoon (Table 3). The SIF values show that the 75 % groundwater locations are undersaturated and remaining 25 % show equilibrium in pre-monsoon, and the 90 % groundwater locations are oversaturated in monsoon. When groundwater equilibrated with respect to fluorite, the activities of Ca+2 and F− shift toward fluorite saturation, consequently, high fluoride concentrations are dependent upon mechanisms that decrease Ca+2 in the pre-monsoon and monsoon. In nutshell, the rainfall triggers a shift in the unsaturated to saturated values of SIC (n = 38) and SIF (n = 19) in the monsoon.
The equilibrium constant of calcite (Kcal.) varied from 0.01 × 102 to 1.41 × 102 in the pre-monsoon, 0.02 × 102 to 3.30 × 102 in the monsoon (Table 3). In the pre-monsoon, the Kcal. values are less than 0.97 × 102 (Hem 1970) indicating significant calcite weathering due to sufficient hydrogen ion concentration (a strong weathering agent). The equilibrium constant of fluorite (Kfluor.) is greater than 10−10.6 except five locations in the monsoon (Parkhurst and Appelo 1999) indicating that the dissociation of fluorite is very slow in groundwater system. These spatial and seasonal variations in the solubility of calcite and fluorite might be due to (a) differences in local geological settings (b) effective CO2 pressure (\( { \log }P_{{{\text{CO}}_{ 2} }} \)), and (c) experiencing seasonal fluctuations in groundwater level in the groundwater system.
The Factor analysis of dissolved F− along with physico-chemical parameters indicates five factors (Table 4). The total variability accounted for five factors was 75.09 and 82.90 % in the pre-monsoon and monsoon seasons, respectively. The first factor (F1) contributes 21.88 % of the total variance in the pre-monsoon season and was strongly loaded on EC, Ca+2, Mg+2, Na+, HCO3 −, and Cl− indicating dominance of carbonate weathering and leaching of salts in the groundwater system. Similar loading is observed in the monsoon season, especially NO3 − enrichment, an indication of anthropogenic activities in the village and urban areas. The F2 (accounts for 16.44 and 20.72 % of the total variance in the pre-monsoon and monsoon) is mainly associated with high loadings of pH, TDS, and F− indicating geogenic origin of the fluoride in the study area. This factor also shows that the geogenic parameters determine the alkalinity of the groundwater that favors substitution of fluoride for hydroxyl ion on clay surfaces at high pH. Weak or negative loading on SiO2 and Na+ support lack of silicate origin of fluoride. In this study, phosphate ion was not detected in groundwaters. Therefore, phosphate minerals cannot be a significant source of fluoride in the study area. Hence, it appears that fluorite (CaF2) is a plausible source of fluoride ion in groundwater: CaF2(s) ↔ Ca+2 + 2 F−. Fluoride solubility is controlled by pH and Ca+2 content. Fluoride is most soluble at high pH and low calcium levels, because at high levels of calcium the insoluble mineral CaF2 (fluorite) is formed (Handa 1975).
Conclusion
The groundwater quality in the Sidhi district was mostly alkaline. The cation and anion concentrations reflect the seasonal variability in the weathering pattern of carbonate minerals and revealed the presence of temporary hardness in groundwaters. The landuse classification, spatial distribution, and seasonal variation identify urban areas and some locations in village areas as fluoride affected subsurface hotspots in the Sidhi district. The geochemical shift in the SIC and SIF values shows an inverse relationship between calcite dissolution and fluorite precipitation. In a qualitative way, SIC, SIF, and HCO3 −/Ca+2 ratio depends on the spatial and seasonal variations in \( { \log }P_{{{\text{CO}}_{ 2} }} \) and weathering of carbonate minerals in alkaline condition. In nut shell, the high dissolved fluoride was associated with alkaline pH, low \( { \log }P_{{{\text{CO}}_{ 2} }} \) and high HCO3 −/Ca+2 ratio. Because of the prolonged intake of higher concentration of F− bearing groundwater, the human population, which depends on agricultural activities for their livelihood, suffers from the fluorosis and related health disorders. In this alarming situation, when, the self-buffering mechanism of geogenic fluoride enrichment is identified in the groundwater system, supply and management of fluoride affected subsurface water in arid and semi-arid regions around the Central India is of utmost importance.
References
Abu Rukah Y, Alsokhny K (2004) Geochemical assessment of groundwater contamination with special emphasis on fluoride concentration, North Jordan. Chemic der Erde 64:171–181
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, New York
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36(6):433–487
Banerjee R, Agarwal M, Mathanwar SR, Roy MK, Maithani PB, Chaki A (2010) Shear-controlled hydrothermal polymetallic mineralisation in southeastern part of Vindhyan–Mahakoshal Basin, Sidhi district, Madhya Pradesh. Gondwana Geol Mag 12:1–18
Carrillo-Rivera JJ, Cardona A, Edmunds WM (2002) Use of abstraction regime and knowledge of hydrogeological conditions to control high-fluoride concentration in abstracted groundwater: San Luis Potosí basin, Mexico. J Hydrol 261(1–4):24–47
Census of India (2011) Provisional population totals. Ministry of home affairs. Government of India
CGWB (2010) Report on ground water quality in shallow aquifers of India. Central Ground Water Board Ministry of Water Resources, Government of India
Chae GT, Yun ST, Mayer B, Kim KH, Kim SY, Kwon JS, Kim K, Koh YK (2007) Fluorine geochemistry in bedrock ground water of South Korea. Sci Total Environ 385:272–283
Chidambaram S, Prasad MBK, Manivannan R, Karmegam U, Singaraja C, Anandhan P, Prasanna MV, Manikandan S (2013) Environmental hydrogeochemistry and genesis of fluoride in groundwaters of Dindigul district, Tamilnadu (India). Environ Earth Sci 68(2):333–342
Edmond JM, Palmer MR, Measures CI, Grant B, Stallard RF (1995) The fluvial geochemistry and denudation rate of the Guayana Shield in Venezuela, Colombia, and Brazil Geochim. Cosmochim Acta 59:3301–3325
Edmunds WM, Andrews JN, Burgess WG, Kay RLF, Lee DJ (1984) The evolution of saline and thermal groundwater in the Carnemenellis granite. Miner Mag 48:407–424
Falvey DA (1999) Groundwater geochemistry. In: Thomas J (ed) Sustainability. Earthwise Magazine- British Geological Survey, New Basford, Nottigham, 13:22
Farooqi A, Masuda H, Kusakabe M, Naseem M, Firdous N (2007) Distribution of highly arsenic and fluoride contaminated groundwater from east Punjab, Pakistan, and the controlling role of anthropogenic pollutants in the natural hydrological cycle. J Geochem 41:213–234
Gaciri SJ, Davies TC (1993) The occurrence and geochemistry of fluoride in some natural waters of Kenya. J Hydrol 143:395–412
Godfrey S, Wate S, Kumar P, Swami A, Rayalu S, Rooney R (2006) Health-based risk targets for fluorosis in tribal children of rural Madhya Pradesh. In: India 32nd WEDC international conference, Colombo
Handa BK (1975) Geochemistry and genesis of fluoride containing ground waters in India. Ground Water 13(3):275–281
Hem JD (1970) Study and interpretation of the chemical characteristics of natural water, 2nd edn. US Geological Survey Water Supply Paper 1473
Hitchon B (1995) Fluoride in formation waters. Alberta basin, Canada. Appl Geochem 10:357–367
Huh Y, Tsoi MY, Zaitsev A, Edmond JM (1998) The fluvial geochemistry of the rivers of Eastern Siberia: I. tributaries of the Lena River draining the sedimentary platform of the Siberian Craton. Geochim Cosmochim Acta 62:1657–1676
Kabata-Pendias A, Pendias H (2001) Trace element in soils and plants, 3rd edn. CRC Press, Boca Raton
Karunakaran, C (1974) Fluorine bearing minerals in India, their geology, mineralogy and geochemistry. Indian Academy of Geological Science, proceedings, symposium on fluorosis, Hyderabad, pp 3–18
Khaiwal R, Garg VK (2007) Hydro-chemical survey of groundwater of Hisar City and assessment of defluoridation methods used in India. Environ Monit Assess 132(1–3):33–43
Kim K, Jeong GY (2005) Factors influencing natural occurrence of fluoride-rich groundwaters: a case study in the southeastern part of the Korean peninsula. Chemosphere 58:1399–1408
Lee JU, Chon HT, John YW (1997) Geochemical characteristics of deep granitic groundwater in Korea. J Korea Soc Groundw Environ 4:199–211
Mehrotra RC, Verma S, Singh BK (1979) Physico-chemical behavior of hydrated aluminum oxides and associated clay minerals occurring around Pipra, district Sidhi, India. J Therm Anal 16:453–462
Naseem S, Rfique T, Bshir E, Bhanger MI, Laghari A, Usmani TH (2010) Lithological influences on occurrence of high-fluoride groundwater in Nagar Parkar area, Thar Desert, Pakistan. Chemosphere 78:1313–1321
Nordstrom DK, Jenne EA (1977) Fluorite solubility equilibria in selected geothermal waters. Geochim Cosmochim Acta 41:175–188
Panigrahy BK, Raymahashay B (2005) River water quality in weathered limestone: a case study in upper Mahanadi basin, India. J. Earth Syst Sci 111(5):533–543
Parkhurst DL, Appelo CAJ (1999) User’s guide to phreeqc (version 2)—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. USGS water resources investigation report 99-4259. Denver: U.S. Geological Survey. 312
Patel SC, Khalkho R, Patel SK, Sheikh JM, Behera D, Chaudhari S, Prabhakar N (2014) Fluoride contamination of groundwater in parts of eastern India and a preliminary experimental study of fluoride adsorption by natural haematite iron ore and synthetic magnetite. Environ Earth Sci 72:2033–2049
Pertti L, Backman B (1995) The occurrence and geochemistry of fluorides with special reference to natural waters in Finland. Report of investigation Geological Survey of Finland
Raiswell R, Thomas AG (1984) Solute acquisition in glacial melt waters Fjallsjokull (south-east Iceland), bulk melt waters with closed-system characteristics. J Glaciol 30:35–43
Rao NS (2009) Fluoride in groundwater, Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environ Monit Assess 152:47–60
Rao NS, Devadas DJ (2003) Fluoride incidence in groundwater in an area of Peninsular India. Environ Geol 45:243–251
Rao NVR, Rao KS, Schuiling RD (1993) Fluorine distribution in waters of Nalgonda district, Andhra Pradesh, India. Environ Geol 21:84–89
Robertson FN (1986) Occurrence and solubility controls of trace elements in groundwater in alluvial basins. Regional aquifer systems in the US. Am Water Res Assoc 7:69–80
Roy A, Bandyopadhyay BK (1990) Cleavage development in Mahakoshal Group of rocks of leemanabad-Sihora area, Jabalpur District, Madhya Pradesh. Indian Miner 44(2–3):111–128
Saxena VK, Ahmed S (2003) Dissolution of fluoride in ground water: a water–rock interaction study. Environ Geol 40:1084–1087
Short HE, McRobert GR, Bernard TW, Mannadiyar AS (1937) Mannadinagar endemic fluorosis in Madras presidency. Ind J Med Res 25:553–561
Stumm W, Morgan JJ (1970) Aquatic chemistry. Wiley Interscience, New York
Susheela AK (1999) Fluorosis management programme in India. An assessment of fluoride in the coastal aquifer of the Bara tract in Bharuch district, Gujarat, India. Curr Sci 77(10):1250–1256
WHO (2004) Guidelines for drinking water quality, 3rd edn. World Health Organization, Geneva
Acknowledgments
The authors are grateful to Ministry of Human Resource Development, Government of India and Indian School of Mines, Dhanbad for funding the Ph.D. thesis of Ms. Asmaa Naaz (Registration no. 2013DR0060). We are also thankful to the department of Environmental Science and Engineering Dhanbad for providing the logistic support to carry out field monitoring and laboratory analysis. We greatly appreciate the anonymous reviewers for their valuable comments, criticism, and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naaz, A., Kumar, B., Narayan, C. et al. Assessment of Fluoride Pollution in Groundwaters of Arid and Semi-arid Regions of Tonalite–Trondjhemite Series in Central India. Water Qual Expo Health 7, 545–556 (2015). https://doi.org/10.1007/s12403-015-0171-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-015-0171-9