Abstract
FK506 binding proteins, a member of peptidyl-prolyl cis-trans isomerase (PPIase), are essential for plant growth; however, the molecular function of FKBPs remains largely unknown. In this report, we isolated and identified an FK506 binding protein, OsFKBP42b, that regulates rice growth and development. The OsFKBP42b mutation led to reductions in plant height, panicle length, and seed setting rate. Scanning electron microscopy of the wild-type and the mutant stems showed no difference in cell size. OsFKBP42b was expressed in rice various organs, especially in panicles, leaves, and stems. Subcellular localization suggested that OsFKBP42b was a plasma membrane protein. Furthermore, we found that OsFKBP42b interacted with the ATP binding cassette B proteins, OsABCB1 and OsABCB14, by yeast two-hybrid and bimolecular fluorescence complementation assays. Taken together, our results provide new insights into OsFKBP42b in rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global extreme weathers, including drought, high temperature, and flood, occur frequently and seriously affect agricultural production. Rice, a food for two-thirds of the world's population, has a small genome and can be used as a model crop for genomics research (Yang et al. 2022). Identification of rice gene function is helpful to rice molecular design and breeding and to ensure global food security.
The FK506 binding proteins (FKBPs) are the superfamily of peptidyl-prolyl cis-trans isomerases (PPIases) (Schiene and Fischer 2000; He et al. 2004; Ahn et al. 2010). Members of the FKBP family have different molecular weights from 12 kDa (hFKBP12) to over 77 kDa (wFKBP77) (Ahn et al. 2010). There are 23 and 29 members of the FKBP family in the genome of Arabidopsis thaliana and rice, respectively (Ahn et al. 2010; Gollan and Bhave 2010). In plants, FKBP members are reported to be involved in regulating cell differentiation, stress responses, brassinosteroid signaling, and auxin transport (Ahn et al. 2010; Cheung et al. 2020). Disruption of the Arabidopsis AtFKBP42, named as a twisted dwarf (TWD1), exhibited dwarfism, epinastic growth of leaves and cotyledons, shorter hypocotyls in the light, and reduced BR sensitivity (Geisler et al. 2003; Henrichs et al. 2012; Chaiwanon et al. 2016). AtFKBP42 functionally interacts with the BR receptor kinases BRI1 and BAK1 to regulate brassinosteroid signaling in Arabidopsis (Chaiwanon et al. 2016). In Arabidopsis, overexpression of PaFKBP12 from Polytrichastrum alpinum increased the plant size, and had enhanced tolerance towards heat, salt, and drought stresses (Alavilli et al. 2018). AtFKBP12 was shown to interact with an ATPase, AtFIP37, in vitro, which was proposed to participate in RNA splicing (Faure et al. 1998). Recently, AtFKBP12 was identified to be a positive regulator of flowering time in A. thaliana by interacting with CONSTANS (CO, Serrano-Bueno et al. 2020). Ectopically expressing OsFKBP12 in A. thaliana was found to increase the susceptibility of the plant to the pathogen and salt stress (Cheung et al. 2020). Furthermore, the unconventional GTPase, OsYchF1, an interactor of OsFKBP12, was involved in both biotic and abiotic stress responses (Cheung et al. 2020). The expression level of OsFKBP20 was induced by heat, and over-expression of OsFKBP20 in yeast enhanced the tolerance of yeast to high temperatures (Nigam et al. 2008). However, the function of FKBPs in rice has many unknowns, especially in the molecular level.
In this study, we confirmed that OsFKBP42b positively regulates plant growth and development in rice. Compared with the wild type, the osfkbp42b mutant showed dwarfism, shorter panicles, and decreased seed setting rate. OsFKBP42b was expressed in rice various organs and encodes a plasma membrane protein. Yeast two-hybrid and bimolecular fluorescence complementation assays indicated that OsFKBP42b interacted with the ATP binding cassette B proteins, OsABCB1 and OsABCB14.
Results
Characterization of OsFKBP42a and OsFKBP42b
There are two OsFKBP42 genes (OsFKBP42b and OsFKBP42a) located on chromosomes 11 and 12 in rice (Ahn et al. 2010). OsFKBP42a and OsFKBP42b were predicted to contain 375 and 370 amino acids, respectively (Fig. 1). OsFKBP42a and OsFKBP42b have a mitochondrial precursor proteins import receptor domain, a TPR repeat region and an FKBP-type peptidyl-prolyl cis-trans isomerase domain (Fig. 1). The homologous protein BLAST revealed that FKBP42 exists among rice, Zea mays, A. thaliana and Sorghum bicolor. OsFKBP42a and OsFKBP42b showed approximately 90% amino acid identity, indicating that these two proteins may have similar functions or are functionally redundant.
Amino acid sequence alignment of OsFKBP42b and its homologs OsFKBP42a (Oryza sativa), GRMZM2G133624 (Zea mays), SORBI_3005G034300 (Sorghum bicolor), AT3G21640 (Arabidopsis thaliana). Black and pink indicate that fully or partially conserved amino acids, respectively. Red underline represents the domain of FKBP-type peptidyl-prolyl cis-trans isomerase
Expression Pattern and Subcellular Localization of OsFKBP42a and OsFKBP42b
Based on the public gene expression database the Rice eFP Browser (http://bar.utoronto.ca/efp rice/cgi-bin/efpWeb.cgi) and the RiceXPro (https://ricexpro.dna.affrc.go.jp/Zapping/), we found that OsFKBP42a and OsFKBP42b had similar expression patterns and were expressed in various rice tissues (e.g., roots, stems, leaves, seeds) (Figs. S1–S4). To verify the expression pattern of OsFKBP42a and OsFKBP42b, we used the qRT-PCR method to analyze these two genes with different organs of the japonica rice Nipponbare. As shown in Fig. 2, OsFKBP42a and OsFKBP42b have similar spatial–temporal expression patterns. Transcripts of OsFKBP42a and OsFKBP42b were expressed in roots, panicles, leaves, stems, and sheaths (Fig. 2).
To examine the subcellular localization of the OsFKBP42a and OsFKBP42b proteins, we fused the OsFKBP42a and OsFKBP42b coding sequences with a GFP tag, respectively. Then, OsFKBP42a-GFP and OsFKBP42b-GFP were introduced into rice protoplasts. Laser confocal microscope observation indicated that GFP fluorescence could be observed in the plasma membrane (Fig. 3), which is similar to the location of the plasma membrane protein OsCAMP (Lam et al. 2007).
Disruption of OsFKBP42b Resulted in Defects of Rice Growth and Development
To study the function of OsFKBP42 on rice growth and development, we isolated an OsFKBP42b mutant osfkbp42b from the CRISPR-Cas9 library of Nipponbare (Oryza sativa), but not obtained an OsFKBP42a mutant. The osfkbp42b mutant plants contained two bases ‘TA’ deletion in the second exon of OsFKBP42b (Fig. 4A), which resulted in premature termination of the OsFKBP42b protein translation (Fig. S5). Compared with the wild type, the osfkbp42b mutant plants showed dwarfism, short panicles, and low grain setting rate (Fig. 4B–E). However, tiller number, grain size, and grain weight were not altered in the osfkbp42b mutant (Fig. S6). To further confirm the OsFKBP42b function, we performed genetic complementation experiments using the pCUBi1390 vector. As shown in Fig. 4F, pCUBi1390-OsFKBP42b complemented the osfkbp42b mutant phenotypes.
OsFKBP42b influenced plant morphology and grain setting rate. A Diagram of the OsFKBP42b mutation site. The mutation position of OsFKBP42b was highlighted in red. sativa)Plant morphologies of the wild type and osfkbp42b plants at the mature stage. Scale bar, 10 cm. C Plant height of the wild type and osfkbp42b plants at the mature stage. D Grain setting rate of the wild type and osfkbp42b plants. E Panicle length of the wild type and osfkbp42b plants. F Complementation of the osfkbp42b mutant plants. Scale bar, 10 cm. ** indicated the significance of differences between the wild type and osfkbp42b as determined by Student’s t-test analysis
To investigate the cytological cause of the dwarf phenotype in the osfkbp42b mutant, we observed the first internode at the heading stage by the scanning electron microscope (SEM) (Fig. S7A). The cell length and width in osfkbp42b were similar to the wild type (Fig. S7A). Four cell cycle-related genes, H1, E2F2, CYCA2.1, and CYCA2.2 were remarkably repressed in osfkbp42b (Fig. S7B). This result indicated that the dwarf phenotype in the osfkbp42b mutant might be caused by decreased cell number but not cell size.
The OsFKBP42b Mutation Affected Auxin Pathway
In Arabidopsis, the OsFKBP42b homologous gene FKBP42 regulates auxin transport, and the FKBP42 mutant twd1 exhibited dwarfism (Geisler et al. 2003). We predicted that the disruption of OsFKBP42b affects the auxin pathway. Firstly, we treated the wild-type and the osfkbp42b mutant with exogenous different concentrations of IAA. As shown in Fig. 5A, IAA inhibited the growth of the wild-type and the mutant seedlings.
Auxin contents were increased in the osfkbp42b plants. A Seedling phenotypes of the wild type and osfkbp42b treated with 0, 0.1 and 1 μM IAA. Bars, 1 cm. B IAA-GLU contents in the wild type and osfkbp42b plants. C Contents of IAA in the wild type and osfkbp42b plants. D IAN contents in the wild type and osfkbp42b plants. E IAM contents in the wild type and osfkbp42b plants. F TAM contents in the wild type and osfbkp42b plants. G IPYA contents in the wild type and osfkbp42b plants. H IAA-ASP contents in the wild type and osfkbp42b plants. Error bars indicate SD with biological triplicates. Asterisks indicate the significance of differences between the wild type and osfkbp42b as determined by Student’s t-test analysis: **P < 0.01
To determine whether the disruption of OsFKBP42b alters auxin concentration in rice, we measured the auxin concentration in the wild type and osfkbp42b mutants. The results showed that the free auxin concentration of osfkbp42b was significantly higher than in the wild type (Fig. 5C). In addition, the concentrations of IAA-GLU, IAN, TAM, and IPYA were also significantly higher than those of the wild type, but IAA-ASP and IAM contents had no difference between the wild type and osfkbp42b (Fig. 5B, D–H).
OsFKBP42b Interacted with OsABCB1 and OsABCB14
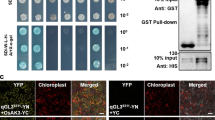
To understand the molecular mechanism by which OsFKBP42b regulates rice growth and development, we performed a yeast-two hybrid (Y2H) screen assay. Among the proteins we identified, two were OsABCB1 and OsABCB14, which encode the ATP Binding Cassette B (ABCB) subfamily proteins (Fig. 6). We further confirmed the protein interaction with bimolecular fluorescence complementation (BiFC) assays (Fig. 7). OsABCB14 was shown to function in rice auxin transport and Fe homeostasis (Xu et al. 2014). Knock-down of OsABCB14 has decreased auxin concentrations and polar auxin transport rates (Xu et al. 2014). OsABCB1 and OsABCB14 were expressed in leaves, stems, roots, panicles, and seeds (Figs. S8 and S9).
Discussion
OsFKBP42a and OsFKBP42b are Two Conserved FKBP Proteins in O. sativa
Compared to the extensive studies on mammalian FKBPs function, little is known about FKBP function in plants, especially crops. In A. thaliana, FKBP domain-containing proteins were reported to regulate cell differentiation, abiotic stresses, redox signaling, auxin transport, and photosystem assembly (Pérez-Pérez et al. 2004; Lima et al. 2006; Aviezer-Hagai et al. 2007). In rice, OsFKBP42a and OsFKBP42b have approximate 90% amino acid identity (Fig. 1). Similar to its homologs in Zea mays, A. thaliana, and Sorghum bicolor, the FKBP domain was conserved (Fig. 1). OsFKBP42a and OsFKBP42b have similar expression patterns (Fig. 2), and subcellular localization indicated that OsFKBP42a and OsFKBP42b are two plasma membrane proteins (Fig. 3). These results implied that OsFKBP42a and OsFKBP42b may have functional redundancy.
OsFKBP42b Influenced Rice Growth and Development
In Arabidopsis, the FKBP42 mutant twd1 exhibited dwarfism, and FKBP42 regulates auxin transport and BR signaling (Geisler et al. 2003). FKBP42 shares 65.25% amino acid sequence identity with OsFKBP42b (Fig. 3). Compared with the wild type, the osfkbp42b mutant showed some defects in growth and development, such as dwarfism, short panicles, and reduced grain setting rate (Fig. 4), but not as severe as the TWD1/FKBP42 mutation phenotype. This may be due to the functional redundancy between OsFKBP42a and OsFKBP42b. As shown in Fig. 5A, IAA inhibited the growth of the wild-type and the osfkbp42b mutant seedlings, but auxin concentrations (e.g., free IAA, IAA-GLU, IAN, TAM, and IPYA) in osfkbp42b were significantly increased (Fig. 5B–H). This implies rice and Arabidopsis FKBP42s both act as regulators in the auxin pathway.
OsFKBP42b Interacts with Two ABCB Subfamily Proteins, OsABCB1 and OsABCB14
Yeast two-hybrid and in vivo bimolecular fluorescence complementation assays were employed in our study to detect the protein interacting partners of OsFKBP42b. Two OsFKBP42b interacting proteins were identified, OsABCB1 and OsABCB14 (Fig. 6). We further confirmed the protein interaction with bimolecular fluorescence complementation (BiFC) assays (Fig. 7). OsABCB1 and OsABCB14 belong to the ATP Binding Cassette B subfamily protein (Xu et al. 2014). In Arabidopsis thaliana, the first identified ABCB subfamily protein is AtPGP1/AtABCB1, which regulates hypocotyl cell elongation in the light (Dudler and Hertig 1992; Sidler et al. 1998). AtABCB4, AtABCB6, and AtABCB19 are all involved in auxin transport and root development (Kaneda et al. 2011; Kamimoto et al. 2012). According to recent studies, AtFBKP42/TWD1 controls auxin transport and plant development by differential activation of multiple ABCB transporters (Jenness et al. 2022; Liu et al. 2022). In rice, OsABCB14, a homology of AtABCB19, is highest expressed in the tubular tissues and regulates auxin transport and Fe homeostasis (Xu et al. 2014). In addition, OsABCB11, OsABCB8, OsABCB13, OsABCB23, and OsABCB24 were induced by drought stress, whereas OsABCB6, OsABCB9, and OsABCB8 were induced by salt stress (Saha et al. 2015), suggesting that they are potentially involved in abiotic stresses.
In this report, OsFKBP42b is characterized as a positive regulator in rice growth and development, and interacts with OsABCB1 and OsABCB14. Our study provides a new insight into the function of the rice OsFKBP42b gene.
Materials and Methods
Plant Materials
The OsFKBP42b mutant was isolated from the CRISPR/Cas9 mutant library of Nipponbare (O. sativa). All plants were grown in a natural field condition in Huai’an cCty, Jiangsu Province, China. For auxin measurement and gene expression analysis, the mutant and Nipponbare were grown in a growth chamber with 14 h light and 10 h dark at 30/25 °C.
Scanning Electron Microscopy (SEM)
Stem samples of the wild type and the mutant at the heading stage were collected and fixed in 2.5% glutaraldehyde. The fixed samples were picked up the suction for 1.5 h and then were dehydrated with alcohol solutions. The samples were observed and photographed by JSM-840 (JEOL, Tokyo, Japan).
Sequence Analysis
The Blastp search program of the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/) was used to identify the homologous proteins of OsFBKP42b. The homologous proteins and OsFBKP42b were compared with the DNAMAN software
Subcellular Localization
For examining the subcellular localization of OsFKBP42b, OsABCB1(LOC_Os08g45030), and OsABCB14 (LOC_Os04g38570), the complete ORF of OsFKBP42b, OsABCB1, and OsABCB14 without the stop codon was amplified and inserted into pAN580‐GFP at the XbaI and BamHI sites. Then, the recombinant plasmid was transformed into rice protoplasts as previously described (Liu et al. 2020). Plasma membrane protein OsCAMP1-mCherry was used as a control. The GFP fluorescence was imaged with a Zeiss confocal laser scanning microscopy LSM700.
Gene Expression Analysis
Various tissues of Nipponbare at the heading stage, such as roots, stems, sheaths, leaves, and panicles, were collected and ground by liquid nitrogen. Total RNA was extracted with an RNA Prep Pure Plant kit (#CW0591S, CWBIO, Jiangsu, China). First‐strand cDNA was reverse transcribed using an oligo(dT)18 primer for nuclear-encoded genes. Quantitative Real‐time PCR was performed using an SYBR Premix Ex TaqTM kit (TaKaRa) on a CFX96 Touch Real-time PCR Detection System (Bio-rad, America) with three biological replicates. The primers for qRT-PCR are listed in Table S1. The primers used for cell cycle-related genes were listed in the reference (Zhang et al. 2016). The rice UBQ gene was used as a reference gene.
Auxin Treatment and Determination
For auxin treatment, the seeds of the wild type and the mutant were sterilized with 75% ethanol for 5 min and rinsed with sterile water. Sterilized seeds were plated in Yoshida rice nutrient salt mixture (Coolaber, NSP1040, Beijing, China) with different concentrations of IAA and grown under 30℃ at a photoperiod of 14-h light/10-h dark in a growth chamber for 10 d.
For auxin measurement, ~1 g of fresh shoots or of 14-day-old wild type and the mutant seedlings grown on Yoshida rice nutrient solution was collected and washed using sterile deionized water, respectively. The samples were ground into powder in liquid nitrogen. IAA concentrations were measured by gas chromatography-selected reaction monitoring mass spectrometry (Nanjing WEBiolotech Biotechnology Co., Ltd).
Yeast Two-Hybrid and Bimolecular Fluorescence Complementation (BiFC) Assays
The coding sequences of OsFBKP42b, OsABCB1, and OsABCB14 were amplified and inserted into the bait vector pXGY18 and the prey vector pXGY17 using a ClonExpress II One Step Cloning Kit (#C112-02, Nanjing Vazyme Biotech Co., Ltd.), respectively. Five combinations of plasmids were transformed into the yeast strain, NMY51 as previously described (Xu et al. 2017). The primers for the pXGY18 and pXGY17 vector constructs are listed in Table S1.
For the bimolecular fluorescence complementation assay, OsFBKP42b, OsABCB1, and OsABCB14 were cloned into pVYNE and pVYCE, respectively. The recombinant plasmids were transformed into Agrobacterium EHA105 and co-transformed into tobacco (Nicotiana benthamiana) leaves as previously described (Waadt et al. 2008).
Data availability
All data supporting this article are provided within the article (and its supplementary files).
References
Ahn JC, Kim DW, You YN, Seok MS, Park JM, Hwang H, Kim BG, Luan S, Park HS, Cho HS (2010) Classification of rice (Oryza sativa L. Japonica nipponbare) immunophilins (FKBPs, CYPs) and expression patterns under water stress. BMC Plant Biol 10:253
Alavilli H, Lee H, Park M, Yun DJ, Lee BH (2018) Enhanced multiple stress tolerance in Arabidopsis by overexpression of the polar moss peptidyl-prolyl isomerase FKBP12 gene. Plant Cell Rep 37(3):453–465
Aviezer-Hagai K, Skovorodnikova J, Galigniana M, Farchi-Pisanty O, Maayan E, Bocovza S, Efrat Y, von Koskull-Döring P, Ohad N, Breiman A (2007) Arabidopsis immunophilins ROF1 (AtFKBP62) and ROF2 (AtFKBP65) exhibit tissue specificity, are heat-stress induced, and bind HSP90. Plant Mol Biol 63(2):237–255
Chaiwanon J, Garcia VJ, Cartwright H, Sun Y, Wang ZY (2016) Immunophilin-like FKBP42/TWISTED DWARF1 interacts with the receptor kinase BRI1 to regulate brassinosteroid signaling in Arabidopsis. Mol Plant 9(4):593–600
Cheung MY, Auyeung WK, Li KP, Lam HM (2020) A rice immunophilin homolog, OsFKBP12, is a negative regulator of both biotic and abiotic stress responses. Int J Mol Sci 21(22):8791
Dudler R, Hertig C (1992) Structure of an mdr-like gene from Arabidopsis thaliana. Evolutionary implications. J Biol Chem 267(9):5882–5888
Faure JD, Gingerich D, Howell SH (1998) An Arabidopsis immunophilin, AtFKBP12, binds to AtFIP37 (FKBP interacting protein) in an interaction that is disrupted by FK506. Plant J 15(6):783–789
Geisler M, Kolukisaoglu HU, Bouchard R, Billion K, Berger J, Saal B, Frangne N, Koncz-Kalman Z, Koncz C, Dudler R, Blakeslee JJ, Murphy AS, Martinoia E, Schulz B (2003) TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19. Mol Biol Cell 14(10):4238–4249
Gollan PJ, Bhave M (2010) Genome-wide analysis of genes encoding FK506-binding proteins in rice. Plant Mol Biol 72(1–2):1–16
He Z, Li L, Luan S (2004) Immunophilins and parvulins. Superfamily of peptidyl prolyl isomerases in Arabidopsis. Plant Physiol 134(4):1248–1267
Henrichs S, Wang B, Fukao Y, Zhu J, Charrier L, Bailly A, Oehring SC, Linnert M, Weiwad M, Endler A, Nanni P, Pollmann S, Mancuso S, Schulz A, Geisler M (2012) Regulation of ABCB1/PGP1-catalysed auxin transport by linker phosphorylation. EMBO J 31(13):2965–2980
Jenness MK, Tayengwa R, Bate GA, Tapken W, Zhang Y, Pang C, Murphy AS (2022) Loss of multiple ABCB auxin transporters recapitulates the major twisted dwarf 1 phenotypes in Arabidopsis thaliana. Front Plant Sci 13:840260
Kamimoto Y, Terasaka K, Hamamoto M, Takanashi K, Fukuda S, Shitan N, Sugiyama A, Suzuki H, Shibata D, Wang B, Pollmann S, Geisler M, Yazaki K (2012) Arabidopsis ABCB21 is a facultative auxin importer/exporter regulated by cytoplasmic auxin concentration. Plant Cell Physiol 53(12):2090–2100
Kaneda M, Schuetz M, Lin BS, Chanis C, Hamberger B, Western TL, Ehlting J, Samuels AL (2011) ABC transporters coordinately expressed during lignification of Arabidopsis stems include a set of ABCBs associated with auxin transport. J Exp Bot 62(6):2063–2077
Lam SK, Siu CL, Hillmer S, Jang S, An G, Robinson DG, Jiang L (2007) Rice SCAMP1 defines clathrin-coated, trans-golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. Plant Cell 19(1):296–319
Lima A, Lima S, Wong JH, Phillips RS, Buchanan BB, Luan S (2006) A redox-active FKBP-type immunophilin functions in accumulation of the photosystem II supercomplex in Arabidopsis thaliana. Proc Natl Acad Sci USA 103(33):12631–12636
Liu X, Cao PH, Huang QQ, Yang YR, Tao DD (2020) Disruption of a rice chloroplast-targeted gene OsHMBPP causes a seedling-lethal albino phenotype. Rice 13(1):51
Liu J, Ghelli R, Cardarelli M, Geisler M (2022) Arabidopsis TWISTED DWARF1 regulates stamen elongation by differential activation of ABCB1,19-mediated auxin transport. J Exp Bot 73(14):4818–4831
Nigam N, Singh A, Sahi C, Chandramouli A, Grover A (2008) SUMO-conjugating enzyme (Sce) and FK506-binding protein (FKBP) encoding rice (Oryza sativa L.) genes: genome-wide analysis, expression studies and evidence for their involvement in abiotic stress response. Mol Genet Genomics 279(4):371–383
Pérez-Pérez JM, Ponce MR, Micol JL (2004) The ULTRACURVATA2 gene of Arabidopsis encodes an FK506-binding protein involved in auxin and brassinosteroid signaling. Plant Physiol 134(1):101–117
Saha J, Sengupta A, Gupta K, Gupta B (2015) Molecular phylogenetic study and expression analysis of ATP-binding cassette transporter gene family in Oryza sativa in response to salt stress. Comput Biol Chem 54:18–32
Schiene C, Fischer G (2000) Enzymes that catalyse the restructuring of proteins. Curr Opin Struct Biol 10(1):40–45
Serrano-Bueno G, Said FE, de Los Reyes P, Lucas-Reina EI, Ortiz-Marchena MI, Romero JM, Valverde F (2020) CONSTANS-FKBP12 interaction contributes to modulation of photoperiodic flowering in Arabidopsis. Plant J 101(6):1287–1302
Sidler M, Hassa P, Hasan S, Ringli C, Dudler R (1998) Involvement of an ABC transporter in a developmental pathway regulating hypocotyl cell elongation in the light. Plant Cell 10(10):1623–1636
Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J (2008) Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J 56(3):505–516
Xu Y, Zhang S, Guo H, Wang S, Xu L, Li C, Qian Q, Chen F, Geisler M, Qi Y, de Jiang A (2014) OsABCB14 functions in auxin transport and iron homeostasis in rice (Oryza sativa L.). Plant J 79(1):106–117
Xu Y, Yang J, Wang Y, Wang J, Yu Y, Long Y, Wang Y, Zhang H, Ren Y, Chen J, Wang Y, Zhang X, Guo X, Wu F, Zhu S, Lin Q, Jiang L, Wu C, Wang H, Wan JM (2017) OsCNGC13 promotes seed-setting rate by facilitating pollen tube growth in stylar tissues. PLoS Genet 13(7):e1006906
Yang Y, Yu J, Qian Q, Shang L (2022) Enhancement of heat and drought stress tolerance in rice by genetic manipulation: a systematic review. Rice 15(1):67
Zhang S, Wu T, Liu S, Liu X, Jiang L, Wan JM (2016) Disruption of OsARF19 is critical for floral organ development and plant architecture in rice (Oryza sativa L.). Plant Mol Biol Rep 34:748–760
Acknowledgments
This study was supported by the Huaishang Talents, Huai'an Academy of Agricultural Sciences Initiation and Development of Scientific Research Fund for High-level Introduced Talents (0062019016B), the Hunan Province Natural Science Fund (2019JJ50714), Outstanding Backbone Young Teachers of Jiangsu Qinglan Project and Natural Science Foundation of Jiangsu Province (BK20191055).
Author information
Authors and Affiliations
Contributions
LX, WD, and WYJ designed the research. WD is responsible for rice field planting. WYJ, WYY, WGZ, and WD performed the qRT-PCR experiment, yeast two-hybrid assays and BiFC assay. PG, CWJ, FYT, and LX are responsible for scanning electron microscope and subcellular localization. LX and WD wrote and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, D., Wang, Y., Pan, G. et al. OsFKBP42b Regulates Rice Growth and Development Through Interacting with OsABCB1 and OsABCB14. J. Plant Biol. 66, 349–357 (2023). https://doi.org/10.1007/s12374-023-09396-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-023-09396-3