Abstract
Floral organ development is fundamentally important to plant reproduction and seed quality, yet its underlying regulatory mechanisms are still largely unknown, especially in crop plants. In this study, we characterized rice null mutant osarf19, which was isolated from a T-DNA insertion pool. The mutant displayed three types of abnormal florets: an enlarged and degenerated palea, and an additional lemma. It also showed enlarged plant architecture, including elongated basal internodes and leaves. Cellular morphology and quantitative real-time PCR (qRT-PCR) analyses showed that cell elongation caused the enlarged organs. Transgenic RNA interference (RNAi) lines of OsARF19 had similar phenotypes to the osarf19 mutant, confirming the role of OsARF19 in floral and vegetative organ development. OsARF19 is expressed in various tissues, especially young panicles and basal internodes, which are elongated. OsARF19 was induced by IAA (indole-3-acetic acid) treatment and functioned in the nucleus. By qRT-PCR analysis, we found that disruption of OsARF19 increases expression levels of OsYUCCA and OsPIN family members, while reducing OsGHs transcription activity. The high auxin performance greatly upregulated two floral organ regulators, OsMADS29 and OsMADS22, possibly responsible for palea abnormalities in osarf19. Our data provide new knowledge on the mechanisms of floral organ development, as well as possibilities in breeding for ideal plant architecture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The initiation and differentiation of floral organs are of fundamental importance in the plant life cycle. In rice, normal development of floral organs is essential for reproduction and seed quality. Multiple genes influence floret formation in rice, and most of them are classified into groups based on the ABCDE model, that was originally derived from the ABC model proposed for Arabidopsis (Krizek and Fletcher 2005; Theissen 2001; Thompson and Hake 2009). RAP1A and RAP1B that belong to Class A (Moon et al. 1999) affect palea development. OsMADS2 and OsMADS16, Class B genes, control formation of the lodicule and paleolate (Xiao et al. 2003; Yadav et al. 2007). OsMADS3 and OsMADS58 in class C determine the identities of pistils and carpel (Yamaguchi et al. 2006; Li et al. 2011a). OsMADS13, a Class D protein, plays a role in ovule development (Dreni et al. 2007). OsMADS1, a Class E gene, functions in balancing meristem growth, lateral organ differentiation, and determinacy (Khanday et al. 2013). In addition to the ABCDE model, many other factors such as phytohormones also affect floret development. These include auxins (McSteen 2010), cytokinins (Khanday et al. 2013), and jasmonic acid (Cai et al. 2014).
Auxin, a morphogen-like hormone, regulates multiple aspects of plant growth and development, including leaf development, apical dominance, shoot and root elongation, lateral root initiation, and vascular differentiation (Benkova et al. 2003). Auxin responses are mediated by a class of transcription factors called auxin response factors (ARFs), which are repressed by Aux/IAA proteins in the absence of auxin (Guilfoyle and Hagen 2007).
In previous studies, the biological functions of several ARFs were reported in Arabidopsis (Arabidopsis thaliana L.) and rice (Oryza sativa L.). For example, MP/ARF5 can affect leaf initiation, embryo patterning, flower patterning, and vascular differentiation (Aida et al. 2002; Garrett et al. 2012; Hardtke and Berleth 1998; Hardtke et al. 2004; Przemeck et al. 1996). NPH4/ARF7 and ARF19 affect leaf expansion and promote lateral root formation via direct activation of LBD/ASL genes in Arabidopsis (Okushima et al. 2007; Wilmoth et al. 2005). Besides, ARF19 can also bind to the promoter of BAT1, a putative acyltransferase, and modulates brassinosteroid levels (Choi et al. 2013). ARF6 and ARF8 regulate floral organ development in Arabidopsis. The double-null mutant arf6 arf8 arrests flower development, leading to infertile closed buds with short petals, short stamen filaments, and non-dehisced anthers (Nagpal et al. 2005; Tabata et al. 2010).
In rice, OsARF1 was shown to be essential for growth in vegetative organs and seed development (Attia et al. 2009; Waller et al. 2002). OsARF2, together with DROOPING LEAF, promotes awn development in rice (Toriba and Hirano 2014). ARF12 and OsARF25 regulate root elongation and affects iron accumulation (Qi et al. 2012). OsARF16 is involved in regulating auxin redistribution, phosphate starvation response, and iron deficiency (Shen et al. 2014, 2015). OsARF23 and OsARF24 control cell growth by regulating the actin-binding protein RMD (Li et al. 2014). Zhang et al. (2015) reported that OsARF19-overexpressing lines can increase leaf angles by enhancing BR signaling. Meanwhile, the overexpression line of OsARF19 also showed slender seeds, narrow leaves, and dwarfism (Zhang et al. 2015).
Even though several studies on the relationship of auxin and floral organ development have been reported in Arabidopsis, the detailed mechanisms in rice are far from conclusive. In this study, we isolated a T-DNA insertion mutant that displayed abnormal florets as well as altered plant architecture. PCR and quantitative real-time PCR (qRT-PCR) analyses indicated that the T-DNA was inserted into gene OsARF19 (LOC_Os06g48950), blocking its transcription. To confirm the function of OsARF19 affecting those phenotypes, we generated transgenic plants by knocking down the transcription level of OsARF19. Knockdown lines displayed similar phenotypes to the T-DNA insertion mutant. We also determined the expression patterns and subcellular localization of OsARF19, which was expressed mainly in young florets, leaves, lamina joint, and elongated basal internodes, and functioned in the nucleus. The combination of morphological and transcription analyses showed that OsARF19 plays important roles in floral organ development, internode elongation, and leaf morphogenesis.
Materials and Methods
Plant Materials and Growth Conditions
The osarf19 mutant was identified by abnormal florets and enhanced plant height from 400T-DNA tagged lines generated in O. sativa var. japonica cv. Dongjin, seeds of which were donated by Professor Gynheung An, Crop Biotech Institute, Kyung Hee University, Korea (Jeon et al. 2000; Jeong et al. 2002, 2006). Plants were grown to maturity in the experimental field at Nanjing Agricultural University (Nanjing, China) in the normal growing season.
Cellular Morphology Analysis
Fourth internodes and 3rd leaves counted from the top of wild-type and osarf19 plants were collected after heading. Samples were fixed in FAA (10 % formalin, 50 % ethanol, and 5 % acetic acid) and dehydrated in a graded ethanol series (Li et al. 2006). For histological analysis, tissues were infiltrated with xylene and embedded in paraplast plus. Materials were sectioned and viewed with a Leica light microscope (DFC402C). Scanning electron microscopy was performed with a JSM-6360LV (Jeol) as described previously (Li et al. 2006).
Genotyping
We obtained flanking sequence information for mutants from OryGenesDB (http://orygenesdb.cirad.fr/). Confirmation of the insertion site was performed by PCR, carried out in a 50-μl mixture containing 20 ng genomic DNA of wild type (WT) and mutant, 0.2 mM dNTP, 0.5 units of Taq polymerase (TaKaRa), 1 mM primers, and 5 μl 10 × Taq buffer. PCR was performed as follows: 95 °C for 5 min, followed by 33 cycles of 95 °C for 30 s, annealing for 30 s, 72 °C for 40 s, and a final elongation step at 72 °C for 5 min. The gene-specific primers were P1 (5’-TTGTAAGCGGCAAGAGG-3’), P2 (5’-CCATTTATGGAAAAGTGAG-3’), and P3 (5’-ATCAATTCCACAGTTTTCG-3’).
Plasmid Construction, Plant Transformation, and GUS Staining
A 230-bp cDNA fragment of OsARF19 sequence was amplified using primers OsARF19R1-F (5’-GGGGTACCCCGATGAGGACATTCACCAAGG-3’), OsARF19R1-R (5’-CCGAGCTCGGTTCACGCAACCGACAAA-3’), OsARF19R2-F (5’-AACTGCAGAATTCACGCAACCGACAAA-3’), and OsARF19R2-R (5’-CGGGATCCCGGATGAGGACATTCACCAAGG-3’) and products were subcloned into the binary vector LH-FAD2-1390RNAi. The LH-FAD2-1390RNAi plasmids were introduced into Agrobacterium tumefaciens strain EHA105 by heat shock, and the rice cv. Dongjin was transformed in accordance with a previously published method (Hiei et al. 1994). A 1.4-kb genomic promoter fragment upstream of the ATG start codon was amplified by PCR using Dongjin genomic DNA as the template and cloned into the binary vector pCAMBIA1305 to drive the beta-glucuronidase (GUS) reporter gene expression. The gene-specific primers were GUSF (5’-CCATGATTACGAATTCGCTCGTGCCAGTGAGATTA-3’) and GUSR (5’-TTGGCTGCAGGTCGACTCTCTCACTTCTGCTCCCA-3’). Transgenic plants were generated as described above. GUS histochemical staining was performed as described previously (Ma et al. 2012). Images were captured using Leica Application Suite 3.3.
RNA Isolation and Quantitative Real-Time PCR Analysis (qRT-PCR)
Total RNA samples were extracted from young leaves and young roots of 2-week-old seedlings, shoot apices of 1-month-old seedlings, mature leaves, mature roots, leaf sheaths, culms, mature seeds, and panicles during the floret organ development stage of wild type and osarf19 using an RNA Prep Pure Plant Kit (Tiangen Co., Beijing) and reverse transcribed using a SuperScript II Kit (TaKaRa). QRT-PCR was performed using an SYBR Premix Ex TaqTM Kit (TaKaRa) on an ABI Prism 7500 real-time PCR system with the actin gene used as an internal control. The 2-DDCT method was used to analyze relative changes in gene expression (Livak and Schmittgen 2001). All qRT-PCR primers used for cell cycles genes (KN, H1, MCN2, MCN3, CYCT1, CDT2, CYCD4, CDC20, CDKA1,CDKA2, CAK1, CAK1A, CDKB, E2F2, CYCA2.1, CYCA2.2, CYCA2.3), cell wall synthesis genes (CESA6, IRX10L, GT8, UGA4e, CSLF6), rice PIN family genes (OsPIN1a, OsPIN1b, OsPIN2, OsPIN5a, OsPIN5b, OsPIN8, OsPIN9, OsPIN10a), OsYUCCAs (OsYUCCA1, 2, 3, 4, 5, 6, 7), OsGHs (OsGH3.7, 3.8, 3.9, 3.10, 3.11, 3.12), ABC model-related genes (OsMADS22, OsMADS29, OsMADS18, OsMADS3, OsMADS2, CFO1, OsMADS1), OsARF1-25, and OsActin are listed in Supplementary Table 1.
Subcellular Localization of OsARF19
To determine the cellular localization of OsARF19, green fluorescent protein (GFP) was fused to the C-terminus of OsARF19 under control of the 35S promoter in the pAN580 vector. The gene-specific primers were OsARF19–GFPF (5’-GCCCAGATCAACTAGTATGATGAAGCAGGCGCAGCAGC-3’) and OsARF19–GFPR (5’-CGGACTTAAGACTAGTCGCAGTATTCCAATACCTG-3’). The nuclear marker Ghd7–mCherry was constructed using primers Ghd7–mCherryF (5’-CGGAGCTAGCTCTAGAATGTCGATGGGACCAGCAGC-3’) and Ghd7–mCherryR (5’-TCGAGACGTCTCTAGATCTGAACCATTGTCCAAGC-3’). The OsARF19–GFP fusion and GFP were transiently co-transferred into rice protoplasts with the Ghd7–mCherry constructs as described by Bart et al. (2006). Fluorescence images were observed using a Zeiss LSM510 confocal laser microscope.
IAA Treatment
Seeds were surface sterilized with 5.0 % NaClO for 10 min, washed three times with sterilized water, and germinated on wet plates in a 35 °C growth chamber for 3 days. After germination, seedlings were moved to plates containing 0.1 μM indole-3-acetic acid (IAA) solution for 0, 10 min, 30 min, 1 h, 3 h, and 6 h. All samples were used for mRNA extraction and RT-PCR analysis as described above.
Results
Identification of the Mutant Osarf19
To elucidate important factors involved in floral organ development, we screened 400T-DNA tagged lines and identified one mutant with partially abnormal florets and enhanced plant height. Using FLS (flanking sequence) and T-DNA vector pGA2717 information from OryGenesDB (http://orygenesdb.cirad.fr/) and sequencing, we found the T-DNA segment was integrated into the 8th exon of LOC_Os06g48950, which encodes for OsARF19 (Fig. 1a). PCR analysis confirmed that the insertion site was homozygous (Fig. 1b). RT-PCR and qRT-PCR results demonstrated that OsARF19 transcripts barely accumulated in osarf19 (Fig. 1c, d). We concluded that osarf19 was a knockout mutant.
Identification of the osarf19 mutation. a T-DNA insertion site in mutant osarf19. Triangle represents the T-DNA vector. The grey box represents the exon, and the black line represents the intron. P1, P2, and P3 are primers for insertion site determination. b PCR analysis to confirm the integration of T-DNA into OsARF19. The upper band indicates the OsARF19 gene fragment, and the lower band indicates the T-DNA insertion fragment. c Reverse transcription PCR analysis to confirm the knockout status of osarf19. The upper bands show OsARF19 expression (32 cycles) in wild type (WT) and mutant, respectively, and the bottom bands show OsActin (30 cycles) expression as the control. d Quantitative real-time RT-PCR analysis to confirm the knockout status of osarf19. Values are given as mean ± SD (n = 3). Double asterisk indicate significant difference between WT and osarf19 at P = 0.01 by Student’s t test
Osarf19 Displayed Abnormal Floral Organs, and Altered Plant Height, Leaf Shape, and Seed Size
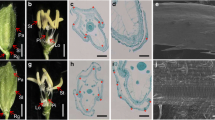
Compared with wild type (O. sativa var. japonica cv. Dongjin), most of the osarf19 florets normally developed, yet the others displayed three types of abnormalities (Fig. 2a–l). The first was an additional lemma-like organ on the same side of palea (Fig. 2e, f), accompanied by a crack when harvested (Fig. 2o). The second was an enlarged palea with a curved tip (Fig. 2g, h), generating an unclosed floret. And the third was variably degenerated paleas, from slight degeneration to complete loss (Fig. 2i–l). The heterozygous plants showed no abnormal florets, similar to WT (Table 1). The abnormal paleas and lemmas could still be observed when mature seeds were harvested (Fig. 2m–q). Each type of abnormal florets only possessed a relatively low proportion, but that was significantly different from WT (Table 1).
Disruption of OsARF19 affected floral organ development and increased plant height. a Floret in the wild type (WT) panicle, bar = 10 mm. b Floret of wild type (WT), bar = 3 mm. c Normal floret in the osarf19 panicle, bar = 10 mm. d Normal floret of the osarf19, bar = 3 mm. e Floret with additional lemma in the osarf19 panicle, bar = 10 mm. f Floret with additional lemma of osarf19, bar = 3 mm. g Floret with enlarged palea in the osarf19 panicle, bar = 10 mm. h Floret with enlarged palea of osarf19, bar = 3 mm. i Florets with degenerated palea in the osarf19 panicle, bar = 10 mm. j–l Florets with degenerated palea of osarf19, bar = 3 mm. m Mature grain from normal florets of wild type (WT). n Mature grain from normal florets of osarf19. o Mature grain from florets with additional lemma in osarf19. p Mature grains from florets with enlarged palea in osarf19. q Mature grain from florets with degenerate palea in osarf19, m–q bar = 3 mm. r Wild type and osarf19 plants after heading, bar = 10 cm. s Individual culm internode and panicle of wild type (WT) and osarf19 after heading, bar = 10 cm. t Lengths of individual culm internode and panicle of wild type (WT) and osarf19 after heading. Values are means ± SD (n = 3). Double asterisk and asterisk indicate significant differences between WT and osarf19 at P = 0.05 and P = 0.01 by Student’s t test
The homozygous mutant osarf19 showed an enlarged plant architecture in all development stages, including the tiller stage (Supplementary Fig. 1) and rice filling stage (Fig. 2r). The heterozygotes showed no obvious differences with WT in plant architecture (Supplementary Fig. 1). Plant height of osarf19 was slightly increased due to elongated culm internodes (Fig. 2s), especially the basal internode that was much longer than that of WT (Fig. 2s, t). Fifth, 4th, 3rd, and 2nd internodes of osarf19 were twice, 94 %, 58 %, and 25 % longer than those of WT, respectively, whereas the 1st internodes and panicles were almost the same (Fig. 2t). Similarly, the leaves of osarf19 had a more slender phenotype. The 2nd and 3rd uppermost leaves showed significantly increased length (Supplementary Fig. 2a, c), whereas the 1st and 2nd leaves slightly decreased in width (Supplementary Fig. 2b, d).
Besides, we investigated other agronomic traits of the osarf19 mutant. Seed width and thickness were obviously decreased in both de-hulled and hulled grains (Table 2), but seed length was similar to WT (Table 2). One thousand-grain weight significantly reduced and seed set of osarf19 (84.14 ± 1.25 %) was also lower than WT (94.09 ± 1.8 %), especially in abnormal florets. Unlike the additional lemma-type florets (84.32 ± 4.21 %), the enlarged palea (11.64 ± 6.22 %) and degenerated palea (50.32 ± 10.4 %) type florets were more infertile than normal florets of osarf19 (Table 2). Panicle number/plant and seed number/plant for osarf19 were both comparable to those of WT (Table 2). Besides, we detect the phenotypes of a F2 population of 210 individuals derived from a cross of osarf19 and WT. In the F2 population, there were 47 individuals exhibiting similar phenotypes to osarf19. The ratio of normal plants to abnormal ones fit a 3:1 ratio which indicated a monogenic inheritance of the mutation. The abnormal phenotypes co-segregated with homozygous T-DNA insertion fragments. The overall results indicated that OsARF19 was related to floret development and plant architecture, as well as affecting yield traits.
RNAi Lines of OsARF19 Display Similar Phenotypes to Knockout Mutant Osarf19
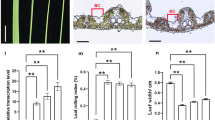
To verify that the mutant phenotypes were due to disruption of OsARF19, we first tried to construct a complementary vector, but in vain. We failed to get a positive clone after amplifying nearly 10-kb genome fragments and trying to recombinate them to complementary vector. Instead, we generated transgenic plants carrying an OsARF19 RNA interference (RNAi) construct (Fig. 3h). A plasmid containing two 230-bp fragments of OsARF19 cDNA inserted in forward and reverse orientations into the LH–1390–RNAi vector was introduced into cv. Dongjin. We obtained positively knockdown (RNAi-8) and empty vector (Vc) lines for function analyses. Compared with the Vc lines, RNAi-8 showed significantly reduced transcripts of OsARF19 (Fig. 3g). Then, we examined the floret development in both lines. Similar to the osarf19, most of florets in the RNAi-8 line were normally organized, but a small proportion displayed abnormalities as shown in Fig. 3b–f. All three types of abnormal florets (an additional lemma, an enlarged palea, and a degenerated palea) were observed in RNAi-8 (Fig. 3d–f). These results confirmed that repression of OsARF19 was indeed the cause of abnormal floral organ development in the osarf19 mutant.
Phenotypes of the OsARF19–RNAi line. a Positive OsARF19 knockdown (RNAi-8) and empty vector (Vc) lines at the heading stage, bar = 10 cm. b Floret from the Vc line, bar = 3 mm. c Normal florets from RNAi–8, bar = 3 mm. d–f Three abnormal floret types of RNAi–8 lines, floret with degenerate palea (d), additional lemma (e), and enlarged palea (f) from RNAi–8, bar = 5 mm. g Relative expression levels of OsARF19 in Vc and RNAi–8 lines. h Schematic diagram of OsARF19–RNAi construct. FAD2 linker, the intron of Arabidopsis FAD2 gene (At3g12120.1). UBi promoter, maize ubi promoter. Values are means ± SD (n = 3). Double asterisk and asterisk indicate significant differences between WT and osarf19 at P = 0.05 and P = 0.01 by Student’s t test
Despite abnormalities, the RNAi-8 plant showed accelerated growth rate and increased plant height at the heading stage (Fig. 3a). The knockdown line showed a more slender architecture compared with the Vc line. Harvested seeds of the RNAi line also displayed a slender shape; seed length was almost the same to that of Vc, but the width was significantly reduced (Supplementary Fig. 3a–d). These mutant phenotypes simulated the effects of reduced OsARF19 transcripts and confirmed that the various phenotypic alterations in the osarf19 mutant were caused by diminished OsARF19 expression.
Mutation in OsARF19 Increases Cell Elongation
Regulation of cell proliferation and expansion is essential for organ growth (Duan et al. 2014; Horiguchi et al. 2006; Potter and Xu 2001; Sugimoto-Shirasu and Roberts 2003). The enlarged basal internodes and leaves of the osarf19 mutant prompted us to study how OsARF19 affects organ size. We firstly investigated whether cell length or cell number was the cause of the enlarged phenotypes. We investigated cell size in the 4th internode and 3rd leaf. As shown in Fig. 4a–d, cells in the 4th internode of osarf19 were almost twice the size of those in WT. Cells in the 3rd leaf of osarf19 were also longer than in WT (Supplementary Fig. 4).
Osarf19 enhanced cell elongation in internodes. a Inner epidermal cells of a 4th elongated internode from wild type (WT) observed by scanning electron microscope (SEM). b Inner epidermal cells of a 4th elongated internode from osarf19 observed by scanning electron microscope (SEM). c Longitudinal section of a 4th elongated internode from wild type (WT). d Longitudinal section of a 4th elongated internode from osarf19. e Average lengths of inner epidermal cells of 4th internodes in wild type (WT) and osarf19. f Relative expression levels of cell wall synthesis-related genes, CESA6, IRX10L, GT8, UGA4e, and CSLF6. g Relative expression levels of cell cycle-related genes, KN, H1, MCN2, MCN3, CYCT1,CDT2, CYCD4, CDC20, CDKA1,CDKA2, CAK1, CAK1A, CDKB, E2F2, CYCA2.1, CYCA2.2, and CYCA2.3. Values are mean ± SD (n = 3). Double asterisk and asterisk indicate significant differences between WT and osarf19 at P = 0.05 and P = 0.01 by Student’s t test, a–d, bar = 100 um
As the cell wall is the major factor restricting cell elongation, many genes involved in cell wall synthesis are reported to regulate cell elongation. We investigated the expression levels of genes previously identified to be involved in cell wall synthesis (Ning et al. 2011). Our data showed expression levels of CESA6, IRX10L, GT8, UGA4e, and CSLF6 were significantly upregulated in osarf19 (Fig. 4f). Along with the morphological observations, this indicated that the enlarged plant architecture in osarf19 was caused by cell elongation. To eliminate the possibility of increased cell number in osarf19, we studied the expression levels of some cell cycle regulators in WT and the mutant (Li et al. 2011b). Expression of most of these genes did not differ significantly between WT and the mutant (Fig. 4g). In fact seven genes were significantly downregulated in osarf19, indicating that cell proliferation might be retarded (Fig. 4g). These results further confirmed that cell elongation rather than cell proliferation was the cause of the enlarged plant architecture.
OsARF19 Expression Pattern and Subcellular Localization
Since the mutant phenotypes of osarf19 were involved in both vegetative and reproductive tissues, we expected that transcripts of OsARF19 would be ubiquitous. We studied the expression patterns of OsARF19 by qRT-PCR. OsARF19 transcripts were present in various tissues, but preferentially in the leaves, lamina joint, basal internodes, young panicles or florets, and roots (Fig. 5m). The transcripts in internodes and panicles slowly decreased during maturation of the plant, and OsARF19 transcripts were barely detectable in mature seeds (Fig. 5m). To further determine spatial expression patterns of OsARF19, we generated transgenic plants that contained OsARF19 promoter::GUS fusions. GUS activities were observed in young panicles, culm internodes, leaf sheaths, leaf blades, lamina joint, stamens, and the primary root (Fig. 5a–k). Higher GUS activity was detected in the basal elongated internodes than the upper ones (Fig. 5a–d). Thus, OsARF19 is a temporally and spatially regulated gene. The abundant transcripts of OsARF19 in specific tissues were consistent with influencing roles in stem, leaf, and floral organ development.
Expression pattern and subcellular localization of OsARF19. a–k GUS staining of various tissues from OsARF19pro::GUS transgenic lines, bar = 5 mm. l OsARF19 transcription levels after 0, 15, 30, 1, 3, and 6 h of 10 uM IAA treatment. m OsARF19 transcription levels in various tissues, viz. 1st elongated internode, 3rd elongated internode, 4th elongated internode, young leaf blades, mature leaf blades, lamina joint, leaf sheath, panicle 1 (panicle before heading), panicle 2 (panicle at heading stage), panicle 3 (panicle after heading), mature seeds, young roots, and mature roots. n Subcellular localization of OsARF19–GFP fusion protein and GFP protein. GHD7 was used as a nuclear marker, bar = 5 um. Values are mean ± SD (n = 3). Double asterisk and asterisk indicate significant differences between WT and osarf19 at P = 0.05 and P = 0.01 by Student’s t test
We also analyzed OsARF19 transcription in plants treated with 0.1 μM IAA. OsARF19 expression was rapidly induced by IAA, peaked at 3 h, and subsequently decreased (Fig. 5l). Thus, OsARF19 is an auxin-dependent regulator, in agreement with its roles in auxin signaling.
To investigate subcellular localization of OsARF19, an OsARF19–GFP (green fluorescent protein) fusion protein and the GHD7–mCherry fusion protein (a nuclear marker) were constructed and co-transfected into rice leaf protoplasts (Xue et al. 2008). OsARF19 co-localized with the nuclear-located GHD7 protein and thus functioned in the nucleus (Fig. 5n).
OsARF19 is Involved in Floral Organ Development
Intensive research has shown that auxin controls plant morphogenesis in a concentration-dependent manner (Friml et al. 2002; Habets and Offringa 2014). The abnormal floret organization, together with enlarged plant architecture, promoted us to determine whether the local auxin concentration and responses were changed in osarf19. We studied the expression of some key regulators involved in endogenous auxin synthesis, deactivation, and distribution. Previous research has shown YUCCA family genes catalyzing tryptamine to N-hydroxytryptamine are responsible for auxin synthesis (Cheng et al. 2007; Stepanova et al. 2008; Zhao 2008). The GH family members modulate the adenylation of IAA, and reduce free IAA content (Jain et al. 2006; Takase et al. 2004; Tian et al. 2004). As showed, genes involved in auxin synthesis (OsYUCCA2, 5, 7) were upregulated, and those involved in auxin deactivation (OsGH3. 8, 3. 9, 3.10, 3.12) had reduced activities in osarf19 (Fig. 6a, b). Additionally, the PIN family members can determine local auxin concentration by mediating intercellular transport (Ding and Friml 2010; Qi et al. 2012), and our results showed OsPIN1b, OsPIN5b, OsPIN8, and OsPIN10a were greatly upregulated expressed in the osarf19 (Fig. 6c). Thus, the overall results indicated that the auxin concentration had been increased in osarf19. To further test this idea, we examined whether the auxin signaling was activated in the osarf19 background. By detecting expression of all OsARFs (except OsARF19), ten members (OsARF4, 9, 10, 11, 13, 14, 15, 18, 20, 24) in osarf19 showed significantly upregulated and the others unchanged (Supplementary Fig. 5).
Expression levels of rice YUCCA, GH, PIN family, and floret formation-related genes in wild type (WT) and osarf19. a Expression levels of rice YUCCA family genes, OsYUCCA1, OsYUCCA2, OsYUCCA3, OsYUCCA4, OsYUCCA5, OsYUCCA6, and OsYUCCA7 in wild type (WT) and osarf19, 2 weeks before heading. b Expression levels of rice GH family genes, OsGH3.7, OsGH3.8, OsGH3.9, OsGH3.10, OsGH3.11, and OsGH3.12 in wild type (WT) and osarf19, 2 weeks before heading. c Expression levels of rice PIN family genes, OsPIN1a, OsPIN1b, OsPIN2, OsPIN5a, OsPIN5b, OsPIN8, OsPIN9, and OsPIN10a in wild type (WT) and osarf19, 2 weeks before heading. d Expression levels of floret formation-related genes, OsMADS29, OsMADS22, OsMADS18, CFO1 (chimeric floral organs1), OsMADS1, OsMADS2, and OsMADS3 in normal florets of wild type (WT), osarf19, and abnormal florets of osarf19. Values are mean ± SD (n = 3). Double asterisk and asterisk indicate significant differences between WT and osarf19 at P = 0.05 and P = 0.01 by Student’s t test
We questioned whether such enhanced auxin performance would result in altered expression of floral organ regulators, thus leading to abnormal florets according to the ABC model by qRT-PCR analyses (Kobayashi et al. 2012; Li et al. 2011a; Nayar et al. 2013; Prasad et al. 2005; Sang et al. 2012; Sentoku et al. 2005). As shown in Fig. 6d, the expression levels of those regulators showed no significant difference in normal florets of WT and osarf19, except that OsMADS29 was mildly upregulated in the mutant. Interestingly, in abnormal florets, two genes (OsMDAS29, OsMDAS22) were markedly upregulated compared with WT, and another two genes (CFO1, OsMADS3) showed slight upregulation, while the others unchanged. Besides, auxin response elements (AuxREs, TGTCTC) were detected in the promoters of OsMADS29, 22, 3, and CFO1 (data not shown) which indicated their function might be affected by ARFs (Ulmasov et al. 1997a, b). An earlier report showed that ectopic expression of OsMADS29 and OsMADS22 in transgenic rice plants resulted in aberrant floral morphogenesis with a disorganized palea (Nayar et al. 2013;Sentoku et al. 2005), which was similar to the observations in osarf19. These results suggest that abnormal floret formation is at least partially caused by altered expression of OsMADS29 and OsMADS22, possibly resulting from irregular local auxin concentration and responses (Fig. 6a).
Discussion
The plant hormone auxin is an essential regulator of normal growth and development (Blakeslee et al. 2005). It can exert pleiotropic effects on development by regulating very basic processes such as cell division, growth, and differentiation (Benkova et al. 2003; Friml et al. 2002; Habets and Offringa 2014). These effects are always mediated by Aux/IAA protein degradation and subsequently auxin response factor (ARF) activation (Guilfoyle and Hagen 2007). In this study, we identified a T-DNA insertion mutant, osarf19, in which OsARF19 transcription was blocked (Fig. 1a–d). The mutant displayed various phenotypes, such as abnormal florets, elongated basal internodes, and altered leaf morphology, indicating OsARF19 functions in regulating such processes (Fig. 2a–t). Compared with previous reports on roles of ARF19 in leaf and lateral root development as well as the crosstalk with BR, to our knowledge, this study was firstly to relate OsARF19 with floral organ development (Choi et al. 2013; Okushima et al. 2007; Wilmoth et al. 2005; Zhang et al. 2015). The increased plant height in osarf19 was in agreement with the former finding that overexpression of OsARF19 resulted in a dwarf plant (Zhang et al. 2015). But OsARF19 knockout and overexpression lines shared similar phenotypes in leaf and seed width, which might be due to a high degree of redundancy among ARF family members in rice (Zhang et al. 2015). Using double-stranded RNA interference (RNAi), we obtained transgenic lines having similar phenotypes to osarf19 (Fig. 3a–h). These results indicated that the abnormal phenotypes in osarf19 were indeed caused by disruption of OsARF19.
We studied the expression pattern of OsARF19 by qRT-PCR and GUS staining analyses. OsARF19 was temporally and spatially expressed with its transcripts mainly in the culm internodes, lamina joint, root, leaves, and florets (Fig. 5m). Such tissue-specific expression pattern might be related with its roles in affecting those organ morphologies. The expression of OsARF19 in the lamina joint and root was correlated with its function in regulating the leaf angle and lateral root development (Zhang et al. 2015). Additionally, when analysis focused on a particular organ (such as the culm internodes), transcripts always peaked at an early stage and gradually decreased during maturation (Fig. 5a–d). The elongation of basal rather than uppermost internodes were possibly due to such temporally expression pattern. Yamamoto et al. (2007) reported overexpression of the OsYUCCA family genes could promote auxin synthesis and basal internode elongation at an early stage, which did not occur in wild type. And suppressing expression of OsGH3.8 by RNAi also increased auxin content and caused great basal internode elongation, as well as disorganized florets, while the upper ones were less affected (Yadav et al. 2007). We supposed the elongated basal internodes in osarf19 should result from the high auxin performance, including upregulation of OsYUCCAs, OsPINs, and downregulation of OsGHs (Fig. 6a–c). As is known, control of basal internode length is an important task in plant breeding. The basal internode length and its dry weight content are major plant traits that determine straw strength and correlates with lodging resistance (Islam et al. 2007; Kashiwagi and Ishimaru 2004). The osarf19 mutant could be useful as an experimental or breeding material to modulate basal internode length for ideal plant architecture.
It is well known that the size of an organ is determined by cell proliferation and expansion, and auxin regulates both processes (Horiguchi et al. 2006; Potter and Xu 2001; Sugimoto-Shirasu and Roberts 2003). In this study, the osarf19 mutant exhibited obviously longer cells than WT, responsible for elongated basal internode (Fig. 4a–d). Moreover, expression levels of genes involved in cell elongation (e.g., CESA6, IRX10L, GT8, UGA4e, and CSLF6) were significantly upregulated, further confirming that result. ARFs with a glutamine-rich middle region always functioned as activators of auxin-responsive gene expression while ARFs with proline- and/or serine-rich middle regions might repress the transcription activities (Tiwari et al. 2003; Qi et al. 2012). The OsARF19 containing a glutamine-rich middle region was considered as a transcription activator (Shen et al. 2010). Thus, it was unlikely that the upregulation of cell elongation genes were directly regulated by OsARF19, which might be possibly caused by other OsARFs enhancement (Supplementary Fig. 5).
Despite the effects of mutation on plant architecture, OsARF19 is also critical for floral organ development. First, we obtained three types of abnormal florets in both osarf19 mutant and RNAi lines. Abundant transcripts of OsARF19 were detected in young panicles, supporting a role in floral organ development (Fig. 5m). Recent studies have demonstrated that auxin controls meristem development in a concentration-dependent manner (Friml 2003; Habets and Offringa 2014). Our findings showed that disruption of OsARF19 might lead to increased auxin concentration, with upregulated OsYUCCAs and downregulated OsGHs (Fig. 6a). A recent study demonstrated that OsARF19 can directly bind to promoter of OsGH3-5 and reduce free IAA concentration (Zhang et al. 2015). However, whether OsARF19 could target other OsGH members (OsGH3.8, 3.9, 3.10, 3.12) and feedback regulation on OsYUCCAs and OsPINs is still complex and is worth further investigation.
Meanwhile, the elevated auxin concentration could induce auxin signaling transduction and might alter the expression of downstream genes (Supplementary Fig. 5). According to the ABC model, OsMADS1 was a Class E gene and played a role in determining the identities of palea and lemma (Jeon et al. 2000). Dysfunction of OsMADS1 can result in elongated leafy palea and lemma and an unclosed floret. The expression level of OsMADS1 was not altered in osarf19, which was coordinated with that OsMADS1 functions upstream of ARFs. However, another two genes (OsMADS29, OsMADS22) were markedly upregulated in abnormal florets of osarf19 (Fig. 6d). AuxREs (TGTCTC) were detected in the promoters of such two genes (data not shown), which further confirmed their transcription was regulated by the auxin signaling pathway.
OsMADS22 is a member of the STMADS11-like family of MADS-box genes. Ectopic expression of OsMADS22 in transgenic rice resulted in different aberrant floral morphogenesis (Sentoku et al. 2005), which were similar to the abnormal floret with an additional lemma and degenerated palea in osarf19. OsMADS29 was induced by auxin, and overexpression of OsMADS29 caused aberration flower morphology with a small and degenerated palea (Nayar et al. 2013). The elevated OsMADS29 expression might be correlated with the degenerated palea found in osarf19. Therefore, it is possible that the abnormal florets in osarf19 were due to improper expression of these two genes, and other possible regulators contributing to palea and lemma development could not be discounted (Fig. 6d). And further researches on auxin and such two regulators will be carried out in our future studies.
In this study, new evidence was provided for functions of ARFs in cell elongation and floral organ development. The osarf19 mutant displayed various phenotypes in both vegetative and reproductive organs, revealing its pleiotropic effects at different development stages. Although previous studies reported that ARFs (ARF5, 6, 8) affect flowering pattern in Arabidopsis (Garrett et al. 2012; Nagpal et al. 2005), this is the first report on the effects of OsARF19 on floral organ development in rice. Our results confirmed previous conclusions on the influence of auxin on floral organ development, but raised new questions as to how ARFs coordinate with each other and what are their downstream targets. Our findings provide an important basis to address those questions.
Abbreviations
- ARF:
-
Auxin response factor
- var:
-
Variety
- cv:
-
Cultivar
- FLS:
-
Flanking sequence
- qRT-PCR:
-
Quantitative real-time PCR
- GFP:
-
Green fluorescent protein
- GUS:
-
Beta-glucuronidase
- IAA:
-
Indole-3-acetic acid
References
Aida M, Vernoux T, Furutani M, Traas J, Tasaka M (2002) Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development 129:3965–3974
Attia KA, Abdelkhalik AF, Ammar MH, Wei C, Yang J, Lightfoot DA, El-Sayed WM, El-Shemy HA (2009) Antisense phenotypes reveal a functional expression of OsARF1, an auxin response factor, in transgenic rice. Curr Issues Mol Biol 11(Suppl 1):i29–i34
Bart R, Chern M, Park CJ, Bartley L, Ronald PC (2006) A novel system for gene silencing using siRNAs in rice leaf and stem derived protoplasts. Plant Methods 2:13
Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602
Blakeslee JJ, Peer WA, Murphy AS (2005) Auxin transport. Curr Opin Plant Biol 8:494–500
Cai Q, Yuan Z, Chen M, Yin C, Luo Z, Zhao X, Liang W, Hu J, Zhang D (2014) Jasmonic acid regulates spikelet development in rice. 5:3476
Cheng Y, Dai X, Zhao Y (2007) Auxin synthesized by the YUCCA flavinmonooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19:2430–2439
Choi S, Cho YH, Kim K, Matsui M, Son SH, Kim SK, Fujioka S, Hwang I (2013) BAT1, a putative acyltransferase, modulates brassinosteroid levels in Arabidopsis. Plant J 73:380–391
Ding Z, Friml J (2010) Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc Natl Acad Sci U S A 107:12046–12051
Dreni L, Jacchia S, Fornara F, Fornari M, Ouwerkerk PB, An G, Colombo L, Kater MM (2007) The D-lineage MADS-box gene OsMADS13 controls ovule identity in rice. Plant J 52:690–699
Duan P, Rao Y, Zeng D, Yang Y, Xu R, Zhang B, Dong G, Qian Q, Li Y (2014) SMALL GRAIN 1, which encodes a mitogen-activated protein kinase kinase 4, influences grain size in rice. Plant J 77:547–557
Friml J (2003) Auxin transport—shaping the plant. Curr Opin Plant Biol 6:7–12
Friml J, Benkova E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jurgens G, Palme K (2002) AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108:661–673
Garrett JJ, Meents MJ, Blackshaw MT, Blackshaw LC, Hou H, Styranko DM, Kohalmi SE, Schultz EA (2012) A novel, semi-dominant allele of MONOPTEROS provides insight into leaf initiation and vein pattern formation. Planta 236:297–312
Guilfoyle TJ, Hagen G (2007) Auxin response factors. Curr Opin Plant Biol 10:453–460
Habets ME, Offringa R (2014) PIN-driven polar auxin transport in plant developmental plasticity: a key target for environmental and endogenous signals. New Phytol 203:362–377
Hardtke CS, Berleth T (1998) The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 17:1405–1411
Hardtke CS, Ckurshumova W, Vidaurre DP, Singh SA, Stamatiou G, Tiwari SB, Hagen G, Guilfoyle TJ, Berleth T (2004) Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL 4. Development 131:1089–1100
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Horiguchi G, Ferjani A, Fujikura U, Tsukaya H (2006) Coordination of cell proliferation and cell expansion in the control of leaf size in Arabidopsis thaliana. J Plant Res 119:37–42
Islam MS, Peng S, Visperas RM, Ereful N, Bhuiya MSU, Julfiquar AW (2007) Lodging-related morphological traits of hybrid rice in a tropical irrigated ecosystem. Field Crop Res 101:240–248
Jain M, Kaur N, Tyagi AK, Khurana JP (2006) The auxin-responsive GH3 gene family in rice (Oryza sativa). Funct Integr Genomics 6:36–46
Jeon JS, Lee S, Jung KH, Jun SH, Jeong DH, Lee J, Kim C, Jang S, Yang K, Nam J, An K, Han MJ, Sung RJ, Choi HS, Yu JH, Choi JH, Cho SY, Cha SS, Kim SI, An G (2000) T-DNA insertional mutagenesis for functional genomics in rice. Plant J 22:561–570
Jeong DH, An S, Kang HG, Moon S, Han JJ, Park S, Lee HS, An K, An G (2002) T-DNA insertional mutagenesis for activation tagging in rice. Plant Physiol 130:1636–1644
Jeong D, An S, Park S, Kang H, Park G, Kim S, Sim J, Kim Y, Kim M, Kim S, Kim J, Shin M, Jung M, An G (2006) Generation of a flanking sequence-tag database for activation-tagging lines in japonica rice. Plant J 45:123–132
Kashiwagi T, Ishimaru K (2004) Identification and functional analysis of a locus for improvement of lodging resistance in rice. Plant Physiol 134:676–683
Khanday I, Yadav SR, Vijayraghavan U (2013) Rice LHS1/OsMADS1 controls floret meristem specification by coordinated regulation of transcription factors and hormone signaling pathways. Plant Physiol 161:1970–1983
Kobayashi K, Yasuno N, Sato Y, Yoda M, Yamazaki R, Kimizu M, Yoshida H, Nagamura Y, Kyozuka J (2012) Inflorescence meristem identity in rice is specified by overlapping functions of three AP1/FUL-like MADS box genes and PAP2, a SEPALLATA MADS box gene. Plant Cell 24:1848–1859
Krizek BA, Fletcher JC (2005) Molecular mechanisms of flower development: an armchair guide. Nat Rev Genet 6:688–698
Li N, Zhang DS, Liu HS, Yin CS, Li XX, Liang WQ, Yuan Z, Xu B, Chu HW, Wang J, Wen TQ, Huang H, Luo D, Ma H, Zhang DB (2006) The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 18:2999–3014
Li H, Liang W, Hu Y, Zhu L, Yin C, Xu J, Dreni L, Kater MM, Zhang D (2011a) Rice MADS6 interacts with the floral homeotic genes SUPERWOMAN1, MADS3, MADS58, MADS13, and DROOPING LEAF in specifying floral organ identities and meristem fate. Plant Cell 23:2536–2552
Li Y, Fan C, Xing Y, Jiang Y, Luo L, Sun L, Shao D, Xu C, Li X, Xiao J, He Y, Zhang Q (2011b) Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat Genet 43:1266–1269
Li G, Liang W, Zhang X, Ren H, Hu J, Bennett MJ, Zhang D (2014) Rice actin-binding protein RMD is a key link in the auxin-actin regulatory loop that controls cell growth. Proc Natl Acad Sci U S A 111:10377–10382
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Ma X, Cheng Z, Qin R, Qiu Y, Heng Y, Yang H, Ren Y, Wang X, Bi J, Ma X, Zhang X, Wang J, Lei C, Guo X, Wang J, Wu F, Jiang L, Wang H, Wan J (2012) OsARG encodes an arginase that plays critical roles in panicle development and grain production in rice. Plant J 73:190–200
McSteen P (2010) Auxin and monocot development. Cold Spring Harb Perspect Biol 2:a1479
Moon Y, Jung J, Kang H, An G (1999) Identification of a rice APETALA3 homologue by yeast two-hybrid screening. Plant Mol Biol 40:167–177
Nagpal P, Ellis CM, Weber H, Ploense SE, Barkawi LS, Guilfoyle TJ, Hagen G, Alonso JM, Cohen JD, Farmer EE, Ecker JR, Reed JW (2005) Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 132:4107–4118
Nayar S, Sharma R et al (2013) Functional delineation of rice MADS29 reveals its role in embryo and endosperm development by affecting hormone homeostasis. J Exp Bot 64:4239–4253
Ning J, Zhang B, Wang N, Zhou Y, Xiong L (2011) Increased leaf angle1, a Raf-like MAPKKK that interacts with a nuclear protein family, regulates mechanical tissue formation in the Lamina joint of rice. Plant Cell 23:4334–4347
Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19:118–130
Potter CJ, Xu T (2001) Mechanisms of size control. Curr Opin Genet Dev 11:279–286
Prasad K, Parameswaran S et al (2005) OsMADS1, a rice MADS-box factor, controls differentiation of specific cell types in the lemma and palea and is an early-acting regulator of inner floral organs. Plant J 43:915–928
Przemeck GK, Mattsson J, Hardtke CS, Sung ZR, Berleth T (1996) Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta 200:229–237
Qi Y, Wang S, Shen C, Zhang S, Chen Y, Xu Y, Liu Y, Wu Y, Jiang D (2012) OsARF12, a transcription activator on auxin response gene, regulates root elongation and affects iron accumulation in rice (Oryza sativa). New Phytol 193:109–120
Sang X, Li Y, Luo Z, Ren D, Fang L, Wang N, Zhao F, Ling Y, Yang Z, Liu Y, He G (2012) CHIMERIC FLORAL ORGANS1, encoding a monocot-specific MADS box protein, regulates floral organ identity in rice. Plant Physiol 160:788–807
Sentoku N, Kato H, Kitano H, Imai R (2005) OsMADS22, an STMADS11-like MADS-box gene of rice, is expressed in non-vegetative tissues and its ectopic expression induces spikelet meristem indeterminacy. Mol Genet Genomics 273:1–9
Shen C, Wang S et al (2010) Functional analysis of the structural domain of ARF proteins in rice (Oryza sativa L.). J Exp Bot 61:3971–3981
Shen CJ, Yue RQ, Yang YJ, Zhang L, Sun T, Tie SG, Wang HZ (2014) OsARF16 is involved in cytokinin-mediated inhibition of phosphate transport and phosphate signaling in rice (Oryza sativa L.). Plos One 9:e112906
Shen CJ, Yue RQ, Sun T, Zhang L, Yang YJ, Wang HZ (2015) OsARF16, a transcription factor regulating auxin redistribution, is required for iron deficiency response in rice (Oryza sativa L.). Plant Sci 231:148–158
Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jurgens G, Alonso JM (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133:177–191
Sugimoto-Shirasu K, Roberts K (2003) “Big it up”: endoreduplication and cell-size control in plants. Curr Opin Plant Biol 6:544–553
Tabata R, Ikezaki M, Fujibe T, Aida M, Tian CE, Ueno Y, Yamamoto KT, Machida Y, Nakamura K, Ishiguro S (2010) Arabidopsis auxin response factor6 and 8 regulate jasmonic acid biosynthesis and floral organ development via repression of class 1 KNOX genes. Plant Cell Physiol 51:164–175
Takase T, Nakazawa M, Ishikawa A, Kawashima M, Ichikawa T, Takahashi N, Matsui M (2004) Ydk1-D, an auxin responsive GH3 mutant that is involved in hypocotyl and root elongation. Plant J 37:471–483
Theissen G (2001) Development of floral organ identity: stories from the MADS house. Curr Opin Plant Biol 4:75–85
Thompson BE, Hake S (2009) Translational biology: from Arabidopsis flowers to grass inflorescence architecture. Plant Physiol 149:38–45
Tian CE, Muto H, Higuchi K, Matamura T, Tatematsu K, Koshiba T, Yamamoto KT (2004) Disruption and overexpression of auxin response factor 8 gene of Arabidopsis affect hypocotyl elongation and root growth habit, indicating its possible involvement in auxin homeostasis in light condition. Plant J 40:333–343
Tiwari SB, Hagen G, Guilfoyle T (2003) The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 15:533–543
Toriba T, Hirano HY (2014) The DROOPING LEAF and OsETTIN2 genes promote awn development in rice. Plant J 77:616–626
Ulmasov T, Hagen G, Guilfoyle TJ (1997a) ARF1, a transcription factor that binds to auxin response elements. Science 276:1865–1868
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997b) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971
Waller F, Furuya M, Nick P (2002) OsARF1, an auxin response factor from rice, is auxin-regulated and classifies as a primary auxin responsive gene. Plant Mol Biol 50:415–425
Wilmoth JC, Wang S, Tiwari SB, Joshi AD, Hagen G, Guilfoyle TJ, Alonso JM, Ecker JR, Reed JW (2005) NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J 43:118–130
Xiao H, Wang Y, Liu D, Wang W, Li X, Zhao X, Xu J, Zhai W, Zhu L (2003) Functional analysis of the rice AP3 homologue OsMADS16 by RNA interference. Plant Mol Biol 52:957–966
Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, Zhang Q (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40:761–767
Yadav SR, Prasad K, Vijayraghavan U (2007) Divergent regulatory OsMADS2 functions control size, shape and differentiation of the highly derived rice floret second-whorl organ. Genetics 176:283–294
Yamaguchi T, Lee DY, Miyao A, Hirochika H, An G, Hirano HY (2006) Functional diversification of the two C-class MADS box genes OSMADS3 and OSMADS58 in Oryza sativa. Plant Cell 18:15–28
Yamamoto Y, Kamiya N, Morinaka Y, Matsuoka M, Sazuka T (2007) Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol 143:1362–1371
Zhang S, Wang S, Xu Y, Yu C, Shen C, Qian Q, Geisler M, Jiang DA, Qi Y (2015) The auxin response factor, OsARF19, controls rice leaf angles through positively regulating OsGH3-5 and OsBRI1. Plant Cell Environ 38:638–654
Zhao Y (2008) The role of local biosynthesis of auxin and cytokinin in plant development. Curr Opin Plant Biol 11:16–22
Acknowledgments
This research was supported by the Key Laboratory of Biology, Genetics and Breeding of Japonica Rice in Mid-lower Yangtze River, Ministry of Agriculture of China, The Yangtze River Valley Hybrid Rice Collaboration Innovation Center, Jiangsu Collaborative Innovation Center for Modern Crop Production, and the grants from the 863 Program (2014AA10A603-15), National Science and Technology Support Program (2013BAD01B02-16), Jiangsu Science and Technology Development Program (BE2014394), Jiangsu Province Self- innovation Program (CX(12)1003), and Qing Lan Project.
Author Contributions
S.Z. Zhang J.M. Wan, and L. Jiang conceived and designed the experiments. S.Z. Zhang, T. Wu, X. Liu, and S.J. Liu performed the experiments. S.Z. Zhang and T. Wu analyzed the data. J.M. Wan and L. Jiang contributed reagents/materials/analysis tools and revised the paper. S.Z. Zhang wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1312 kb)
Rights and permissions
About this article
Cite this article
Zhang, S., Wu, T., Liu, S. et al. Disruption of OsARF19 is Critical for Floral Organ Development and Plant Architecture in Rice (Oryza sativa L.). Plant Mol Biol Rep 34, 748–760 (2016). https://doi.org/10.1007/s11105-015-0962-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-015-0962-y