Abstract
The down-regulation of zeaxanthin (Zx) epoxidation is important for the regulation of Zx accumulation in xanthophyll cycle and for the development of non-photochemical quenching (NPQ). The NPQ development and Zx accumulation kinetics in rice, barley, and spinach leaves under light of different intensities were highly similar among the three plants. When the leaves were pre-treated with an inhibitor of Zx epoxidase (ZE), salicylaldoxime (SA), the two kinetics patterns in the leaves under low and moderately high light intensities became similar to those of high light intensity-treated leaves. Therefore, we propose that reversible down-regulation of Zx epoxidation plays an important role in plants, and this reversible down-regulation mechanism is a general mechanism in plants which occurs at room temperature under various light conditions as well as under different stress conditions in the presence of light. This reversible down-regulation is different from the irreversible down-regulation mechanism of ZE which involves ZE protein degradation together with D1 protein degradation under photooxidative conditions. There will be discussion on the mechanisms for the actual regulation of ZE activities involving phosphorylation/dephosphorylation of still unknown regulator(s) and/or by the redox regulation involving NADPH thioredoxin reductase C and thioredoxin m.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While light energy is converted to useful chemical energy during photosynthesis, excess light is harmful to the plants. Most of the excess energy, which is not required for photosynthetic CO2 assimilation, is dissipated as heat via non-photochemical quenching (NPQ) of chlorophyll fluorescence (Horton et al. 1996; Niyogi 2000; Zulfugarov et al. 2010, 2014). Zeaxanthin (Zx), a component of the violaxanthin (Vx) cycle, participates in the non-photochemical protection mechanism in vascular plants and algae (Demmig et al. 1987; Krause and Weis 1991; Pfündel and Bilger 1994; Jahns et al. 2009). In the Vx cycle, also known as the xanthophyll cycle, Vx is reversibly converted to Zx via the intermediate antheraxanthin (Ax). In the forward de-epoxidation reaction, two epoxy groups are removed stepwise by Vx de-epoxidase (VDE) in the thylakoid lumen. The backward epoxidation reaction is catalyzed by Zx epoxidase (ZE) in the chloroplast stroma.
Given the important function of Zx as a photoprotector against photoinhibition, the majority of studies have focused on the regulation of VDE in the Vx cycle. There have been several reports regarding the regulatory factors of de-epoxidation involved in the light-driven lumen acidification and ascorbate availability (Pfündel and Dilley 1993; Hager and Holocher 1994; Neubauer and Yamamoto 1994; Bratt et al. 1995), Vx availability and temperature (Siefermann and Yamamoto 1974; Bilger and Björkman 1991; Arvidsson et al. 1997), Vx orientation (Gruszecki et al. 1999), aggregation of light-harvesting complex II (LHCII), and lipid properties of thylakoid membrane (Jahns 1995; Gruszecki et al. 2006; Jahns et al. 2009; Schaller et al. 2010). A structural study of VDE suggested that the activation of VDE at low pH involves its dimerization that permits the parallel de-epoxidation of two epoxide rings of Vx (Arnoux et al. 2009).
However, there are far fewer studies on the regulation of ZE. Zx retention has been observed in plants under severe stress such as in overwintering evergreen plants, suggesting a long-term regulation mechanism (Adams et al. 2002; Öquist and Huner 2003). Moreover, significant inhibition of Zx epoxidation has been observed under prolonged illumination of high intensity or by chilling under light (Jahns 1995; Reinhold et al. 2008). This short-term down-regulation of Zx epoxidation was found to be independent of trans-thylakoid pH gradient (Xu et al. 1999; Gilmore and Ball 2000). Reinhold et al. (2008) attributed the short-term down-regulation of ZE to its direct modification, possibly by photooxidation.
Recently, we showed that the down-regulation of Zx epoxidation is a key factor that confers chilling-tolerance to the japonica rice cultivar compared with an indica one cultivar (Kim et al. 2017). Although previous studies mostly focused on the activity of VDE as a key regulator of the non-photochemical protection mechanism (Latowski et al. 2004; Szabó et al. 2005; Chen and Gallie 2012; Murchie and Ruban 2019), no significant differences have been observed in the activity of VDE between the two cultivars. In this review, we suggest that reversible down-regulation of Zx epoxidation is important in the leaves of a vascular plant at various light intensities at room temperature, and this reversible down-regulation mechanism is a general mechanism that functions in vascular plants at room temperature under various light conditions as well as under stress conditions in the presence of light. Under stress condition, a rather irreversible down-regulation mechanism is working, as we have mentioned earlier. Still, the actual mechanism for this down-regulation of ZE activity is uncertain. We will discuss about the redox regulated activation of ZE activity in the low light, which is proposed recently (Nikkanen et al. 2019), but cannot explain its down-regulation under higher light. We have proposed that the down-regulation of ZE activity is by phosphorylation of itself or one of its regulators (Kim et al. 2017), but still without suggesting candidate(s) for the regulator(s).
NPQ Development and Zx Accumulation Kinetics in a Dicot and Two Monocot Plant Leaves Under Light of Various Intensities
Faster NPQ development and Zx accumulation kinetics shown in leaves at room temperature under light of higher intensity can be due to down-regulation of ZE activity rather than up-regulation of VDE activity. To prove this hypothesis, we compared NPQ development and Zx accumulation kinetics in rice, barley, and spinach leaves under different light intensities.
The seeds of the rice (Oryza sativa L.) cultivar Dongjin-byeo were grown in a growth chamber under a 14-h light period with a PPFD of 100 μmol m–2 s–1 under a day/light temperature regime of 28 °C/25 °C. Barley (Hordeum vulgare L. ‘Albori’) seeds were grown at a PPFD of 50 μmol m–2 s–1 under a photoperiod of 14 h at 25 °C. For all the experiments, fully expanded first leaves of 1-week-old barley were used. Fresh spinach (Spinacia oleracea L.) leaves were obtained from a local market. The leaves of spinach, rice, and barley were floated on distilled water in Petri dishes with the adaxial side up and dark-adapted at 25 °C for 3 h to remove Zx that is present in plants under non-stressed growth condition. The dark-adapted leaf segments floated on distilled water were then exposed to different PPFDs (50–2100 μmol m–2 s–1) at 25 °C. The light provided using 500-W halogen lamps was passed through a 10-cm-deep water bath.
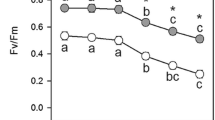
The NPQ development kinetics at room temperature were compared as reported previously (Kim et al. 2017) using the Stern–Volmer equation: NPQ = (Fm − Fm′)/Fm′, where Fm′ is the maximum yield of fluorescence in the light-acclimated leaves and Fm was measured after 10 min of dark adaption at room temperature for photoinhibition at 25 °C. Chlorophyll fluorescence quenching was analyzed using a pulse-amplitude modulated fluorometer (PAM-2000, Walz, Effeltrich, Germany). The illumination of spinach, rice, and barley leaves at four to five different PPFDs varying from low (50–100, 60–150, and 180–300 μmol m–2 s–1 for barley, rice, and spinach, respectively) to moderately high (300–600 μmol m–2 s–1 for barley and rice, and 700–1400 μmol m–2 s–1 for spinach), and high (1000, 1200, and 2100 μmol m–2 s–1 for barley, rice, and spinach, respectively) led to different NPQ development kinetics (Fig. 1a–c). The pattern of kinetics was highly similar among the three plants in each light group: NPQ increased rapidly or exponentially and saturated under light at high PPFDs (high light, HL), increased exponentially and saturated but rather slowly under light at moderately high PPFDs (medium light, ML), and increased initially but then decreased under light at low PPFDs (low light, LL). The saturated level of NPQ was approximately 3.6, 2.4, and 1.5 for spinach, rice, and barley, respectively.
Development of non-photochemical quenching (NPQ) and time course of zeaxanthin formation under light of various intensities at 25 °C in the leaves of spinach (a, d), rice (b, e), and barley (c, f). Light intensity is presented as photosynthetic photon flux density (PPFD) and is expressed as μmol m−2 s−1. After the measurement of NPQ, pigments were extracted with 100% cold-acetone from three segments of leaves at each measuring point and analyzed by a high-performance liquid chromatography system. % VAZ = % (violaxanthin + antheraxanthin + zeaxanthin) (n = 3, mean value ± SD; n = 1 ~ 2 for 0 h samples, rice high light (1200 μmol m−2 s−1) treated samples and barley samples)
As Zx accumulation is closely related to NPQ development, Zx accumulation kinetics at room temperature were also compared among the three plant species under light of various intensities (Fig. 1d–f). After the measurement of NPQ, pigments were extracted with 100% cold-acetone from three segments of leaves at each measuring point, and the Vx cycle components were determined as described by Kim et al. (2017) following the method of Thayer and Björkman (1990) with some modifications. The pattern of accumulation kinetics was highly similar to that of the NPQ development kinetics. Within 2 h of illumination, the relative amount of accumulated Zx reached a steady-state in all light groups. Remarkably, the fluctuations with respect to the relative amount of Zx were more prominent than those of the NPQ development kinetics in the plants under LL. Under HL, approximately 80%, 70%, and 50% of the Vx cycle pigments were converted from Vx to Zx in spinach, rice, and barley, respectively.
Effects of Salicylaldoxime on NPQ Development and Zx Accumulation in the Leaves of Three Vascular Plants Under Light of Various Intensities
To ensure that the differences in the rate of Zx formation at different PPFDs are due to the availability of Vx to de-epoxidase or the activity of epoxidase as reported in Jahns (1995), the leaves of three plants were pre-inhibited using 5 mM salicylaldoxime (SA), an epoxidase inhibitor (Pfündel and Bilger 1994), and then exposed to PPFDs varying from 50 to 2100 μmol m–2 s–1 (Fig. 2). Both the NPQ development kinetics and the rate of Zx formation in the leaves of three plants under low and moderately high PPFDs increased, reaching the level of leaves under high PPFDs. However, the treatment with SA did not significantly alter the availability of Vx in all the three plant species (Fig. 2d–f, Tables S1, S2, S3). This indicates that the differences in the rate of Zx formation in the three plant leaves under different PPFDs depend on the differences in the activity of ZE, and not on the availability of Vx to VDE. Although our data shown in this study are consistent with our conclusion, we cannot exclude the possible side effects of SA.
Development of non-photochemical quenching (NPQ) and time course of zeaxanthin formation under light of various intensities at 25 °C in the salicylaldoxime (SA)-treated leaves of spinach (a, d), rice (b, e), and barley (c, f). Light intensity is presented as photosynthetic photon flux density (PPFD) and expressed as μmol m−2 s−1. To inhibit epoxidation, pre-darkened leaves were infiltrated with 5 mM SA through the cut petiole at 25 °C for 3 h in dark before the light treatment. After the measurement of NPQ, pigments were extracted with 100% cold-acetone from three segments of leaves at each measuring point and analyzed by a high-performance liquid chromatography system. % VAZ = % (violaxanthin + antheraxanthin + zeaxanthin) (n = 3, mean value ± SD; n = 1 ~ 2 for 0 h samples, rice high light (1200 μmol m−2 s−1) treated samples and barley samples)
Reversible Down-Regulation of ZE Activities is a General Mechanism Working on Vascular Plant Leaves Under Light of Various Intensities
The leaves of plants tolerant to photoinhibitory stress are expected to have relatively high VDE activity compared with that in the leaves of sensitive plants. However, as reported earlier, a light-chilling resistant rice cultivar reversibly down-regulates the rate of Zx epoxidation rather than promoting the rate of Vx de-epoxidation, serving the photosystems in thylakoid membranes with the large amount of photoprotectors (Zx) (Kim et al. 2017). Under the same light-chilling condition, in the leaves of a light-chilling-sensitive rice cultivar, large amounts of Zx were re-converted to Ax and Vx due to relatively higher ZE activity, resulting in the slower accumulation of Zx in the thylakoid membranes compared with that in the tolerant plants. Extending this idea, the results of the present study suggest that the down-regulation of Zx epoxidation is a key factor that regulates Zx accumulation in vascular plant leaves (including both monocots and dicots) at various light intensities, and this down-regulation mechanism is a general mechanism that works at room temperature as well as under other stresses in the light including light-chilling.
Irreversible Down-Regulation of ZE Activities During Photoinhibition of PSII
The down-regulation of Zx epoxidation by a different mechanism was proposed by Reinhold et al. (2008) using Arabidopsis leaves under prolonged illumination of high-intensity light or under light-chilling stress. The gradual retardation of Zx epoxidation when authors increased light stress during pre-illumination was referred to the gradual down-regulation of the Zx epoxidase activity. Increasing the light intensity or the illumination time or decreasing the temperature during pre-illumination which decreases the PSII quantum efficiency after the pre-illumination treatment, also delays the epoxidation rates. Authors show that Zx epoxidation was retarded in thylakoids isolated from pre-illuminated leaves and on the basis of this data suggest that modification of the Zx epoxidase is most probably involved in the light-induced down-regulation. They speculated that the down-regulation of Zx epoxidation was due to the modification of ZE induced by photooxidation. The down-regulation of ZE protein by degradation has also been suggested by Schwarz et al. (2015) using Arabidopsis leaves under drought stress, not under high light. In Arabidopsis, pea and tobacco plant leaves under photoinhibitory stress condition, ZE protein degradation is also reported together with D1 protein degradation as a photoprotective mechanism by Bethmann et al. (2019).
The degradation of ZE protein in this irreversible mechanism in induced by photooxidation. However, the content of ROS produced in the chilling-tolerant rice cultivar was less than that produced by chilling-sensitive rice cultivar (Kim et al. 2017). Further, the down-regulated ZE quickly re-activated in the dark, suggesting that the down-regulation is a reversible process. Therefore, this irreversible mechanism is different from the above-mentioned reversible down-regulation mechanism. However, the involvement of irreversible inactivation of ZE by ROS or its degradation cannot be excluded, and this irreversible mechanism can be partially involved under mild stress conditions and is likely involved under severe stress conditions.
ZE Inactivation Mechanism for Reversible Down-Regulation
According to the data in our published paper (Kim et al. 2017) and the data shown in Figs. 1 and 2, we hypothesized that the reversible down-regulation of Zx epoxidation in vascular plants at room temperature under various light conditions as well as under stress conditions in the presence of light might be regulated by phosphorylation of ZE. Based on the research using stn7/stn8 mutants of Arabidopsis, Reinhold et al. (2008) suggest that phosphorylation is not involved in the short-term down-regulation of Zx epoxidation. However, this result still does not exclude the participation of other chloroplast kinases, because there are at least an overall set of 15 chloroplast-localized protein kinases are present in diverse vascular plants (Reiland et al. 2009; Bayer et al. 2012). However, we still cannot answer whether ZE itself is phosphorylated for its inactivation or other reaction partners of ZE that can influence ZE activity are phosphorylated.
Recently, several papers have been published suggesting the redox regulation of the activity of ZE. At light-limiting conditions, both Arabidopsis mutants defective in NADPH thioredoxin (Trx) reductase C (NTRC) (Naranjo et al. 2016) and Arabidopsis trxm mutants with silenced Trx m proteins (Da et al. 2018) accumulate higher levels of Zx than wild-type plants. Although the redox state of ZE was not altered in ntrc mutants (Naranjo et al. 2016), Da et al. (2018) provides evidences suggesting that by either NTRC or via Trx m, the sulfhydryl group of the ZE is reduced and activated through oligomerization. Because of the degradation of ZE in trxm mutants, Da et al. (2018) suggests that the redox-dependent oligomerization of ZE stabilizes and activate ZE, as well.
In conclusion, ZE can be somewhat inactive in darkness possibly with the oxidation of Trx and is activated and stabilized in the LL by the Trx system. However, we do not know how this stabilized ZE or activated ZE is degraded or inactivated under HL. Therefore, under HL, the current redox regulation mechanism need further evidences to explain the irreversible down-regulation of ZE by concomitant degradation of D1 and ZE proteins (Bethmann et al. 2019), and the reversible down-regulation mechanism of ZE activity by phosphorylation (Kim et al. 2017) need further regulators that influence ZE activity by phosphorylation.
References
Adams WW III, Demmig-Adams B, Rosenstiel TN, Brightwell AK, Ebbert V (2002) Photosynthesis and photoprotection in overwintering plants. Plant Biol 4:545–557
Arnoux P, Morosinotto T, Saga G, Bassi R, Pignol D (2009) A structural basis for the pH-dependent xanthophyll cycle in Arabidopsis thaliana. Plant Cell 21:2036–2044
Arvidsson PO, Carlsson M, Stefánsson H, Albertsson PA, Akerlund HE (1997) Violaxanthin accessibility and temperature dependency for de-epoxidation in spinach thylakoid membranes. Photosynth Res 52:39–48
Bayer RG, Stael S, Rocha AG, Mair A, Vothknecht UC, Teige M (2012) Chloroplast-localized protein kinases: a step forward towards a complete inventory. J Exp Bot 63:1713–1723
Bethmann S, Melzer M, Schwarz N, Jahns P (2019) The zeaxanthin epoxidase is degraded along with the D1 protein during photoinhibition of photosystem II. Plant Direct 3:e00185
Bilger W, Björkman O (1991) Temperature dependence of violaxanthin de-epoxidation and non-photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parviflora L. Planta 184:226–234
Bratt CE, Arvidsson PO, Carlsson M, Åkerlund HE (1995) Regulation of violaxanthin de-epoxidase activity by pH and ascorbate concentration. Photosynth Res 45:169–175
Chen Z, Gallie DR (2012) Violaxanthin de-epoxidase is rate-limiting for non-photochemical quenching under subsaturating light or during chilling in Arabidopsis. Plant Physiol Biochem 58:66–82
Da Q, Sun T, Wang M, Jin H, Li M, Feng D, Wang J, Wang H-B, Liu B (2018) M-type thioredoxins are involved in the xanthophyll cycle and proton motive force to alter NPQ under low-light conditions in Arabidopsis. Plant Cell Rep 37:279–291
Demmig B, Winter K, Krüger A, Czygan F-C (1987) Photoinhibition and zeaxanthin formation in intact leaves: a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol 84:218–224
Gilmore AM, Ball MC (2000) Protection and storage of chlorophyll in overwintering evergreens. Proc Natl Acad Sci USA 97:11098–11101
Gruszecki WI, Grudzinski W, Banaszek-Glos A, Matula M, Kernen P, Krupa Z, Sielewiesiuk J (1999) Xanthophyll pigments in light-harvesting complex II in monomolecular layers: localisation, energy transfer and orientation. Biochim Biophys Acta 1412:173–183
Gruszecki WI, Grudzinski W, Gospodarek M, Patyra M, Maksymiec W (2006) Xanthophyll-induced aggregation of LHCII as a switch between light-harvesting and energy dissipation systems. Biochim Biophys Acta 1757:1504–1511
Hager A, Holocher K (1994) Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light-dependent) pH decrease. Planta 192:581–589
Horton P, Ruban AV, Walter RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47:655–684
Jahns P (1995) The xanthophyll cycle in intermittent light-grown pea plants (possible functions of chlorophyll a/b-binding proteins). Plant Physiol 108:149–156
Jahns P, Latowski D, Strzalka K (2009) Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids. Biochim Biophys Acta 1787:3–14
Kim H-S, Hoang MH, Jeon YA, Wu G, Lee CH (2017) Differential down-regulation of zeaxanthin epoxidation in two rice (Oryza sativa L.) cultivars with different chilling sensitivities. J Plant Biol 60:413–422
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Latowski D, Grzyb J, Strzałka K (2004) The xanthophyll cycle-molecular mechanism and physiological significance. Acta Physiol Plant 26:197–212
Murchie EH, Ruban AV (2019) Dynamic non-photochemical quenching in plants: from molecular mechanism to productivity. Plant J. https://doi.org/10.1111/tpj.14601
Naranjo B, Mignee C, Krieger-Liszkay A, Hornero-Méndez D, Gallardo-Guerrero L, Cejudo FJ, Lindahl M (2016) The chloroplast NADPH thioredoxin reductase C, NTRC, controls non-photochemical quenching of light energy and photosynthetic electron transport in Arabidopsis. Plant Cell Environ 39:804–822
Neubauer C, Yamamoto HY (1994) Membrane barriers and Mehler-peroxidase reaction limit the ascorbate available for violaxanthin de-epoxidase activity in intact chloroplasts. Photosynth Res 39:137–147
Nikkanen L, Guinea Diaz M, Toivola J, Tiwari A, Rintamäki E (2019) Multilevel regulation of non-photochemical quenching and state transitions by chloroplast NADPH-dependent thioredoxin reductase. Physiol Plant 166:211–225
Niyogi KK (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3:455–460
Öquist G, Huner NP (2003) Photosynthesis of overwintering evergreen plants. Annu Rev Plant Biol 54:329–355
Pfündel E, Bilger W (1994) Regulation and possible function of the violaxanthin cycle. Photosynth Res 42:89–109
Pfündel E, Dilley R (1993) The pH dependence of violaxanthin deepoxidation in isolated pea chloroplasts. Plant Physiol 101:65–71
Reiland S, Messerli G, Baerenfaller K, Gerrits B, Endler A, Grossmann J, Gruissem W, Baginsky S (2009) Large-scale Arabidopsis phosphoproteome profiling reveals novel chloroplast kinase substrates and phosphorylation networks. Plant Physiol 150:889–903
Reinhold C, Niczyporuk S, Beran KC, Jahns P (2008) Short-term down-regulation of zeaxanthin epoxidation in Arabidopsis thaliana in response to photo-oxidative stress conditions. Biochim Biophys Acta 1777:462–469
Schaller S, Latowski D, Jemioła-Rzemińska M, Wilhelm C, Strzałka K, Goss R (2010) The main thylakoid membrane lipid monogalactosyldiacylglycerol (MGDG) promotes the de-epoxidation of violaxanthin associated with the light-harvesting complex of photosystem II (LHCII). Biochim Biophys Acta 1797:414–424
Schwarz N, Armbruster U, Iven T, Brückle L, Melzer M, Feussner I, Jahns P (2015) Tissue-specific accumulation and regulation of zeaxanthin epoxidase in Arabidopsis reflect the multiple functions of the enzyme in plastids. Plant Cell Physiol 56:346–357
Siefermann D, Yamamoto HY (1974) Light-induced de-epoxidation of violaxanthin in lettuce chloroplasts. III. Reaction kinetics and effect of light intensity on de-epoxidase activity and substrate availability. Biochim Biophys Acta 357:144–150
Szabó I, Bergantino E, Giacometti GM (2005) Light and oxygenic photosynthesis: energy dissipation as a protection mechanism against photo-oxidation. EMBO Rep 6:629–634
Thayer SS, Björkman O (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res 23:331–343
Xu CC, Jeon YA, Hwang HJ, Lee CH (1999) Suppression of zeaxanthin epoxidation by chloroplast phosphatase inhibitors in rice leaves. Plant Sci 146:27–34
Zulfugarov IS, Tovuu A, Dogsom B, Lee CH (2010) PsbS-specific zeaxanthin-independent changes in fluorescence emission spectrum as a signature of energy-dependent non-photochemical quenching in higher plants. Photochem Photobiol Sci 9:697–703
Zulfugarov IS, Tovuu A, Eu YJ, Dogsom B, Poudyal RS, Nath K, Hall M, Banerjee M, Yoon UC, Moon YH, An G, Jansson S, Lee CH (2014) Production of superoxide from photosystem II in a rice (Oryza sativa L.) mutant lacking PsbS. BMC Plant Biol 14:242
Acknowledgements
This research was supported by a 2-year research grant from Pusan National University.
Author information
Authors and Affiliations
Contributions
MHH and HSK performed the experiments and analyzed the data, ISZ analyzed and interpreted the data, and CHL designed the experimental plan and edited the manuscript. All the authors agreed on the contents of the paper and have no conflict of interest.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hoang, M.H., Kim, HS., Zulfugarov, I.S. et al. Down-Regulation of Zeaxanthin Epoxidation in Vascular Plant Leaves Under Normal and Photooxidative Stress Conditions. J. Plant Biol. 63, 331–336 (2020). https://doi.org/10.1007/s12374-020-09260-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-020-09260-8