Abstract
This study aimed to investigate the accelerated ripening of sukkari dates and examine the resulting changes in color, pulp, carbohydrates, vitamins, and antioxidant capacity using a comprehensive set of nine individual treatments. Fresh dates at the Khalal stage were subjected to immersion in normal water for 5 h or hot water for 5 min, with or without the inclusion of NaCl (2%), potassium metabisulfite (PM, 0.5%), and acetic acid (AA, 1.5%), either individually or in combination. The treated dates were then allowed to ripen for 72 h in an aerated incubator at 40 °C. Evaluation of color shade, fruit weight, pulp texture, total soluble carbohydrates (TSC), total soluble sugars (TSS), beta-carotene, total polyphenols (TPC), total flavonoids (TFC), DPPH radical scavenging activity, FRAP value, and overall appearance assessed the efficacy of each treatment. Among the treatments, T6 (normal water with 1.5% AA + 0.5% PM) and T8 (hot water with 1.5% AA + 0.05% PM) exhibited superior acceptance levels, characterized by softness, reduced pungency, a rich brown color, and exceptional visual appeal, with more than 77% and 88% pulp and ripened fruit, respectively. Furthermore, T6 demonstrated higher TSC (96.70 mg g−1 FW), TSS (322.22 mg g−1 FW), DPPH radical scavenging activity (92.84%), and FRAP value (0.40), while exhibiting lower levels of beta-carotene (4.91 mg 100 g−1 FW), TPC (0.14 mg g−1 FW), and TFC (0.31 mg g−1 FW). Similarly, T8 performed exceptionally well across all parameters except for beta-carotene.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Date palms (Phoenix dactylifera) are flowering plants in the Arecaceae family of palms, primarily cultivated for their edible fruit (Al-Shwyeh 2019). In 2020, the global production of palm dates amounted to around 9.5 million tons annually (Fikry et al. 2023). They can withstand a wide range of temperatures and exhibit resistance to adverse environmental conditions, ranging from − 6 to 50 °C. Additionally, they are tolerant to soil and water salinity. Date palms thrive in arid regions characterized by hot, dry weather and minimal rainfall (Allbed et al. 2017). While indigenous to Africa and the Persian Gulf and considered one of the ancient and main staple foods, the exact origin of date palms remains unknown (Shanta et al. 2021).The leading date palm producing countries worldwide include Iraq, Egypt, Saudi Arabia, Tunisia, Algeria, the UAE, Oman, Libya, Pakistan, and Sudan. Currently, there are approximately 2000 different date palm cultivars cultivated globally (Zihad et al. 2021).
The date palm fruit is an exceptional source of refined sugar, concentrated juice, confectionary pastes, and fermentation products owing to its elevated levels of mineral salts and vitamins (Assirey 2015). With a carbohydrate content of 70–80%, it serves as a potent supplier of readily available energy. Additionally, it boasts a rich profile of essential nutrients. The body efficiently absorbs fructose, glucose, and sucrose, which constitute the majority of carbohydrates found in dates. The high dietary fiber content of dates makes them suitable for incorporating into fiber-based food products and dietary supplements, further enhancing their nutritional value (Hazbavi et al. 2015). Furthermore, dates exhibit a notable presence of phenolics, flavonoids, and carotenoids, known for their anti-inflammatory, anti-mutagenic, and therapeutic properties (Tang et al. 2013).
The process of date ripening is intricate, involving the breakdown of chlorophyll, the formation of carotenoids, cell wall degradation, and starch conversion into sugars (Eltayeb et al. 1999). The maturity and ripening stage of dates play a crucial role in determining their sweetness, mouthfeel, and texture. Typically, dates are harvested and marketed in three distinct stages of development: mature firm (Besser or Khalal), half-ripe (Rutab), and fully ripe (Tamer). The selection of the optimal harvest stage is influenced by the cultivar’s characteristics, particularly sugar and soluble tannin content, as well as climatic conditions and consumer demand (Awad 2007). After reaching maturity, dates have a limited shelf life, potentially leading to reduced antioxidant levels. The antioxidant phenolic composition of the fruit undergoes significant changes during the ripening process, which may impact its shelf life. Although extensive research has been conducted on the physical and chemical transformations occurring in date palm fruit during growth and development, limited publications exist on the total phenolic content (TPC), condensed tannin (CTC) contents, and antioxidant activity (AA) of date fruit throughout the maturation stages (Amira et al. 2012). Therefore, understanding the ripening process and evaluating parameters such as TSS (total soluble solids), TSC (total soluble carbohydrates), TPC, TFC (total flavonoid content), CTC, β-carotene, antioxidant capacity like TAC (total antioxidant capacity), DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity, and FRAP (ferric reducing antioxidant power) is essential. To address this, our research focuses on the ripening process and evaluates the aforementioned parameters for the sukkari date palm cultivar grown in the arid region of Saudi Arabia, specifically assessing the Khalal stage of date fruit.
The sukkari date fruit type holds the reputation of being the most renowned and premium variety of dates in Arab nations. It is characterized by its elongated shape and a rich golden brown color, facilitating its differentiation from other variants of date palm fruits. Derived from the Arabic word ‘Sukkar,’ meaning ‘sugar,’ its name aptly captures its exceptionally sweet taste. It is worth noting that common misspellings of this cultivar include sukkari, sukkary, and succary (Siddeeg et al. 2019). Sukkari dates are highly valued for their exceptional sweetness, often described as a luscious caramel-like flavor with hints of honey. They have a soft and tender texture, offering a delightful melt-in-mouth experience (Siddeeg et al. 2019; Mani et al. 2022). Sukkari dates are not only cherished for their exquisite taste but also for their nutritional value. They are an excellent source of natural sugars, dietary fiber, and essential minerals such as potassium, magnesium, and iron. The high fiber content contributes to digestive health and aids in maintaining a healthy weight. These dates also contain vitamins, including vitamin A, vitamin B6, and vitamin K, which contribute to overall well-being (Vayalil 2012; Aljutaily et al. 2021).
The ripening of date fruit involves the application of various techniques. Traditional methods, commonly employed for ripening and curing, continue to be widely practiced. These methods typically entail exposing the fruits to the sun, spreading them out on mats. However, such practices often lead to suboptimal outcomes, as dusty conditions result in poor and inconsistent sundried product quality, accompanied by reduced yield. Furthermore, unfavorable weather conditions, including constant rain and strong winds, contribute to a significant portion of the harvested dates becoming susceptible to mold, fermentation, dust contamination, and damage from birds and insects (Saleem et al. 2005). These challenges have discouraged growers and severely impeded the continued cultivation and propagation of date palm varieties. To mitigate post-harvest losses and enhance produce quality, the adoption of appropriate scientific measures becomes imperative.
Previous studies have explored the ripening and curing of dates utilizing microwave radiation (Asif and Al Taher 1983), hot water, and heat treatments (Hazbavi et al. 2015). Although a few papers exist on the ripening of dates with salt and acetic acid, these studies are specific to particular varieties (Kalra et al. 1977; Saleem et al. 2005). However, limited information is available on the ripening of sukkari dates. Therefore, the primary objective of this study was to develop a method for the accelerated ripening of sukkari date fruit using acetic acid, sodium chloride, potassium metabisulfite, or a combination of these substances. Additionally, we aimed to investigate the physicochemical changes occurring during accelerated ripening and assess the suitability of these treatments as the optimal ripening method for commercial implementation, thereby improving the appearance of date palm fruit.
Materials and Methods
Sample Preparation
The sukkari dates were obtained from the germplasm of Bangladesh Sugarcrop Research Institute (BSRI), Ishurdi, Pabna. The fruits were harvested in the month of August at the fully mature Khalal stage. Healthy and non-infected fruits were selected and brought to the research laboratory of the Department of Physiology and Sugar Chemistry, BSRI, Ishurdi, Pabna. The fruits were given preliminary treatments of cleaning and washing before being subjected to ripening inducer treatments with analytical reagent (AR) grade sodium chloride (2% w/v), acetic acid (AA, 1.5% v/v) and potassium metabisulfite (PM, 0.5% w/v). Several solutions of sodium chloride, acetic acid and potassium metabisulfite were made for the treatments (Table 1). Twenty fruits containing equal sized and maturity was taken in each tray. The samples were immersed in normal water for 5 h or in hot water for 5 min along with/without NaCl (2% w/v), potassium metabisulfite (PM, 0.5% w/v) and acetic acid (AA, 1.5% v/v) individually or in combined form at room temperature, allowed to drain and then spread out separately on stainless steel trays. The samples were incubated for 72 h in an aerated incubator at 40 °C. Each treatment was replicated three times. There is a control in which fresh fruits were incubated for 72 h at 40 °C after cleaning and washing with water to compare the effectiveness of the chemical treatments.
Data Collection of Morphological Parameters
Ripening parameters were estimated soon after harvesting and treatments. Twenty fruits were randomly chosen to determine the ripening percentage using the following equation.
Changes in characteristics such as color, texture, average fruit weight and percent pulp were used to evaluate how well a treatment performed, whether it was applied alone or in combination. Visual observations and comparison with a horticultural color chart were used to record the fruit’s color (Kader 1992). The average fruit weight (g) was calculated by averaging the weights of the ten fruits. Similar methods were used to record average seed weight. Fruit’s flesh or pulp weight was also measured after the seed was removed. Flesh weight ratio was calculated as:
After then, the following formula was used to estimate pulp percentage.
An organoleptic test was performed for determining the acceptability of date fruits under different treatments by the methods of Jellinek (1985). The samples were distributed to a panel consisting of ten scientists from the Bangladesh Sugarcrop Research Institute. Initially, five date palm fruits from each treatment were presented to the panel members, who were asked to evaluate the tastiness of the fruits. Following this, the overall appearance was assessed by the panel members using a 10-point hedonic scale ranging from 1 to 10, where 1 = excellent, 2 = best, 3 = better, 4 = less better, 5 = very good, 6 = good, 7 = fair, 8 = less fair, 9 = poor, and 10 = very poor. The samples were evaluated twice, and the average values were recorded. The texture of the fruits was assessed using a hardness-testing device, as described by Baloch et al. (2003) with slight modifications. Briefly, the device operates on the principle of an aneroid sphygmomanometer. It consists of a glass barrel with an internal piston, a stainless steel bar with a 1 mm2 penetrating surface, and a rubber blow ball attached to a pressure gauge. The barrel is connected to the pressure gauge, and the piston is mounted on the stainless steel bar. The barrel is fixed vertically on a stand so that the needle just touches the surface of the date.
To perform the hardness test, the selected date was placed on the base beneath the tip of the pressure needle. Air pressure was gradually applied to the piston, increasing the force on the penetrometer and pressure gauge. The maximum pressure was recorded as soon as the needle punctured the surface of the date. At least five readings were taken for each date, with ten dates randomly selected from the batch chosen for maturity evaluation. The mean pressure readings (mm Hg) were recorded. The fruits were categorized based on the recorded pressure values as follows: very soft (≥ 150 mm Hg), over soft (≥ 200 mm Hg), soft (≥ 250 mm Hg), less soft (≥ 275 mm Hg), and hard (≥ 300 mm Hg).
Total Soluble Carbohydrate (TSC) and Total Soluble Sugar Content (TSS)
The homogenization of a 200 mg sample was carried out in 5 mL of 95% ethanol, followed by centrifugation at 3500 rpm for 10 min. The resulting supernatant was collected. Subsequently, the process was repeated using 70% ethanol (5 mL), and the combined supernatant was stored in a refrigerator at 4 °C for the determination of total soluble carbohydrates (TSC) and total soluble sugars (TSS) content.
TSC content was determined according to the method described earlier (Khoyerdi et al. 2016). Briefly, a 0.1 mL aliquot was combined with 1 mL anthrone solution (comprising 200 mg anthrone mixed with 100 mL of 72% sulfuric acid). The mixture underwent heating at 100 °C for 10 min, followed by cooling. The determination of total soluble carbohydrates utilized a standard curve, with a detection wavelength of 625 nm measured using a UV–Vis spectrophotometer (Jasco V-630, Jasco international co., ltd., Tokyo, Japan). The outcomes were expressed as mg g−1 fresh weight.
For the analysis of sucrose content, 0.2 mL of the supernatant was combined with 0.1 mL of KOH (30%) and subjected to heating at 100 °C for 10 min. Following cooling to room temperature, 3 mL of anthrone solution (150 mg anthrone mixed with 100 ml 70% sulfuric acid) was added. After an additional ten minutes, the samples were cooled, and the absorbance was measured at 620 nm. The sucrose concentration was determined using a standard curve, and the outcomes were presented as mg g−1 fresh weight (van Handel 1968).
Estimation of Beta-Carotene
Estimation of beta-carotene was done according to the method described earlier (Sarker and Oba 2018). In brief, a 200 mg fresh sample was ground thoroughly with 10 mL acetone (80%). After centrifugation of the extract for 4 min at 10,000× g, the supernatant was removed and made the final volume 20 mL by adding acetone. The optical density was measured with a spectrophotometer (Jasco V-630, Jasco international co., ltd., Tokyo, Japan) at 480 and 510 nm. The results were quantified as mg beta-carotene per 100 g FW by using the following formula:
Determination of Total Phenolic Content and Total Flavonoid Content
A 200 mg fresh sample was dissolved in 10 mL of ethanol (80%, v/v in water) and underwent sonication at 35 °C for 60 min. Following this, the resulting extracts were filtered through Advantech 5B filter paper (Tokoyo Roshi Kaisha Ltd., Saitama, Japan) and preserved in a refrigerator at 4 °C for further analysis.
Folin-Ciocalteu method was performed to estimate the total phenolic content (TPC) of the sample (Singleton and Rossi 1965). A reaction mixture comprised 1 mL of the sample, 200 µL of phenol reagent (1 N), and 1.8 mL of distilled water. After vortexing, 400 µL of Na2CO3 (10%, v/v in water) was introduced three minutes later. Subsequently, 600 µL of distilled water was added to reach the final volume of 4 mL, and the mixture was allowed to incubate at room temperature for 1 h. Absorbance was measured at 725 nm using a spectrophotometer (Jasco V-630, Jasco international co., ltd., Tokyo, Japan). The phenolic acid content was quantified based on a standard calibration curve of Gallic acid and expressed in µg g−1 fresh weight.
The total flavonoid content (TFC) was estimated following the method described by Ghimeray et al. (2014). In brief, 500 µL of the extract was combined with 100 µL of Al (NO3)3(10%, w/v) and 100 µL of potassium acetate (1 M) solution. Subsequently, 3.3 mL of distilled water was added to reach a final volume of 4 mL. The reaction mixture was vortexed and left to incubate at room temperature for 40 min, followed by measuring the absorbance at 415 nm using a UV–Vis spectrophotometer (Jasco V-630, Jasco international co., ltd., Tokyo, Japan). The total flavonoid content was quantified as mg g−1 of Quercetin equivalent on a fresh weight basis.
Estimation of Antioxidant Capacity
The fresh sample (200 mg) was dissolved in 10 mL of ethanol (80%, v/v in water) followed by sonication at 35 °C for 60 min. Afterward, the extracts were filtered (Advantech 5B filter paper, Tokoyo Roshi Kaisha Ltd., Saitama, Japan) and kept in a refrigerator (4 °C for further analysis).
DPPH Free Radical Scavenging Assay
The DPPH free radical scavenging assay was conducted followed by the method described earlier (Islam et al. 2020). Initially, DPPH powder (5.914 mg) was dissolved in methanol (100 mL) to create a stock solution, maintaining an absorbance range between 1.1 and 1.3 using a spectrophotometer. Subsequently, 1 mL of the extract was vigorously shaken with 3 mL of the DPPH solution and left in a dark room for 30 min at room temperature. A blank sample was generated by mixing 1 mL of distilled water with the DPPH solution instead of the extract. The absorbance was measured at 517 nm using a UV–Vis spectrophotometer (Jasco V-630, Jasco international co., ltd., Tokyo, Japan). The scavenging capacity of the samples was calculated using the following formula, and the results were expressed as a percentage (%):
Ferric Reducing Power Assay
The reducing potential of date extract was examine as described before (Oyaizu 1986). In this assay, 1 mL of plant extract was added to 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide (w/v), mixed and incubated at 50 °C for 20 min. Then 2.5 mL of 10% TCA (trichloroacetic acid) was added to it, and the resultant mixture was centrifuged for 10 min at 3000 rpm. The supernatant (5 ml) was added to distilled water (5 mL) and 1 mL of 0.1% FeCl3 solution (w/v), and the absorbance was taken at 700 nm in a UV–Vis spectrophotometer (Jasco V-630, Jasco international co., ltd., Tokyo, Japan).
Total Antioxidant Capacity Assay
The total antioxidant capacity assay was performed using phosphomolybdenum method (Adnan et al. 2019), where ascorbic acid was used as a standard. In this assay, 0.3 mL of plant extract was mixed with 3 mL of reagent solution (prepared by using 28 mM sodium phosphate, 4 mM ammonium molybdate, and 0.6 M sulfuric acid) in the test tubes. The tubes were covered and incubated in a water bath for 90 min at 95 °C. After cooling, the absorbance of the mixture was taken at 695 nm in a UV–Vis spectrophotometer (Jasco V-630, Jasco international co., ltd., Tokyo, Japan) and the calculated values were presented as equivalent to ascorbic acid in mg AAg−1 of fresh sample.
Statistical Analysis
All results were expressed as mean ± standard deviation (SD) in table and mean ± standard error (SE) in graphs, with three replications. Graphs were prepared using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Data presented in tables and graphs were analyzed using the Statistix 10 software (Analytical Software, Tallahassee, FL, USA) following a completely randomized design (CRD). Mean differences were compared using the Tukey HSD test, with p values ≤ 0.05 considered significant. Principal component analysis (PCA) was performed using OriginPro 10.0 (OriginLab Corporation, Northampton, MA, USA), and the Sankey diagram was created using the open-source statistical software R version 4.2.2, accessed on June 27, 2022.
Results
Changes in Fruit Color, Texture, Taste, and Overall Appearance
The color of a fruit serves as a crucial indicator, playing a pivotal role in the identification of fruit ripeness. The comprehensive spectrum of colors exhibited by sukkari dates is found in Table 2.
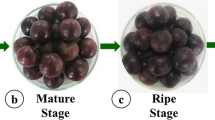
The impact of chemical treatments on the color of sukkari dates was significantly pronounced, particularly with the utilization of acetic acid and potassium metabisulfite treatments, resulting in an acceptable ripening color (Fig. 1). The collected data further elucidated that the combination of 1.5% acetic acid and 0.5% potassium metabisulfite ratio (T6) yielded a desired brown hue, enhancing the attractiveness of the fruit. Notably, the treatment involving 1.5% acetic acid and 0.05% potassium metabisulfite combined with hot water (T8) exhibited an exceptional color shine, surpassing the outcomes of all other treatments. Conversely, the color produced solely by potassium metabisulfite treatment did not meet the desired standards of appeal.
Fruit texture, particularly the firmness of the flesh, serves as a significant indicator of maturity when combined with other quality parameters. The fruits in the control group (T0) exhibited a hard and compact texture. However, when treated with a combination of acetic acid, potassium metabisulfite, and hot water (T2, T4, T6, and T7), the fruits acquired a very soft, sticky, and juicy texture (Table 2). It was observed that these treated samples were generally soft, with some of them being loose and sticky. Additionally, the fruits subjected to these treatments displayed increased flaccidity on the surface, while their firmness was enhanced toward the center. This observation suggests that the ripening process commenced at the fruit surface and gradually progressed toward the fruit core.
The taste of the fruits in the control condition exhibited a pungent flavor. However, the pungency was significantly reduced under different treatment conditions. Among the treated samples, those subjected to T5, T6, T7, and T8 treatments displayed a notably sweet taste. Conversely, T4 treatment resulted in fruit spoilage, leading to an unpleasant taste and odor.
In terms of overall ranking, the treatment involving normal water and potassium metabisulfite (T3) resulted in an amber to light brown color of the fruits, which was comparatively less attractive. The fruits treated with 2% NaCl and 1.5% acetic acid (T4) also influenced the appearance, albeit to a lesser extent. Furthermore, T4 treatment caused the fruits to become excessively soft and emit a foul odor.
Effect of Treatments on Morphophysiological Changes in Sukkari Dates
In general, the weight of the fruits and the percentage of pulp were observed to decrease across different treatment conditions when compared to the control group (Table 3). A noteworthy reduction was noted in the fruit weight across all treatments, while the percentages of pulp and the ratio of flesh weight experienced a significant decrease specifically in treatments T1 (− 13.19% and − 10.42%), T2 (− 13.29% and − 10.52%), and T3 (− 12.92% and − 10.15%) in comparison with the control group. On the other hand, higher ripening % was observed in T2 (90%), T4 (88.33%), T6 (88.33%), T7 (90%) and T8 (90%).
Total Soluble Carbohydrate (TSC) and Total Soluble Sugar Content (TSS)
Under different treatment conditions, both TSC and TSS exhibited notable variations (Fig. 2). Remarkably, the maximum TSC content was observed in treatments T6 and T8, displaying an increase of 59.8% and 60.8%, respectively, compared to the control group. Furthermore, treatments T5, T6, and T7 demonstrated significantly higher TSS levels, exhibiting enhancements of 23.7%, 21.09%, and 20.68% respectively, compared to the control group.
Total carbohydrate content (TSC) (A), total sugar content (TSS) (B) of sukkari date palm under different treatment condition. Values are expressed as mean ± S.E. (n = 3). Different letters indicate significant differences (p < 0.05) among the treatments within each parameter using Duncan’s multiple range test
Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
It was shown that the different treatment had a significant effect (p < 0.05) on the phenolic and flavonoid content of sukkari date palm (Fig. 3). Among the different treatments, the higher TPC was accumulated in T8 (0.76 mg g−1 FW) followed by T5 (0.76 mg g−1 FW) which is 31.02% and 30.94%higher than control group. On the other hand, higher TFC content was found in T5 (0.77 mg g−1 FW) followed by T7 (0.72 mg g−1 FW) and T8 (0.70 mg g−1 FW) which is 25.42%, 20.64% and 18% higher than control group. Besides, lower TPC (− 73.72% and − 73.82%) and TFC (− 49.14% and − 45.67%) were recorded in T3 and T6 treatments, respectively, compared to control group.
Total phenolic content (TPC) (A), total flavonoid content (TFC) (B) of sukkari date palm under different treatment condition. Values are expressed as mean ± S.E. (n = 3). Different letters indicate significant differences (p < 0.05) among the treatments within each parameter using Duncan’s multiple range test
Beta-Carotene Content
Higher beta-carotene was recorded in T3 (7.95 mg 100 g−1 FW) and T0 (7.72 mg 100 g−1 FW), while opposite result was observed in T4 (4 mg 100 g−1 FW) (Fig. 4).
Antioxidant Capacity
DPPH Free Radical Scavenging Assay
The results of DPPH radical scavenging activity of extracts affected by different treatment were shown in Fig. 5. The highest DPPH was recorded in T5 (66%) and the lowest was in T4 (− 59.2%) compared to control. Even though all the treatments exhibited a higher DPPH radical scavenging activity, T0, T2, T3, T5, T7, and T8 outperformed the others which are statistically similar.
DPPH free radical scavenging activity (%) (A). Ferric reducing antioxidant power assay (FRAP) (B). Total antioxidant capacity (C) of sukkari date palm under different treatment condition. Values are expressed as mean ± S.E. (n = 3). Different letters indicate significant differences (p < 0.05) among the treatments within each parameter using Duncan’s multiple range test
Ferric Reducing Power Assay
The reducing power demonstrated significant differences (p < 0.05) across various treatments (Fig. 5).The control condition exhibited the lowest ferric reducing antioxidant power (FRAP), which increased under the different treatment conditions. The highest FRAP values were observed in treatments T5 (72.87%) and T8 (71.55%) compared to the control.
Total Antioxidant Capacity Assay
Phosphor molybdenum assay is a quantitative method to evaluate water-soluble and fat-soluble antioxidant capacity (total antioxidant capacity). The results of total antioxidant capacity of date under different treatment are shown in Fig. 5. Total antioxidant capacity was higher in T1 (15.13%), T8 (15%) and lower in T5 (− 34.38%) compared to control.
Discussion
Fruit color is crucial to the marketing value and quality index. Similarly, variation in the color is closely related to the ripening. During ripening and curing, dates undergo a color change that produces a range of colors from yellow to brown. The fruit’s biological processes, which are triggered by chemical treatments, tend to modify the color (Saleem et al. 2010). All the treatments exerted a positive effect on this important property. The sukkari date while undergoing ripening changes color from light yellow at khalal to golden brown at the rutab stage. But the type of treatment used, and the level of ripening affected the color shade differently (Table 2). The fruits ripened under the influence of different chemicals changed color much earlier compared to the control treatment. This effect was highly pronounced in the treatments using a combination of acetic acid and potassium metabisulfite that gave a satisfactory ripening color. The results also suggested that three substances like sodium chloride, acetic acid and potassium metabisulfite might be involved in adjustment of physiological and biochemical process like antioxidants, secondary metabolites and enzymes to bring about the desired color change during ripening. The positive effect of sodium chloride and acetic acid on ripening of ‘Khasab’ dates was previously narrated (Afshari-Jouybari et al. 2014).
The fruits ripened by the control treatment exhibited a reduced softness throughout. However, the application of acetic acid in combination with potassium metabisulfite and hot water treatment resulted in a significant increase in the fruit’s texture, rendering it notably softer. The surface ripening of the dates using salt and vinegar acid was another finding made by others (Yektankhodaei et al. 2007). However, most of the dates after treatment with combination of NaCl and acetic acid (T4) became over soft and developed slightly disagreeable taste and flavor.
Fruits gave higher weight and pulp contents in control while it tends to decrease under various treatment conditions. This might be the reason of breakdown of starch into sugar (Adi et al. 2019). This finding is similar to the results from ‘Khasab’ date where weight and pulp percentage was lower in fruits treated with vinegar acid alone or in combination with salt as compared to the control samples (Asif and Al Taher 1983). However, a dissimilar finding was also narrated where treated dates had higher fruit weight and pulp contents than control (Saleem et al. 2005), where salt treated fruits gave the highest result. In the present study, a slight increase in fruit weight or pulp was seen in the case of T4, T5, T6, T7 and T8 where NaCl, AA and PM were used in various combination.
Apparently, all the treatments helped induce ripening of sukkari date to a certain degree (Table 3). However, the extent of ripening varied with the nature of the chemical and amount of the chemical application. In the context of unripe fruits, invertase predominantly exists in an intracellular or endoform configuration, where it can interact with the cellular protoplasm, leading to the formation of an insoluble compound. Once ripening begins, the invertase enters the extracellular space, or ectoform, and easily dissolves in water. Moreover, the softening of dates is facilitated by the action of hydrolytic enzymes, including polygalacturonase and cellulase. These enzymes serve to catalyze the hydrolysis of pectin and cellulose, which are integral structural components responsible for maintaining cell cohesion (Ayour et al. 2023; Alsawmahi et al. 2018; Rastegar et al. 2012). The findings of the present study about the softening brought on by NaCl and acetic acid are consistent with certain earlier reports (Afshari-Jouybariet al. 2014; Kalra et al. 1977). These agents may cause softening and ripening by tearing the epidermal cells and the protoplasm, whereby invertase gets activated. It was observed that heat treatment of fruits killed the protoplasm but induced ripening by activating enzymes (Yi et al. 2021). Further, dissociation of cell walls occurs during natural softening of fruits (Alina et al. 2023). In our study, the effect of NaCl and acetic acid on sukkari dates was more pronounced compared to the control treatment. Although all of the treatments contributed to inducing the ripening of dates, the combinatorial use of NaCl, acetic acid (A.A.), and potassium metabisulfite (P.M.) resulted in a significant level of ripening. The findings are consistent with previous results that reported a progressive increase in ‘dong’ formation of ‘Khadrawi’ and ‘Shamran’ date cultivars as salt concentration increased. Furthermore, the addition of vinegar acid enhanced this effect (Kalra et al. 1977).
Total soluble sugar and carbohydrate content of date fruit was increased as the fruit ripened. Sugar content of date is one of the most important commercial characteristics that is significant for both fresh consumption and fruit processing (Fadel 2008). Dates are composed of 70% carbohydrates, which include principally reducing sugars like glucose and fructose, non-reducing sugars like sucrose, and trace amounts of polysaccharides like cellulose and starch (Al-Shahib and Marshall 2003; Al-Farsi and Lee 2008). The amount of reducing sugars starts to increase at the Kimri stage and maximum amount of sucrose rises at the Khalal stage. Due to moisture loss, the weight drops at the Rutab stage, and sucrose is converted into reducing sugars (Tafti and Fooladi 2006). At the Khalal and early rutab stages of ripening, sucrose makes up around 60% of the dry weight of dates (Samarawira 1983).
The moisture content is related to sugar content since dates with low moisture content contain high sugar and vice versa (Tafti and Fooladi 2006). It is indicated that recently harvested dates have higher water contents than total soluble solids. It shows that a decrease in moisture or water contents results in more concentrated fruits TSS. Further, water evaporates during incubation for ripening/ curing, and moreover, the insoluble fruit materials get solubilized simultaneously because of breaking down of polymers through ripening. In our study, it is indicated clearly that the treatment in combination with acetic acid and potassium metabisulfite with or without hot water has responded well giving a higher percentage of both TSC and TSS (Fig. 2). However, in the presence of NaCl, TSC exhibited a slight increase, while TSS experienced a significant reduction. Although the exact mechanism remains undisclosed by the current experiment, we hypothesize that this phenomenon may be attributed to the conversion of sucrose into reducing sugars under the influence of NaCl. Earlier study reported that the activity of NaCl was significantly more potent than that of acid, and in treatments combining it with acetic acid, the action of acid was more potent and effective than when applied alone (Thatai and Kalra 1982; Asif and Al Taher 1983; Cheema 1986; Ali 1989). However, some of the dates after treatment with sodium chloride and those from combination treatments became over soft and developed a disagreeable taste and flavor.
Secondary metabolites of different groups of molecules called polyphenols are substances that occur naturally (phenolic acids, flavonoids, phytoestrogens etc.) found in large quantities in cereals, fruits, vegetables, and beverages (Zihad et al. 2021). The presence of hydroxyl groups and the conjugated ring structure in phenolic compounds, as well as their capacity to scavenge free radicals, all contribute to their significant antioxidant activity. The most effective free radical scavengers against the majority of oxidizing chemicals among the several polyphenol groups are flavonoids (Saeed et al. 2012). Some studies reported that phenolic substance were high in the inedible Kimri stage of date and declined progressively as the date matured to Tamer stage in natural ripening condition (Zhu et al. 2002; Ahmed and Ramaswamy 2006). In our study, we used full matured dates of Khalal stage where the levels of TPC and TFC were observed slightly increased in the treatment combination involving acetic acid and potassium metabisulfite, with the addition of either NaCl or hot water (Fig. 3). The slight variation in phenolic compound might be the reason of varietal differences (Ghnimi et al. 2017) and antioxidative nature of preservatives used in the treatments (Carocho et al. 2018). On the other hand, beta-carotene decreased under most of the treatments compared to control. Concurrently, within these treatments, there was a progressive enhancement in the taste, flavor, and ripening of the dates. This phenomenon strongly supports the role of antioxidants in the maturation of dates. One of the primary functions of polyphenols in maturation is their transformation from a soluble form (resulting in astringent taste) to an insoluble form (rendering them tasteless), likely attributed to their interaction with proteins (Ashraf and Hamidi-Esfahani 2011).
DPPH is a suitable free radical used to determine the antioxidant properties or radical scavenging capacity. Free radicals, which are produced during the lipid peroxidation process, are thought to have a significant impact on a variety of chronic pathologies, including cancer and cardiovascular illnesses. The DPPH radical is frequently used to assess the capacity of various natural products to scavenge free radicals and is recognized as a model molecule for free radicals originating in lipids (Shahdadi et al. 2015).
One of the primary causes of several medical disorders in the human body is oxidative stress, which results from an imbalance between the body’s natural defense mechanisms and the production of free radicals (McCord 2000). Several pathological conditions, such as degenerative diseases, cancer, and inflammatory diseases (Kohen and Nyska 2002), are caused by these unstable free radicals and reactive oxygen species (ROS), which can come from either internal or external sources (X-rays, industrial chemicals, tobacco smoke, air pollutants, etc.). They harm cellular macromolecules like protein, lipid, and DNA through electron pairing (Juan et al. 2021). The DPPH free radical scavenging assay was used in this study to assess the date palms capacity to scavenge free radicals. The DPPH-based free radical scavenging method is one of the most accurate and simple ways to assess the antioxidant activity of natural compounds, with the benefit of being unaffected by adverse effects (Livani et al. 2013). The present study displayed almost similar DPPH radical scavenging activity of extracts in various treatment conditions (Fig. 5). Higher levels of phenolic compounds may have potential role on increased the DPPH radical scavenging activity as the fruits have been shown to have a strong relationship between their phenolic content and free radical scavenging (Gao et al. 2000; Jiménez-Escrig et al. 2001; Arabshahi-Delouee and Urooj 2007).
Antioxidant action is typically attributed to a variety of processes, including reducing capacity, prevention of chain initiation, radical scavenging, and peroxide’s breakdown (Islam et al. 2022). The ability of bioactive substances to donate electrons, which reflects their reducing power, has been linked to antioxidant activity, according to numerous studies (Yen et al. 1993; Siddhuraju et al. 2002). Reducing power is thus a crucial indicator of the antioxidant activity of plant extracts. Different studies have indicated that the electron donation capacity (reflecting the reducing power) of bioactive compounds is associated with antioxidant activity (Beyhan et al. 2010). In this work, date extracts were assessed for their reducing capacity, or ability to donate electrons in the transition of Fe (III) to Fe (II), which is contributed through the reducing ability of the extracts. In a prior investigation, extracts from the reducing power exhibited substantial differences from the Khalal stage to the rutab stage, but no such variations were seen between the rutab stage and the Tamer stage. Khalal stage, which had the highest concentration of total phenolics, was the most effective reducing agent, whereas Tamer stage, which had the lowest concentration of phenolics, had the poorest reducing activity (Shahdadi et al. 2015). In our study, FRAP activity showed a linear increase with both TPC and TFC concentrations, and this relationship was most pronounced when using acetic acid and potassium metabisulfite, either with NaCl or hot water.
Total antioxidant capacity is a comprehensive method for assessing the antioxidant activity of plant extracts, and phenolics, flavonoids, and other reducing chemicals in the extract may contribute to some extent of the total antioxidant capacity (Kumar et al. 2014). The phosphomolybdenum method was used in this experiment to examine the overall antioxidant activity of the date extracts. The present study indicated that date fruits that were artificially ripened had higher levels of polyphenols than controls, which boosted their overall antioxidant capability. Significant amounts of polyphenols are found in the date extracts in both previous study (Sheikh et al. 2016) as well as present study, and the differences in these amounts can be directly related to the observed activity. This is because the total antioxidant capacity is directly proportional to the amount of polyphenols present in the extract (Zhu et al. 2004).
Principal Component Analysis and Sankey Diagram
The PCA analysis was conducted to determine the relationship between various morphophysiological parameters, phenolic components, antioxidant characteristics, and their scavenging activity of sukkari date fruits under all treatment settings during artificial ripening (Fig. 6). The elements PC1 and PC2 together describe 57.5% of the data variability. Two groups may be found in this figure across all treatment scenarios. From the figure, the treatments T0, T3, and T4 make up one group, while T1, T2, T5, T6, T7 and T8make up the other groups. Most of the parameters closely associated with the second group. The findings also indicated a positive correlation among Pulp%, FLW ratio, FW, and β-carotene, while exhibiting an inverse association with ripening percentage.
Principal component analysis (PCA) to elucidate the variable treatment relationships under nine treatments. The lines starting from the central point of the biplots display negative or positive associations of different variables, and their proximity specifies the degree of correlation with specific treatment
Conversely, distinct treatments were observed to form coherent clusters in accordance with the evaluated parameters within the Sankey diagram (Fig. 7). An assessment of the treatments with regard to their overall visual attributes revealed that T6 and T8 exhibited a notably superior performance, in contrast to the other treatments, which demonstrated comparatively inferior results.
Conclusion
In conclusion, our study highlights the substantial impact of various chemical treatments on sukkari dates. The combination of 1.5% acetic acid and 0.5% potassium metabisulfite (T6) yielded optimal color and texture, while T8, incorporating hot water, enhanced the color further. Taste improved in T5, T6, T7, and T8, with a notable shift from pungency to sweetness. Some treatments, such as T3 and T4, had less favorable effects on appearance and resulted in spoilage. Importantly, T6 and T8 treatments led to higher total soluble carbohydrates (TSC) and total soluble solids (TSS), indicating enhanced sugar content. Phenolic and flavonoid content increased in T8 and T5, while beta-carotene levels varied. Antioxidant activity was significantly influenced by the treatments, with T5 exhibiting the highest DPPH radical scavenging activity. Ferric reduction power was notably higher in T5 and T8. Total antioxidant capacity was higher in T1 and T8 but decreased in T5. These findings underline the potential for specific chemical treatments to enhance the quality, ripening, and antioxidant properties of sukkari dates, offering opportunities to improve their appeal and nutritional value. Further, artificial ripening of date fruits will help to ensure consistent quality and year-round availability, meeting consumer demand and supporting a stable market supply chain.
Data Availability
Not applicable.
References
Adi, D.D., I.N. Oduro, and C. Tortoe. 2019. Physicochemical changes in plantain during normal storage ripening. Scientific African 6: e00164. https://doi.org/10.1016/j.sciaf.2019.e00164.
Adnan, M., M.N.U. Chy, S. Rudra, A. Tahamina, R. Das, M.A.H. Tanim, T.I. Siddique, A. Hoque, S.M. Tasnim, and A. Paul. 2019. Evaluation of Bonamia semidigyna (Roxb.) for antioxidant, antibacterial, anthelmintic and cytotoxic properties with the involvement of polyphenols. Oriental Pharmacy and Experimental Medicine 19: 187–199. https://doi.org/10.1007/s13596-018-0334-x.
Afshari-Jouybari, H., A. Farahnaky, and S. Moosavi-Nasab. 2014. The use of acetic acid, sodium chloride solutions, and incubation to accelerate the ripening of ‘Mazafati’date. International Journal of Fruit Science 14 (1): 95–106. https://doi.org/10.1080/15538362.2013.818396.
Ahmed, J., and H.S. Ramaswamy. 2006. Physico-chemical properties of commercial date pastes (Phoenix dactylifera). Journal of Food Engineering 76: 348–352. https://doi.org/10.1016/j.jfoodeng.2005.05.033.
Al-Farsi, M.A., and C.Y. Lee. 2008. Nutritional and functional properties of dates: A review. Critical Reviews in Food Science and Nutrition 48 (10): 877–887. https://doi.org/10.1080/10408390701724264.
Ali, L. 1989. Effect of different chemical treatment on the physico-chemical characteristics and shelf-life of date fruit (Phoenix dactylifera L.). Faisalabad: University of Agriculture.
Alina, M.R., C.C. Mureșan, A. Pop, G.S. Marțiș, A.E. Mureșan, A.N. Postolache, F. Stoica, I.C. Crivei, I.D. Veleșcu, and R.N. Rațu. 2023. An overview of ripening processes. In New discoveries in the ripening processes, ed. Romina Alina Marc and Crina Carmen Mureșan. London: IntechOpen. https://doi.org/10.5772/intechopen.114015.
Aljutaily, T., H. Barakat, M.M.A. Moustafa, and M. Rehan. 2021. Incorporation of sukkari date in probiotic-enriched fermented camel milk improves the nutritional, physicochemical, and organoleptical characteristics. Fermentation 8 (1): 5. https://doi.org/10.3390/fermentation8010005.
Allbed, A., L. Kumar, and F. Shabani. 2017. Climate change impacts on date palm cultivation in Saudi Arabia. The Journal of Agricultural Science 155 (8): 1203–1218. https://doi.org/10.1017/S0021859617000260.
Alsawmahi, O.N., F.Y. Al-Juhaimi, A.M. Alhamdan, K. Ghafoor, I.A.M. Ahmed, B.H. Hassan, K.A. Ehmed, D. Abdelkarim, M. Younis, N. Alashmawe, and O.Q. Adiamo. 2018. Enzyme activity, sugar composition, microbial growth and texture of fresh Barhi dates as affected by modified atmosphere packaging. Journal of Food Science and Technology 55 (11): 4492–4504. https://doi.org/10.1007/s13197-018-3378-5.
Al-Shahib, W., and R.J. Marshall. 2003. The fruit of the date palm: its possible use as the best food for the future? International Journal of Food Sciences and Nutrition 54: 247–259. https://doi.org/10.1080/09637480120091982.
Al-Shwyeh, H.A. 2019. Date palm (Phoenix dactylifera L.) fruit as potential antioxidant and antimicrobial agents. Journal of Pharmacy and Bioallied Sciences 11 (1): 1–11. https://doi.org/10.4103/jpbs.JPBS_168_18.
Amira, E.A., S.E. Behija, M. Beligh, L. Lamia, I. Manel, H. Mohamed, and A. Lotfi. 2012. Effects of the ripening stage on phenolic profile, phytochemical composition and antioxidant activity of date palm fruit. Journal of Agricultural and Food Chemistry 60 (44): 10896–10902. https://doi.org/10.1021/jf302602v.
Arabshahi-Delouee, S., and A. Urooj. 2007. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chemistry 102 (4): 1233–1240. https://doi.org/10.1016/j.foodchem.2006.07.013.
Ashraf, Z., and Z. Hamidi-Esfahani. 2011. Date and date processing: A review. Food Reviews International 27 (2): 101–133. https://doi.org/10.1080/87559129.2010.535231.
Asif, M.I., and O.A.A. Taher. 1983. Ripening of Khasab dates by sodium chloride and acetic acid. Date Palm Journal 2 (1): 121–128.
Assirey, E.A.R. 2015. Nutritional composition of fruit of 10 date palm (Phoenix dactylifera L.) cultivars grown in Saudi Arabia. Journal of Taibah University for Science 9 (1): 75–79. https://doi.org/10.1016/j.jtusci.2014.07.002.
Awad, M.A. 2007. Increasing the rate of ripening of date palm fruit (Phoenix dactylifera L.) cv. Helali by preharvest and postharvest treatments. Postharvest Biology and Technology 43 (1): 121–127. https://doi.org/10.1016/j.postharvbio.2006.08.006.
Ayour, J., H. Harrakand, and M. Benichou. 2023. Cell wall enzymatic activity control: A reliable technique in the fruit ripening process. In New discoveries in the ripening processes, ed. Romina Alina Marc and Crina Carmen Mureșan. London: IntechOpen. https://doi.org/10.5772/intechopen.113752.
Baloch, A.K., S.A. Saleem, N.G. Dar, W.A. Baloch, and M.K. Baloch. 2003. Influence of microwave radiation on ripening of Dhakki dates. Journal of Food Processing and Preservation 27 (3): 181–193. https://doi.org/10.1111/j.1745-4549.2003.tb00511.x.
Beyhan, Ö., M. Elmastas, and F. Gedikli. 2010. Total phenolic compounds and antioxidant capacity of leaf, dry fruit and fresh fruit of feijoa (Acca sellowiana, Myrtaceae). Journal of Medicinal Plants Research 4 (11): 1065–1072.
Carocho, M., P. Morales, and I.C.F.R. Ferreira. 2018. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends in Food Science and Technology 71: 107–120. https://doi.org/10.1016/j.tifs.2017.11.008.
Cheema, W.A. 1986. Effect of different doses of chemicals and gamma irradiation on physio-chemical changes and storage behavior of date fruit. M. Sc. Thesis. Dept. Hort. Uni. Agric., Faisalabad, Pakistan.
Eltayeb, E.A., A.S. Al-hasni, and S.A. Farooq. 1999. Changes in soluble sugar content during the development of fruits in some varieties of Omani date palm (Phoenix dactylifera). Pakistan Journal of Biological Sciences 2 (1): 255–258. https://doi.org/10.3923/pjbs.1999.255.258.
Fadel, M.A. 2008. Sugar content estimation of date (Phoenix dactylifern L.) fruits in Tamr stage. Agricultural Engineering International: CIGR Journal. https://cigrjournal.org/index.php/Ejounral/article/view/1166. Accessed 15 May 2024.
Fikry, M., Y.A. Yusof, A.M. Al-Awaadh, S.A.H.M. Baroyi, N.S.M. Ghazali, K. Kadota, S. Mustafa, H.A. Saad, N.N.A.K. Shah, and S. Al-Ghamdi. 2023. Assessment of physical and sensory attributes of date-based energy drink treated with ultrasonication: Modelling changes during storage and predicting shelf life. Processes 11 (5): 1399. https://doi.org/10.3390/pr11051399.
Gao, X., L. Björk, V. Trajkovski, and M. Uggla. 2000. Evaluation of antioxidant activities of rosehip ethanol extracts in different test systems. Journal of the Science of Food and Agriculture 80 (14): 2021–2027. https://doi.org/10.1002/1097-0010(200011)80:14%3c2021::AID-JSFA745%3e3.0.CO;2-2.
Ghimeray, A.K., P. Sharma, P. Phoutaxay, T. Salitxay, S.H. Woo, S.U. Park, and C.H. Park. 2014. Far infrared irradiation alters total polyphenol, total flavonoid, antioxidant property and quercetin production in tartary buckwheat sprout powder. Journal of Cereal Science 59 (2): 167–172. https://doi.org/10.1016/j.jcs.2013.12.007.
Ghnimi, S., S. Umer, A. Karim, and A. Kamal-Eldin. 2017. Date fruit (Phoenix dactylifera L.): An underutilized food seeking industrial valorization. NFS Journal 6: 1–10. https://doi.org/10.1016/j.nfs.2016.12.001.
Hazbavi, I., M.H. Khoshtaghaza, A. Mostaan, and A. Banakar. 2015. Effect of postharvest hot-water and heat treatment on quality of date palm (cv. Stamaran). Journal of the Saudi Society of Agricultural Sciences 14 (2): 153–159. https://doi.org/10.1016/j.jssas.2013.10.003.
Islam, M.J., J.W. Kim, M.K. Begum, M.A.T. Sohel, and Y.S. Lim. 2020. Physiological and biochemical changes in sugar beet seedlings to confer stress adaptability under drought condition. Plants 9 (11): 1511. https://doi.org/10.3390/plants9111511.
Islam, M.J., B.R. Ryu, M.H. Rahman, E.J. Cheong, M.H. Wang, J.D. Lim, M.A. Hossain, and Y.S. Lim. 2022. Cannabinoid accumulation in hemp depends on ROS generation and interlinked with morpho-physiological acclimation and plasticity under indoor LED environment. Frontiers in Plant Science 13: 984410. https://doi.org/10.3389/fpls.2022.984410.
Jellinek, G. 1985. Sensory evaluation of food. Theory and practice. Sussex: Ellis Horwood Ltd.
Jiménez-Escrig, A., M. Rincón, R. Pulido, and F. Saura-Calixto. 2001. Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. Journal of Agricultural and Food Chemistry 49 (11): 5489–5493. https://doi.org/10.1021/jf010147p.
Juan, C.A., J.M. Pérez de la Lastra, F.J. Plou, and E. Pérez-Lebeña. 2021. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. International Journal of Molecular Sciences 22 (9): 4642. https://doi.org/10.3390/ijms22094642.
Kader, A.A. 1992. Postharvest technology of horticultural crops (2nd edn). University of California, Division of agriculture and natural resources. Publication 3311, pp. 296–301.
Kalra, S.K., J.S. Jawanda, and S.K. Munshi. 1977. Studies on softening of Doka dates by sodium chloride and acetic acid. Indian Journal of Horticulture 34 (3): 220–224.
Khoyerdi, F.F., M.H. Shamshiri, and A. Estaji. 2016. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Scientia Horticulturae 198: 44–51. https://doi.org/10.1016/j.scienta.2015.11.028.
Kohen, R., and A. Nyska. 2002. Invited review: oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicologic Pathology 30 (6): 620–650. https://doi.org/10.1080/01926230290166724.
Kumar, S., R. Sandhir, and S. Ojha. 2014. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Research Notes 7: 1–9. https://doi.org/10.1186/1756-0500-7-560.
Livani, F., M. Ghorbanli, and A. Sateeyi. 2013. Changes in antioxidant activity and content of phenolic compounds during the ripening process of elm-leaved blackberry fruit. International Journal of Agronomy and Plant Production 4 (1): 88–93.
Mani, V., M. Arfeen, H.A. Mohammed, H.A. Elsisi, S. Sajid, Y. Almogbel, M. Aldubayan, M. Dhanasekaran, and A. Alhowail. 2022. Sukkari dates seed improves type-2 diabetes mellitus-induced memory impairment by reducing blood glucose levels and enhancing brain cholinergic transmission: In vivo and molecular modeling studies. Saudi Pharmaceutical Journal 30 (6): 750–763. https://doi.org/10.1016/j.jsps.2022.03.016.
McCord, J.M. 2000. The evolution of free radicals and oxidative stress. The American Journal of Medicine 108 (8): 652–659. https://doi.org/10.1016/S0002-9343(00)00412-5.
Oyaizu, M. 1986. Studies on product of browning reaction prepared from glucosamine. The Japanese Journal of Nutrition and Dietetics 44 (6): 307–315. https://doi.org/10.5264/eiyogakuzashi.44.307.
Rastegar, S., M. Rahemi, A. Baghizadeh, and M. Gholami. 2012. Enzyme activity and biochemical changes of three date palm cultivars with different softening pattern during ripening. Food Chemistry 134 (3): 1279–1286. https://doi.org/10.1016/j.foodchem.2012.02.208.
Saeed, N., M.R. Khan, and M. Shabbir. 2012. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complementary and Alternative Medicine 12: 1–12. https://doi.org/10.1186/1472-6882-12-221.
Saleem, S.A., A.K. Baloch, M.K. Baloch, W.A. Baloch, and A. Ghaffoor. 2005. Accelerated ripening of Dhakki dates by artificial means: Ripening by acetic acid and sodium chloride. Journal of Food Engineering 70 (1): 61–66. https://doi.org/10.1016/j.jfoodeng.2004.09.013.
Saleem, S.A., A.A. Saddozai, M. Asif, and A.K. Baloch. 2010. Impact of artificial ripening to improve quality and yield for the export of ‘dhakki’dates. In IV International date palm conference 882, 1125–1134. https://doi.org/10.17660/ActaHortic.2010.882.130.
Samarawira, I. 1983. Date palm, potential source for refined sugar. Economic Botany 37: 181–186. https://doi.org/10.1007/BF02858783.
Sarker, U., and S. Oba. 2018. Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chemistry 252: 72–83. https://doi.org/10.1016/j.foodchem.2018.01.097.
Shahdadi, F., H.O. Mirzaei, and A. DaraeiGarmakhany. 2015. Study of phenolic compound and antioxidant activity of date fruit as a function of ripening stages and drying process. Journal of Food Science and Technology 52: 1814–1819. https://doi.org/10.1007/s13197-013-1177-6.
Shanta, F.H., B.K. Rahut, M.J. Islam, M.O.K. Azad, M.A.T. Sohel, M.R.R. Rajib, M. Shams-Ud-Din, and M. Adnan. 2021. Development of value added drinks from date palm juice (Phoenix sylvestris). Heliyon 7 (11): e08322. https://doi.org/10.1016/j.heliyon.2021.e08322.
Sheikh, B.Y., S.M.N.K. Zihad, N. Sifat, S.J. Uddin, J.A. Shilpi, O.A.A. Hamdi, H. Hossain, R. Rouf, and I.A. Jahan. 2016. Comparative study of neuropharmacological, analgesic properties and phenolic profile of Ajwah, Safawy and Sukkari cultivars of date palm (Phoenix dactylifera). Oriental Pharmacy and Experimental Medicine 16: 175–183. https://doi.org/10.1007/s13596-016-0239-5.
Siddeeg, A., X.A. Zeng, A.F. Ammar, and Z. Han. 2019. Sugar profile, volatile compounds, composition and antioxidant activity of Sukkari date palm fruit. Journal of Food Science and Technology 56: 754–762. https://doi.org/10.1007/s13197-018-3534-y.
Siddhuraju, P., P.S. Mohan, and K. Becker. 2002. Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): A preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chemistry 79 (1): 61–67. https://doi.org/10.1016/S0308-8146(02)00179-6.
Singleton, V.L., and J.A. Rossi. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture 16: 144–158. https://doi.org/10.5344/ajev.1965.16.3.144.
Tafti, A.G., and M.H. Fooladi. 2006. A study on the physico-chemical properties of Iranian Shamsaei date at different stages of maturity. World Journal of Dairy and Food Sciences 1 (1): 28–32.
Tang, Z.X., L.E. Shi, and S.M. Aleid. 2013. Date fruit: Chemical composition, nutritional and medicinal values, products. Journal of the Science of Food and Agriculture 93 (10): 2351–2361. https://doi.org/10.1002/jsfa.6154.
Thatai, S.K., and S.K. Kalra. 1982. Saline water treatments for softening of Doka dates. Journal of Research, Punjab Agricultural University 19 (4): 320–323.
van Handel, E. 1968. Direct microdetermination of sucrose. Analytical Biochemistry 22 (2): 280–283. https://doi.org/10.1016/0003-2697(68)90317-5.
Vayalil, P.K. 2012. Date fruits (Phoenix dactylifera Linn): An emerging medicinal food. Critical Reviews in Food Science and Nutrition 52 (3): 249–271. https://doi.org/10.1080/10408398.2010.499824.
Yektankhodaei, M., A. Bagheri, I. Mohamadpour, and Y.A. Karami. 2007. Artificial ripening of Khuneizi date using physical and chemical methods. Acta Horticulturae 736: 87–93. https://doi.org/10.17660/ActaHortic.2007.736.6.
Yen, G.C., P.D. Duh, and C.L. Tsai. 1993. Relationship between antioxidant activity and maturity of peanut hulls. Journal of Agricultural and Food Chemistry 41 (1): 67–70. https://doi.org/10.1021/jf00025a015.
Yi, M., J. Kong, and Z. Yu. 2021. Effect of heat treatment on the quality and energy metabolism in “Golden Delicious” apple fruit. Journal of Food Biochemistry 45 (8): e13759. https://doi.org/10.1111/jfbc.13759.
Zhu, Q.Y., R.M. Hackman, J.L. Ensunsa, R.R. Holt, and C.L. Keen. 2002. Antioxidative activities of oolong tea. Journal of Agricultural and Food Chemistry 50 (23): 6929–6934. https://doi.org/10.1021/jf0206163.
Zhu, Y.Z., S.H. Huang, B.K.H. Tan, J. Sun, M. Whiteman, and Y.C. Zhu. 2004. Antioxidants in Chinese herbal medicines: A biochemical perspective. Natural Product Reports 21 (4): 478–489. https://doi.org/10.1039/b304821g.
Zihad, S.M.N.K., S.J. Uddin, N. Sifat, F. Lovely, R. Rouf, J.A. Shilpi, B.Y. Sheikh, and U. Göransson. 2021. Antioxidant properties and phenolic profiling by UPLC-QTOF-MS of Ajwah, Safawy and Sukkari cultivars of date palm. Biochemistry and Biophysics Reports 25: 100909. https://doi.org/10.1016/j.bbrep.2021.100909.
Acknowledgements
The authors express their gratitude to the scientists of the Bangladesh Sugarcrop Research Institute who participated in the organoleptic test. Additionally, the authors extend their thanks to all the staff members of the Physiology and Sugar Chemistry Division at the Bangladesh Sugarcrop Research Institute for their valuable technical support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Ethical Approval
In this study, no animal or human subjects were involved. The authors wish to clarify that for the sensory analysis, all panelists were provided with a written consent form detailing the procedures, ranking scale, potential risks, and consent for the future publication of the research findings.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alam, T., Islam, M.J., Habib, M.A. et al. Effects of Various Chemical Treatments on Ripening Acceleration and Quality Attributes of Sukkari Dates. Sugar Tech (2024). https://doi.org/10.1007/s12355-024-01449-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12355-024-01449-2