Abstract
This study assessed variation in fruit color coordinates and internal quality attributes during fruit development in ‘Kinnow’ and ‘W. Murcott’ mandarins. With progression of fruit development, green colored fruit, with high titratable acidity (TA) (2.92 ± 0.51%), ascorbic acid (42.11 ± 3.33 mg ml-100), low maturity index values (2.50 ± 0.31) and juice content (25.07 ± 1.75%), developed into yellowish-orange colored fruits, with low TA (0.82 ± 0.2%) and ascorbic acid content (28.72 ± 1.48 mg/100 ml) as well as a concomitant improvement in maturity index (14.76 ± 0.85) and juice yield percentage (47.46 ± 3.90%), at full maturity stages. β-carotene content also significantly increased (p < 0.05) with the advancement of fruit maturity and varied from 0.89 ± 0.35 to 26.28 ± 4.6 µg g−1 FW. Regardless of maturity stages, β-carotene was found to be higher in the peel of fruits than in the pulp and also showed a significant correlation with color coordinate values. At the full maturity stage, ‘Kinnow’ exhibited maximum color development, β-carotene content, total soluble solids (TSS), maturity index value and juice yield percentage compared to ‘W. Murcott’. The maturity index showed a maximum association with peel C* for ‘W. Murcott’ peel and for pulp b* in ‘Kinnow’ mandarin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus fruits are consumed fresh or as fruit juice and have health-related benefits due to their primary and secondary metabolites (Lado et al. 2016; Liu et al. 2012; Sidana et al. 2013). In India, citrus are cultivated over an area of 1050 thousand hectares with a total production of 12.74 MT (National Horticulture Board 2017). Citrus acreage in the Punjab province is 60980 ha, with ‘Kinnow’ mandarin contributing an area of 55470 ha and annual citrus production of 1.31 MT (Anonymous 2020a). The dominance of mandarins over the sweet orange varieties is due to their high yield potential and greater juice content. The flavor of mandarin fruit is actually the blend of basic taste, aroma and taste-bud sensations that are anticipated synchronously by the brain during eating (Goff and Klee 2006; Zou et al. 2016). Since non-climacteric in nature, citrus fruit ripens on the tree and its maturity assessment relies on the visual examination conducted at field level. In European Union markets, the maturity indices used to determine fruit quality comprise juice percentage, total soluble solids (TSS), acidity level and their ratios (TSS/acidity), with fruit surfaces having minimum coloration (Lado et al. 2014). However, in domestic markets, consumption of fresh citrus fruits primarily depends on the preferences of consumers, who decide their acceptability primarily based on fruit peel coloration. It is worth mentioning that the color of citrus fruits shows deviation with respect to climate, diurnal and seasonal temperature as well as growing conditions (Cronje et al. 2013; Porras et al. 2014; Nawaz et al. 2019b). The color break in Clementine mandarins is related to onset of lower soil temperature in late summer or early fall (Mesejo et al. 2012). In tropical zones, the maturity index in citrus fruits does not rely on peel coloration since this invariably fails to exhibit a change in colour (Stewart and Wheaton 1973). In these areas, other factors like fruit size and shape can be considered as maturity indices for commercial harvest in citrus fruit (Nawaz et al. 2018, 2019a). There are wide variations in mandarin fruits with regard to internal quality attributes such as juice yield, TSS, titratable acidity (TA) and their ratio since maturity indices and color do not in isolation designate the internal quality of harvested fruits (Tadeo et al. 2008). Furthermore, evidence demonstrated that peel and pulp maturation are not fully coordinated. Deciding the norms regarding the precise and consistent determination of citrus fruit maturity is not an effortless task since it includes physiological alterations occurring in two distinct and non-dependent tissues: color variations taking place in the fruit peel and compositional alterations appearing in the flesh (Tadeo et al. 2008). Thus, internal fruit attributes such as TSS, TA and solid/acid ratio as well as antioxidants (ascorbic acid) are also obscure quality parameters that require standard laboratory procedures and approaches for meticulous estimation. Hence, to ascertain the harvest quality of fruit for the commercial and nutritional needs of the market, it is imperative to investigate the dynamics of fruit colour coordinates and quality components during fruit development in ‘Kinnow’ and ‘W. Murcott’ mandarins. Therefore, the objectives of this research were: 1) to determine fruit color and quality changes in two mandarin cultivars during development and 2) to elucidate the relationship between fruit coloration and maturity index at different developmental stages in these mandarins.
Materials and Methods
Fruit Materials
Fruits of ‘Kinnow’ (Citrus nobilis Lour × Citrus deliciosa Tenora) and ‘W. Murcott’ mandarins (also called ‘Afourer’, which is an open pollinated seed of ‘Murcott’ [C. reticulata × C. sinensis] Kahn and Chao 2004) were harvested during the 2019–20 period from an orchard of the Punjab Agricultural University (PAU), Fruit Research Station, Jallowal-Lesriwal, Jalandhar (latitude, 31° 19′ 33.6540″ N and longitude, 75° 34′ 34.2480″ E, located at an altitude of 228 m). The experimental plants were 5 years old, budded on ‘Carrizo’ citrange rootstock (Citrus sinensis Osb. X Poncirus trifoliate L. Raf.) and spaced at 6 m × 3 m. The soil type of the plot was sandy loam and plants were treated with a uniform dose of fertilizers according to guidelines laid down by the Punjab Agricultural University, Ludhiana, India. Fruit sampling was carried out during the morning hours at monthly intervals from August to January until optimum maturity (TSS: 12.10 ± 0.77 °Brix for ‘Kinnow’; 10.70 ± 0.70 °Brix for ‘W. Murcott’) (Mahajan et al. 2018; Anonymous 2020b). Healthy fruits from the selected cultivars were randomly picked at each interval with the help of fruit clippers and immediately transported to the laboratory of the Department of Fruit Science, PAU, Ludhiana. At each sampling stage, 20 fruits per replication were used for analysis. Fruits were cleaned with distilled water and wiped dry at room temperature, following which the peel and pulp (consisting of juice sacs and segment epidermis) portions were separated manually. Fruit juice was extracted using a kitchen citrus juicer and was filtered through a stainless steel sieve with 1.0-mm mesh for further analysis.
Evaluations
Physical Evaluation
Fruit Weight
Average fruit weight was measured with an electronic balance with 0.01‑g accuracy.
Peel Thickness
Peel thickness was measured with a digital Vernier caliper (Mitutoyo, Japan) on both sides of the fruit, and mean data were expressed in millimeter (mm).
Juice Content
The juice content was expressed in percentage (%) and was calculated with the following equation:
The weight of fruit juice and an individual fruit was taken with electronic balance.
Fruit Color Evaluation
Peel, pulp and juice color of fruits were measured using the Color Flex meter (Hunter lab Color Flex, Hunter Associates Inc., Reston, VA, USA) with the method described by Hunter (1975). The instrument was calibrated with standard white and black ceramic tiles before use. Fruit peel and pulp color along the equatorial axis of each fruit at two opposite spots were recorded in CIE coordinates (L*, a*, b*). Similarly, an aliquot of fresh juice was taken in the colorless glass petri dish for color measurement. The color coordinate L* measures the luminosity between black (0) and white (100), a* varies from a negative value (green) to a positive value (red) and b* varies from negative value (blue) to positive value (yellow). Chroma (C*), which determines the length of the color vector in the plane generated by a* and b*, and hue angle (h°), which represents the position of such vectors, were calculated by (a*2 + b*2)1/2 and tan−1(b*/a*), respectively, as given by McGuire (1992).
Chemical Evaluation
Total Soluble Solids (TSS)
For the determination of TSS, one drop of fruit juice was placed on a digital hand refractometer (ATAGO, PAL‑1, Japan) with a range of 0–53 °Brix. During measurement, the temperature of the sample was also noted and a correlation was applied. The final value was expressed in °Brix.
Titratable Acidity (TA)
TA was estimated by titrating 2 ml fresh juice against 0.1 N NaOH solution up to pH 8.1 and phenolphthalein as an indicator (AOAC 2000). The obtained results were expressed as a percentage (%) in terms of citric acid, since this is a dominant organic acid in citrus. Juice acidity: (%) = 0.0064 × \(\frac{\text{Volume of}\,0.1\,\mathrm{N}\:\text{NaOH used}}{\,\text{volume of juice taken}}\) × 100.
Maturity Index
The maturity index is an indicator of fruit sweetness and was calculated as the ratio of TSS/TA.
Ascorbic Acid
Ascorbic acid was estimated using the visual titration method (AOAC 2000). A total of 2 ml fresh fruit juice was added to metaphosphoric acid, and titration was performed against 2, 6‑dichlorophenol-indophenol dye until the appearance of a light pink color that persisted for 15 s. The final results were expressed in mg/100 ml ml of juice.
Total Sugars
Extraction
Total sugars were estimated in a known volume of juice of fresh fruits by using the method suggested by Lane and Eynon (AOAC 1990). Lead acetate and potassium oxalate were added to 10 ml fresh juice to precipitate the extra material in juice and to remove excess lead acetate. The filtered solution was then made up to 100 ml by adding distilled water. This aliquot was further used for total sugar estimation according to the method described by Dubois et al. (1956).
Estimation
Diluted sugar extract with a volume of 30 µl was taken (lying within the dimensions of pure sugar standards) and admixed with 1 ml of phenol reagent (5%), followed by the addition of 5 ml concentrated H2SO4. The test tubes were then subjected to vortexing, and these test tubes were later kept for 10 min at room temperature and another 20 min under moving tap water for cooling. The orange color was developed and its optical density was estimated at 490 nm with distilled water as blank. The final result of total sugars was calculated from the glucose standards (10–100 µg) run simultaneously and was expressed in percentages (%).
Carotenoid Extraction
Carotenoids were determined in the form of β-carotene from the peel and pulp of selected cultivars with the method described by Ranganna (1977). A homogenous representative sample (5 g) of fruit peel and pulp was kept to dry for 3 h in a hot air oven at 40–45 °C; afterwards, this was crushed with 5 g of Na2SO4 using a pestle and mortar, and a petroleum ether and acetone mixture (97:3) was taken for softening the cell wall. All operations were carried out in the presence of dimmed light to prevent isomerization and photodegradation of carotenoids. This extraction was filtered through filter paper, and color intensity was read at 452 nm in Spectronic 20D+ (Thermo Fischer Scientific, Madison, WI, USA). Petroleum ether was used as a blank, and β-carotene content in fruit peel and pulp was calculated as µg g−1 FW.
Statistical Analysis
All physical and chemical evaluations were recorded in quadruplicate and presented as means ± standard deviations. The statistical difference between time intervals was identified from one-way analysis of variance (ANOVA) using the least significance difference (p < 0.05). The correlation coefficients between fruit color coordinates and maturity index were obtained through Pearson’s correlation coefficients (p ≤ 0.01) using SAS 9.3 (the SAS system for Windows, Version 9.3, SAS Institute, Cary, NC, USA).
Results and Discussion
Fruit Physical Quality (Fruit Weight, Peel Thickness and Juice Content)
Fruit weight during the fruit development period varied from 29.57 ± 6.31 g in ‘W. Murcott’ to 167.70 ± 8.86 g in ‘Kinnow’ (Table 1). The maximum increment in fruit weight was noted from August to September and continued to significantly increase (p < 0.05) up to October; thereafter, fruit growth was non-significant in both cultivars. The increment in fruit weight could be attributed to an increase in cell size and an accumulation of food substances in the intercellular spaces in fruits (Bollard 1970). A similar analysis in ‘Nagpur’ mandarins was also described by Bhatnagar et al. (2012), who showed that fruit weight varied from 65 to 140 g between July and November. Ladaniya and Mahalle (2011), during their investigations on the sweet orange cultivar ‘Mosambi’, recorded a linear increase in fruit weight from 180 to 250 days after fruit set.
Peel thickness in two commercial cultivars altered from 4.60 ± 0.22 to 2.64 ± 0.14 mm. Thicker fruit peel was observed in immature green colored fruits, which declined during fruit development, with the minimum value being recorded in the fully matured fruits (Table 1). Between the two cultivars, greater peel thickness was observed in ‘Kinnow’ during August (green color fruit stage; 4.60 ± 0.22 mm) compared to the ‘W. Murcott’ cultivar (3.78 ± 0.38 mm), which may be due to cultivar differences as reported by Muramatsu et al. (1999) in citrus fruits.
Juice percent was also considered as another parameter in determining the maturity index of citrus fruits (Deka et al. 2006). This varied from 25.07 ± 1.75 to 47.46 ± 3.90% during the fruit development period (Table 1), which is in line with the findings of Altaf et al. (2008) in mandarin fruits. Juice content increased from August to November with significant variations (p < 0.05), showing no changes thereafter. Comparing the two cultivars, ‘Kinnow’ exhibited higher physical fruit quality in terms of fruit weight, juice content and fruit peel thickness compared to ‘W. Murcott’ during the different developmental stages.
Fruit Internal Quality (TSS, TA, Total Sugars, Ascorbic Acid and Maturity Index)
Various internal quality parameters of fruits including TSS, total sugars and TA content were significantly influenced during fruit developmental stages in both mandarin cultivars (Table 2). TSS content increased linearly from August (7.29 ± 0.38 °Brix for ‘W. Murcott’; 7.54 ± 0.84 °Brix for ‘Kinnow’) to January (10.70 ± 0.76 °Brix for ‘W. Murcott’; 12.10 ± 0.77 °Brix for ‘Kinnow’), with significant variations (p < 0.05). Similarly, total sugar content was lowest during August (1.96 ± 0.08% for ‘W. Murcott’; 2.31 ± 0.2% for ‘Kinnow’) and then exhibited an increasing trend with considerable changes (p < 0.05) until full maturity (6.47 ± 0.54% for ‘W. Murcott’; 7.32 ± 0.43% for ‘Kinnow’). The results of the present study regarding TSS and sugars are in agreement with earlier investigations by Hardy and Sanderson (2010) in citrus fruits.
The highest TA was recorded in immature fruits in the August sampling (2.61 ± 0.39% for ‘Kinnow’; 2.92 ± 0.51% for ‘W. Murcott’), then exhibited a declining trend with noticeable variations (p < 0.05) and was observed to be lowest at the full maturity stage (0.82 ± 0.2% for ‘Kinnow’; 0.91 ± 0.23% for ‘W. Murcott’). A similar trend for TA in mandarin fruits during maturity at different locations was reported by Roakaya et al. (2016). During fruit development, acids are rapidly consumed in citrus fruits as part of respiration processes, as reported by Giovannoni (2001). Higher ascorbic acid accumulation was observed in green colored fruits during the August–September period (39.28 ± 1.44 to 42.11 ± 3.33 mg/100 ml), with slight statistical alterations between months as displayed in Table 1. During the color development phase, ascorbic acid was converted to 2‑3-dioxy-L-gluconic acid by losing L‑ascorbic acid (Mapson 1970). Giuffre (2019) also discovered the declining pattern of ascorbic acid in Bergamot (Citrus bergamina Risso) fruits, ranging from 867 (October) to 341 g L−1 (March) during the fruit ripening period. The maturity Index, which correlates TSS/TA ratio, is an important parameter associated with quality attributes of citrus fruits, although it is most commonly used as a method for the estimation of harvest maturity of sub-tropical mandarins. The maturity index pattern during fruit development demonstrated an inverse correlation between TA and TSS (Table 2). The maturity index was observed to be lower (2.89 ± 0.53 for ‘Kinnow’; 2.50 ± 0.31 for ‘W. Murcott’) in green colored fruits during the month of August, when TSS was low, while TA was at a relatively higher level. It exhibited an increasing trend with significant variations (p < 0.05) during fruit development, reaching its maximum at full maturity (11.76 ± 2.04 for ‘W. Murcott’; 14.76 ± 0.85 for ‘Kinnow’).
Overall, immature fruits in the month of August possessed a higher level of TA and ascorbic acid concentration with the lowest TSS, total sugars and low maturity index, and vice-versa in mature fruits in the month of January.
Carotenoid Content
During fruit maturation, the carotenoid content of peel and pulp in terms of β-carotene rapidly increased in relation to color development of mandarin fruits (Table 3). Irrespective of fruit development stages, β-carotene was higher in the fruit peel than the pulp in both cultivars, which was also in agreement with the findings of Kato (2012) and Alquezar et al. (2008) in citrus fruits. β-carotene content varied from 0.89 ± 0.35 to 26.28 ± 4.65 µg g−1 FW during the fruit development period. Values in the present study were lower in the range than those observed in the findings of Mansour (2018) in sour orange (Citrus aurantium) at different fruit color stages (0.48 ± 0.01 to 0.81 ± 0.03 mg−1 FW). However, these results are in agreement with the findings of Alquezar et al. (2008) and Rodrigo et al. (2004) in flavedo of orange (Citrus sinensis L.) and Zheng et al. (2016) in grapefruit during fruit development and maturation. In the context of the present cultivars, ‘Kinnow’ (26.28 ± 4.65 µg g−1 FW) in peel and ‘W. Murcott’ in pulp (13.25 ± 4.29 µg g−1 FW) contained higher concentrations of β-carotene at the full fruit maturity stage. Hence, green colored fruits were rich in chlorophyll content, and during different maturity stages breakdown of chlorophyll leads to an enhancement in the concentration of β-carotene, which was responsible for the orange-yellowish color of fruit peels at full maturity. However, variations in β-carotene content of the selected cultivars may be due to climate, temperature, geographical region, precipitation, sunshine exposure and different stages of maturation, which was also observed by Boudries et al. (2007, 2012) in Algerian Date and mandarin cultivars.
Color Attributes
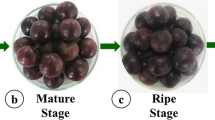
Fruit color variations in peel, pulp and juice of ‘Kinnow’ and ‘W. Murcott’ mandarins during fruit development were evaluated in CIE L*, a*, b*, C* and h° coordinates and are presented in Table 4, while the variation in external fruit color development is shown in Fig. 1. During different fruit development stages, the peel and juice CIE L* values of both the mandarins increased significantly with fruit maturity, varying from 31.14 ± 2.85 to 62.74 ± 3.41, while in fruit pulp, it was recorded as highest in the month of August (73.14 ± 4.8 for ‘Kinnow’; 69.20 ± 3.06 for ‘W. Murcott’), significantly (p < 0.05) declining thereafter until October; it then decreased with non-significant variations and was observed to be lowest in the month of January (54.43 ± 5.66 for ‘Kinnow’; 57.19 ± 2.96 for ‘W. Murcott’). The present findings are in agreement with the observations of Soares et al. (2007) in guava fruit and Singh et al. (2015) in the ‘Star Ruby’ cultivar of grapefruit. In the context of cultivars, the peel of ‘Kinnow’ and the pulp and juice of ‘W. Murcott’ showed more lightness at the full maturity stage.
The fruit peel of both the mandarins retained their green color until October, with a slight variation in a* value (−6.46 ± 1.25 to 2.34 ± 0.23), thereafter turning reddish-yellow in color with considerable differences (p < 0.05) and attaining a value of 34.44 ± 1.59–36.42 ± 6.02 at full maturity. The color break in mandarin fruits during October coincides with the onset of the low seasonal temperature of the region. In contrast, the pulp and juice did not develop reddish coloration at full maturity, as observed in peel. Likewise, at commercial harvest, the peel of ‘W. Murcott’ exhibited a higher a* value, while the pulp and juice of ‘Kinnow’ exhibited a greater a* value than ‘W. Murcott’. The b* value in mandarin fruits significantly (p < 0.05) increased during fruit development and varied from 12.92 ± 1.06 to 62.63 ± 3.58. At full maturity stages, the peel of both cultivars (62.63 ± 3.58 for ‘Kinnow’; 51.70 ± 5.12 for ‘W. Murcott’) displayed a more yellowish color than pulp (37.72 ± 3.45 for ‘Kinnow’; 33.05 ± 2.37; 33.05 ± 2.37 for ‘W. Murcott’) and juice (40.36 ± 1.87 for ‘Kinnow’; 33.05 ± 2.35 for ‘W. Murcott’). The change in color of the fruit peel, and to some extent in the case of pulp and juice, was attributed to an accumulation of β-carotene content at later maturity stages, which was also studied by Arias et al. (2000) in peel and pulp of tomato and by Yoo and Moon (2016) in lemon peel. In the context of the present cultivars, the peel, pulp and juice of ‘Kinnow’ exhibited a greater development of yellowish color than ‘W. Murcott’ cultivars at the final harvest stage.
The hue angle (h°) ranged from 115.90 ± 4.31 to 61.19 ± 3.98 in different portions of the fruits during development. The h° value of fruit peel significantly declined (p < 0.05) with the onset of fruit maturity and with a slight alteration in fruit pulp and juice. At full maturity, ‘W. Murcott’ exhibited a higher hue angle value in fruit pulp and juice (69.69 ± 3.03 and 72.95 ± 3.07, respectively), while ‘Kinnow’ displayed higher h° in fruit peel (61.19 ± 3.9) than ‘W. Murcott’ (54.84 ± 2.18). A similar declining pattern of hue value during fruit development was also reported by Singh et al. (2015) in grapefruits and Barragan-Iglesias et al. (2018) in papaya. The chrome value (C*) significantly (p < 0.05) increased with fruit development and varied from 14.36 ± 1.66 to 71.48 ± 3.91 until full maturity. The purity of color was more in the peel than in the pulp and juice in both mandarins. The present results are in agreement with the findings of Soares et al. (2007) in guava fruits in relation to maturity. ‘Kinnow’ showed more color intensity at fruit maturity than ‘W. Murcott’ in all three fruit portions.
Correlation Analysis
To elucidate the relation of fruit color coordinates L*, a*, b*, C* and h° with the maturity index, Pearson’s correlation coefficient was evaluated and is shown in Table 5. The correlation coefficients analyzed for changes in fruit color coordinates were significantly (p < 0.05) correlated with the maturity index of both the cultivars. In the case of ‘Kinnow’, pulp exhibited a stronger relation with the maturity index compared to the peel. The C*value of pulp (r = 0.996) showed a positive and highly significant correlation (p < 0.01) with the fruit maturity index compared to peel (r = 0.865). The L*value of juice also exhibited a stronger correlation (p < 0.01) with the fruit maturity index than the peel and pulp of ‘Kinnow’ fruits (r = 0.841, r = −0.813 peel and pulp, respectively). Whereas in the case of ‘W. Murcott’ fruits, the C*value of peel (r = 0.997) demonstrated a stronger and more positive correlation with the maturity index than pulp and juice (r = 0.985 and 0.944 for peel and pulp, respectively). In the context of the present cultivars, the change in peel and pulp color of ‘W. Murcott’ and ‘Kinnow’ fruits, respectively, significantly correlated with the onset of the optimum fruit maturity stage.
These correlation coefficients showed that fruit peel and pulp color is an important indicator for internal quality attributes. Similar results of correlation studies were also reported by Lee and Castle (2001) in “Early Gold” and “Budd blood” orange juices and by Conesa et al. (2019) in lemon fruits, thereby justifying the present studies.
Conclusion
This study describes quality and color changes in different portions of fruit during development in ‘Kinnow’ and ‘W. Murcott’ mandarins. The results demonstrate an improvement in fruit quality of ‘Kinnow’ and ‘W. Murcott’ mandarins during development in terms of an increase in fruit weight, TSS, juice content and reduction in peel thickness and acid contents. All portions of fruit exhibited color development with the advancement of fruit maturity. This study demonstrated that for ‘Kinnow’ mandarin, pulp C* coordinate and for ‘W. Murcott’ peel C* can be used as maturity indices to determine the commercial harvest of fruit. In the context of the present cultivars, the ‘Kinnow’ cultivar exhibited greater color development as well as higher maturity index and β-carotene content than ‘W. Murcott’ at the full maturity stage. Hence, our findings have generated valuable information for citrus fruit quality breeding and consumer guidelines.
References
Alquezar B, Rodrigo MJ, Zacarias L (2008) Carotenoid biosynthesis and their regulation in citrus fruits. Tree Sci Biotech 2:23–35
Altaf N, Khan AR, Hussain J (2008) Fruit variability in ‘Kinnow’ mandarin (Citrus reticulata). PAK J BOT 40:599–604
Anonymous (2020a) Introduction. In: Thind SK (ed) Package of Practices for Fruit Crops, Punjab Agricultural University Printing Press, Ludhiana, India, pp 1
Anonymous (2020b) Citrus. In: Thind SK (ed) Package of Practices for Fruit Crops, Punjab Agricultural University Printing Press, Ludhiana, India, pp 13–24
AOAC (1990) Official methods of analysis, 15th edn. Association of official Analytical Chemist, Washington DC
AOAC (2000) Official method of analysis, 15th edn. Association of the Official Analytical Chemists, Washington, DC
Arias R, Lee TC, Logendra L, Janes H (2000) Correlation of lycopene measured by HPLC with the L*, a*, b* color readings of a hydroponic tomato and the relationship of maturity with color and lycopene content. J Agric Food Chem 48:1697–1702
Barragan-Iglesias J, Mendez-Lagunas LL, Rodriguez-Ramirez J (2018) Ripeness indexes and physicochemical changes of papaya (Carica papaya L. cv. Maradol) during ripening on-tree. Sci Hortic 236:272–278
Bhatnagar P, Singh J, Jain MC, Singh B, Manmohan JR, Dashora LK (2012) Studies on seasonal variations in developing fruits of “Nagpur” Mandarin (Citrus reticulata Blanco) under Jhalawar conditions. Asian J Hort 7:263–265
Bollard EG (1970) The physiology and nutrition of developing fruits. In: Hulme AC (ed) The biochemistry of fruits and their products. Academic Press, London, pp 387–425
Boudries H, Kefalas P, Hornero-Mendez D (2007) Carotenoid composition of algerian date varieties (Phoenix dactylifera) at different edible maturation stages. Food Chem 101:1372–1377
Boudries H, Madani K, Touati N, Souagui S, Medouni S, Chibane M (2012) Pulp antioxidant activities, mineral contents and juice nutritional properties of algerian clementine cultivars and mandarin. Afr J Biotechnol 11:4258–4267
Conesa A, Manera FC, Brotons JM, Fernandez-Zapata JC, Simon I, Simon-Grao S, Alfosea-Simon M, Nicolas JJM, Valverde JM, Garcia-Sanchez F (2019) Changes in the content of chlorophylls and carotenoids in the rind of Fino 49 lemons during maturation and their relationship with parameters from the CIELAB color space. Sci Hortic 243:252–260
Cronje PJ, Barry GH, Huysamer M (2013) Canopy position affects pigment expression and accumulation of flavedo carbohydrates of ‘Nules Clementine’ mandarin fruit, thereby affecting rind condition. J Am Soc Hortic Sci 138:217–224
Deka BC, Sharma S, Borah SC (2006) Post-harvest management practices for shelf life extention of “Khasi” mandarin. Indian J Hortic 63:251–255
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Giovannoni J (2001) Molecular biology of fruit maturation and ripening. Annu Rev Plant Biol 52:725–749
Giuffre AM (2019) Bergamot (Citrus bergamia Risso): The Effects of cultivar and harvest date on functional properties of juice and cloudy juice. Antioxidants 8:221. https://doi.org/10.3390/antiox8070221
Goff SA, Klee HJ (2006) Plant volatile compounds: sensory cues for health and nutritional value. SciHortic 311:815–819
Hardy S, Sanderson G (2010) A procedural manual for citrus maturity testing. NSW Department of Industry and Investment
Hunter (1975) The measurement of appearance. Willey, New York
Kahn TL, Chao CT (2004) Sex, seedlessness, and new varieties. University of California, Riverside, p 4
Kato M (2012) Mechanism of carotenoid accumulation in citrus fruit. J Jpn Soc Hortic Sci 81:219–233
Ladaniya MS, Mahalle BC (2011) Fruit maturation and associated changes in ‘Mosambi’ orange (Citrus sinensis). Indian J Agric Sci 81:494–499
Lado J, Rodrigo MJ, Zacarias L (2014) Maturity indicators and citrus fruit quality. Stewart Postharvest Rev 10:1–6
Lado J, Cuellar F, Rodrigo MJ, Zacarias L (2016) Nutritional composition of mandarins. In: Simmonds MSJ, Preedy VR (eds) Nutritional Composition of Fruit Cultivars. Academic Press, pp 419–443
Lee HS, Castle WS (2001) Seasonal changes of carotenoid pigments and color in “Hamlin”, “Earlygold”, and “Budd Blood” orange juices. J Agric Food Chem 49:877–882
Liu Y, Heying E, Tanumihardjo SA (2012) History, global distribution, and nutritional importance of citrus fruits. Compr Rev Food Sci 11:530–545
Mahajan BVC, Chahal TS, Dhillon WS, Kapoor S (2018) Postharvest quality maintenance of ‘W. Murcott’ mandarin using packaging films. Indian J Agric Sci 88:1270–1274
Mansour R (2018) Determination of nutritional composition in citrus fruits (C. aurantium) during maturity. Nutr Food Sci. https://doi.org/10.1108/nfs-05-2018-0141
Mapson LW (1970) In: Hulme AC (ed) The biochemistry of fruits and their products, vol 1. Academic Press, London, pp 369–384
McGuire RG (1992) Reporting of objective color measurements. Hort Sci 27:1254–1255
Mesejo C, Gambetta G, Gravina A, Martinez-Fuentes A, Reig C, Agusti M (2012) Relationship between soil temperature and fruit colour development of ‘Clemenpons’ Clementine mandarin (Citrus clementina Hort ex. Tan). J Sci Food Agric 92:520–525
Muramatsu N, Takahara T, Ogata T, Kojima K (1999) Changes in rind firmness and cell wall polysaccharides during citrus fruit development and maturation. Hort Sci 34:79–81
National Horticulture Board (2017) http://nhb.gov.in. Accessed 25 Mar 2020
Nawaz R, Abbasi NA, Hafiz IA, Khalid A, Ahmad T (2018) Economic analysis of citrus (‘Kinnow’ mandarin) during on-year and off-year in the Punjab Province. Pak J Hortic 5:2376–2454
Nawaz R, Abbasi NA, Hafiz IA, Khalid A (2019a) Color-break effect on ‘Kinnow’ (Citrus nobilis Lour xCitrus deliciosa Tenora) fruit’s internal quality at early ripening stages under varying environmental conditions. Sci Hortic. https://doi.org/10.1016/j.scienta.2019.05.041
Nawaz R, Abbasi NA, Hafiz IA, Khalid A, Ahmad T (2019b) Impact of climate change on ‘Kinnow’ fruit industry of Pakistan. Agrotechnology 8:2732–2786
Porras I, Brotons JM, Conesa A, Manera FJ (2014) Influence of temperature and net radiation on the natural degreening process of grapefruit (Citrus paradise Macf Cultivars ‘Rio Red’ and ‘Star Ruby’). Sci Hortic 173:45–53
Ranganna S (1977) Manual of analysis of fruit and vegetable products. Tata McGraw-Hill, New Delhi
Roakaya PR, Baral DR, Gautam DM, Shrestha AK, Paudyal KP (2016) Effect of altitude and maturity stages on quality attributes of mandarin (Citrus reticulata Blanco). Am J Plant Sci 7:958–966
Rodrigo MJ, Marcos JF, Zacarias L (2004) Biochemical and molecular analysis of carotenoid biosynthesis in flavedo of orange (Citrus sinensis L.) during fruit development and maturation. J Agric Food Chem 52:6724–6731
Sidana J, Saini V, Dahiya S, Nain P, Bala S (2013) A review on citrus—the boon of nature. Int J Pharma Sci Rev Res 18:20–27
Singh S, Aulakh PS, Gill PPS (2015) Seasonal variations in peel colour in relation to fruit development of grapefruit (Citrus paradise Macf.). Int Agric Environ Biotechnol 8:83–87
Soares FD, Pereira T, Marques MOM, Monteiro AR (2007) Volatile and non-volatile chemical composition of the white guava fruit (Psidium guajava) at different stages of maturity. Food Chem 100:15–21
Stewart I, Wheaton TA (1973) Carotenoids in citrus. In: Proceedings of 1stinternational citrus congress, vol 3, pp 325–330
Tadeo FR, Cerco M, Flores MC, Iglesias DJ, Naranjo MA, Ri G, Morillon L, Ollitrault P, Talon M (2008) Molecular physiology of development and quality of Citrus. Adv Bot Res 47:147–223
Yoo KM, Moon B (2016) Comparative carotenoid compositions during maturation and their antioxidative capacities of three citrus varieties. Food Chem 196:544–549
Zheng H, Zhang Q, Quan J, Zheng Q, Wanpeng X (2016) Determination of sugars, organic acids, aroma components, and carotenoids in grapefruit pulps. Food Chem 205:112–121. https://doi.org/10.1016/j.foodchem.2016.03.007
Zou X, Xi W, Hu Y, Nie C, Zhou Z (2016) Antioxidant activity of Citrus fruits. Food Chem 196:885–896. https://doi.org/10.1016/j.foodchem.2015.09.072
Acknowledgements
The research support provided by Punjab Agricultural University has been duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Singh, T.S. Chahal, P.P.S. Gill and S.K. Jawandha declare that they have no competing interests.
Rights and permissions
Springer Nature oder sein Lizenzgeber (z.B. eine Gesellschaft oder ein*e andere*r Vertragspartner*in) hält die ausschließlichen Nutzungsrechte an diesem Artikel kraft eines Verlagsvertrags mit dem/den Autor*in(nen) oder anderen Rechteinhaber*in(nen); die Selbstarchivierung der akzeptierten Manuskriptversion dieses Artikels durch Autor*in(nen) unterliegt ausschließlich den Bedingungen dieses Verlagsvertrags und dem geltenden Recht.
About this article
Cite this article
Singh, J., Chahal, T.S., Gill, P.S. et al. Comparison of Fruit Color and Quality Changes During Fruit Development in ‘Kinnow’ and ‘W. Murcott’ Mandarins. Erwerbs-Obstbau 65, 369–377 (2023). https://doi.org/10.1007/s10341-022-00818-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-022-00818-z