Abstract
Artificial pollination is one of the major constraints in date palm cultivation, while the unavailability or timely availability of pollen during the flowering period elevates the problem. One of the common practices is the use of stored pollen, but while stored at room temperature, pollen viability rapidly deteriorates after two to three months. In the present experiment, pollens were stored at ambient temperature and in a freezer at − 4 °C and a refrigerator at 5 °C using different storage containers for a year. The stored pollens were tested every month for 12 months for their pollen viability using acetocarmine as a staining agent. These stored pollens were then used for pollination, and on-farm fruit retention percentages were calculated. The best result for pollination was observed with the fresh pollen, while pollen stored at − 4 °C in a glass bottle gave the second-best results and can be used as an alternative in cases of pollen scarcity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Date palm (Phoenix dactylifera L.) is one of the most important fruit crops in the world, and its history of cultivation dates back to 4000 BC, making it one of the oldest cultivated fruit crops in the world (Johnson et al. 2013). Date palm is a dioecious plant, i.e., male and female flowers are borne into different plants, and naturally, they are pollinated through the wind. Successful pollination is dependent on pollen quality and viability, pistillate receptivity, pollination method, time of pollination, and environmental factors. In commercial settings, male and female palms are usually not closely planted, and their flowering periods do not coincide with each other, which reduces the chances of natural pollination (Kadri et al. 2019). Moreover, pollination is a time-bound process that needs to be done within 1–5 days after spathe cracking of female inflorescence, and best can be achieved on the day of spathe cracking itself, as delayed pollination may result in a loss of pistillate receptivity (Muralidharan et al. 2020; Shabana et al. 2001; Iqbal et al. 2018). However, asynchronized emergence of male and female inflorescence and delayed pollination may result in the development of unpollinated fruits, which have no commercial importance (Muralidharan et al. 2020). This makes artificial pollination one of the most important but laborious agronomical practices in date palm cultivation. Although, in most cases, males used to flower earlier, in a few cases, females planted flowers first (Sharma et al. 2023). It is always preferred to use fresh pollen for pollination as their efficiency of successful pollination is higher (Salomón-Torres et al. 2021; Sharma et al. 2021); however, in cases where the emergence of female flowers occurs before the emergence of male flowers, the usage of stored pollen from the last year is the only alternative (Rezazadeh et al. 2013; Sharma et al. 2023). Moreover, pollen is a living material and may lose its viability if not properly stored. A few of the earlier reports suggested that the pollens stored at room temperature may lose their viability after 2–3 months (Karim et al. 2022; Mesnoua et al. 2018) and possibly retain their viability at − 15 °C (Kumawat et al. 2022), − 20 °C (Mesnoua et al. 2018), − 30 °C (Karim et al. 2022), or − 196 °C (Anushma et al. 2018). However, practical storage at very low temperatures is not commonly available at farms because of its limited alternative use; thus, the method of storage should be such that it may be easily available among the date palm growers. The objective of the current experiment was to identify the temperature and container at which pollen can be stored to keep them viable and usable for next year and to further cross-examine the stored pollen under field conditions in a fruit setting.
Material and Methods
Pollen Collection and Storage
The experiment was conducted at Date Palm Research Station, Sardarkrushinagar Dantiwada Agricultural University, Mundra-Kachchh, Gujarat, India (22° 49′ 25.1″ N and 69° 43′ 13.6″ E) for 2 years. The pollen was collected from a selected male plant for both years. The male spathe from the selected male was harvested using a sickle from the base of the spathe when the spathe matured and just started to crack. The spathe cover was removed, and the inflorescence was shade-dried for a week separately. The dried inflorescence was shaken to collect the pollen. The collected pollen was then passed through a sieve to remove any dust or flower-based impurities. The pollen collected from different inflorescences was then uniformly mixed, and 5 g of each pollen was filled in all the containers and then kept at different temperatures as per the treatment (Table 1). Three sets of each container were kept at different temperatures for each treatment as a replication.
In Vitro Evaluation of Pollen Viability
Pollen viability was observed as per the method suggested by Maryam et al. (2015) using 1% (w/v) acetocarmine as a staining agent. One drop of acetocarmine is dropped on the glass slide, along with a drop of pollen suspension. Slides were covered using a cover slip, and tissue paper was used to remove extra stains. The sample was kept for an hour, and the slides were observed under a compound microscope (Olympus CKX31) at 200X magnification. If the pollens are stained red, it shows their viability; if they are transparent, then they are not viable. The experiment was conducted under a completely randomized design and replicated three times. Three sets of observations were made per replication from different parts of the slide and then averaged. The observations were made from March onwards (the month of pollen collection), continued for a year (up to February), and repeated for the second year. The percentage of viable pollen was calculated by
Effect of Stored Pollen on Fruit Retention
A separate set of pollen was also collected and stored as per the treatment and used for pollination. Pollen collected in the first year was used in the second year of experimentation for pollination, and pollen collected in the second year was used in the third year of experimentation for pollination. Thirty-nine uniform plants of date palm germplasm MDP-22 aged 8 years were selected with a spacing of 9 m × 9 m and were treated with the same set of agronomical practices. Thirty-six plants (3 each as 3 replications) were pollinated as per the treatment, and the remaining three were pollinated using fresh pollen as a control. Six inflorescences were kept in all the selected plants, and five strands were selected in three bunches of each plant for observation. Each inflorescence per plant was pollinated with the same amount of pollen (2 g) stored at different temperatures and containers from the previous season as per the treatment. In both years, pollination was done in March, when the female spathe cracks. An initial number of flowers per strand was recorded for the selected strand and was compared with the number of fruits at the time of harvest as a percentage. Unpollinated parthenocarpic fruits (if any) were removed and were not counted at the time of harvest.
Statistical Analysis
Statistical analysis was done using “R” with the “agricolae” package, and treatments’ significance was measured at p = 0.05, while graphical representation was made using "ggplot2," "ggthemes”, and “tidyverse” packages of “R” (R Core Team 2019; Mendiburu 2019; Wickam 2016, 2017; Arnold 2019).
Results and Discussions
In Vitro Evaluation of Pollen Viability
The microscopic observation of pollen viability shows a clear difference among the various storage methods and containers. The results were polled for both years, and the effect of different storage conditions in different containers and temperatures on date palm pollen viability in different months (from the first month (March) to the twelfth month (February)) is presented in Fig. 1, and their interaction effect is presented in Fig. 2. Among different temperatures (Fig. 1a), a sudden drop in pollen viability was observed in the pollen stored at ambient temperature after July, which also coincided with the rise in atmospheric humidity with the initiation of the monsoon. While the pollen stored at − 4 °C and 5 °C shows a decline in pollen viability after September, which suggests that the date palm pollen mostly starts losing its viability naturally after three months of flowering, The pollen stored at ambient temperature showed pollen viability of less than 25% (20.2%) after 12 months, while the highest pollen viability was shown by pollen stored at 4 °C (67.65%), which was closely followed by those kept at 5 °C (63.69%); however, both were significantly different among themselves. It suggests the possible option of pollen storage at − 4 °C for 12 months, contrary to storing at ambient conditions. Among the different containers (Fig. 1b), lower pollen viability was observed in earthen pots or polythene bags compared to PET bottles and glass bottles. Earthen pots are moisture sensitive and capture moisture from the atmosphere, while the moisture resistance of polythene bags is also limited to a certain extent and is comparatively poorer than glass bottles and PET bottles. Higher pollen viability was observed after twelve months (February) in the pollen stored in glass bottles (59.37%). In the interaction effect (Fig. 2), the highest pollen viability was observed in the pollen stored in a glass bottle at − 4 °C, with 74.77% viability after 12 months. In general, pollen remains most viable at the time of anthesis or just after the anthesis (Pinillos and Cuevas 2007). A continuous decline in pollen viability is more of a continuous variable than a dichotomous condition, which may vary from variety to variety (Thompson et al. 1994; Kelen and Demirtas 2003). It has been noted that the life of the pollen is majorly dependent on temperature and humidity, and dry pollen remains more viable compared to wet ones (Broussard et al. 2023). The ambient temperature and the presence of high humidity led to the absorption of humidity, which might have resulted in the loss of pollen viability. The origin of hydrolysis reactions of sugars due to higher enzymatic activity under ambient temperature was higher compared to that of cold storage (Yao et al. 2010). With the change in temperature and humidity, the life of the pollen may vary, even for a short period of time (Koubouris et al. 2009). Du et al. (2009) observed that at room temperature, there is a severe loss of water and viability due to the high temperature, which leads to high respiration and metabolism. They also noted that at low temperatures, respiration reduces enzymatic and metabolic activities, leading to better pollen viability, which also supports current observations. In the current experiment, higher pollen viability for pollen stored in a glass bottle at − 4 °C is due to the impermeable body of glass and the presence of low temperatures in storage, which further increases the storage life of the pollen. Similar results were obtained by El Kadri and Ben Mimoun (2020), who obtained pollen with higher pollen viability at − 20 °C followed by 4 °C for several Tunisian cultivars. A few earlier experiments supported the possible storage of pollen at sub-zero temperatures of − 15 °C, − 20 °C, − 30 °C (Kumawat et al. 2022; Mesnoua et al. 2018; Karim et al. 2022) or − 196 °C (Anushma et al. 2018). However, higher pollen viability does not guarantee a fruit set (Akond et al. 2012; Mesnoua et al. 2018), and thus a field trial is needed.
Effect of Stored Pollen on Fruit Set and Retention
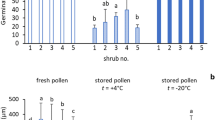
During the on-farm trial, which is also a verification of the viability and longevity test, the use of stored pollen for fruit set and retention of different treatments was compared with those pollinated with fresh pollen. The best result concerning fruit set and retention at the time of harvest was observed with the fresh pollen in both years (63.02%) and (60.10%) pooled to 61.56%. However, among different storage temperatures and containers, the best treatment interaction was obtained with pollen stored in a glass bottle stored at − 4 °C (53.48%), which shows lower fruit retention than fresh pollen by 8.08%≈8%. It suggests that fresh pollen is the best for pollination, and in their absence, stored pollen at − 4 °C can be useful. In a few earlier experiments, it was noted that pollen diluted to 1:19 (Sharma et al. 2021), 1:15 (Al-Wusaibai et al. 2012), and 1:9 (Munir 2019) is sufficient for successful pollination, suggesting that even a small percentage of viable pollen may suffice for pollination. However, the current result suggests that the mere presence of pollen does not guarantee fruit set, and higher fruit set and retention are expected only if the pollens are viable and of high quality (Salomón-Torres et al. 2021). The best pollen adhesion takes place when they are fresh and the adhesion of the pollen also decreases with time (Dutta et al. 2013). It also supports our earlier observation on pollen storage, where the highest pollen viability was observed for pollen stored at − 4 °C in a glass bottle. This might be due to the reduced pollen viability, which also impacted the fruit set as non-viable pollens do not germinate and develop pollen tubes, ultimately leading to overall fertilization (Table 2).
To conclude, the overall results showed that most of the date palm pollens can be stored for at least 1 year at − 4 °C in a glass bottle. Although the pollen viability is reduced, it is sufficient to pollinate to get a productive yield and can be used as an alternative in case of a pollen shortage in the current year.
Conclusions
Date palm growers can effectively store date palm pollen in-house under freeze conditions at − 4 °C in an airtight glass bottle for 1 year. This could be helpful to overcome the shortage of fresh pollen.
References
Akond, A.S.M.G.M., C.T. Pounders, E.K. Blythe, and X. Wang. 2012. Longevity of crape-myrtle pollen stored at different temperatures. Scientia Horticulturae 139: 53–57. https://doi.org/10.1016/j.scienta.2012.02.021.
Al-Wusaibai, N.A., A.B. Abdallah, M.S. Al-Husainai, H. Al-Salman, and M. Elballaj. 2012. A comparative study between mechanical and manual pollination in two premier Saudi Arabian date palm cultivars. Indian Journal of Science and Technology 5 (4): 2487–2490. https://doi.org/10.17485/ijst/2012/v5i4.4.
Anushma, P.L., L. Vincent, P.E. Rajesekharan, and S. Ganeshan. 2018. Pollen storage studies in date palm (Phoenix dactylifera L.). International Journal of Chemical Studies 6 (5): 2640–2642.
Arnold, J.B. 2019. ggthemes: Extra Themes, Scales and Geos for ‘ggplot2’. R package version 4.2.0. https://CRAN.R-project.org/package=ggthemes.
Baidiyavadra, D.A., C.M. Muralidharan, and K.M. Sharma. 2019. Fresh date production in Kachchh (India): Challenges and prospects. Medicinal Plants 11 (3): 218–227. https://doi.org/10.5958/0975-6892.2019.00029.7.
Broussard, M.A., M. Coates, and P. Martinsen. 2023. Artificial pollination technologies: A review. Agronomy 13 (5): 1351. https://doi.org/10.3390/agronomy13051351.
Du, G., J. Xu, C. Gao, J. Lu, Q. Li, J. Du, M. Lv, and X. Sun. 2009. Effect of low storage temperature on pollen viability of fifteen herbaceous peonies. Biotechnology Reports 21: e00309. https://doi.org/10.1016/j.btre.2019.e00309.
Dutta, S.K., M. Srivastav, H. Rymbai, R. Chaudhary, A.K. Singh, A.K. Dubey, and K. Lal. 2013. Pollen-pistil interaction studies in mango (Mangifera indica L.) cultivars. Scientia Horticulturae 160: 213–221. https://doi.org/10.1016/j.scienta.2013.05.012.
El-Kadri, N., and M.B. Mimoun. 2020. In vitro germination of different date palm (Phoenix dactylifera L.) pollen sources from Southern Tunisia under the effect of three storage temperatures. International Journal of Fruit Science 20 (3): S1519–S1529. https://doi.org/10.1080/15538362.2020.1815116.
Iqbal, M., K. Usman, M. Munir, and M.S. Khan. 2018. Quantitative and qualitative characteristics of date palm cv. Gulistan in response to pollination times. Sarhad Journal of Agriculture 34 (1): 40–46. https://doi.org/10.17582/journal.sja/2018/34.1.40.46.
Johnson, D.V., J.M. Al-Khayri, and S.M. Jain. 2013. Seedling date palm (Phoenix dactylifera L.) as genetic resources. Emeritus Journal of Food and Agriculture 25 (11): 809–830. https://doi.org/10.9755/ejfa.v25i11.16497.
Kadri, K., M. Elsafy, S. Makhlouf, and M.A. Awad. 2021. Effect of pollination time, the hour of daytime, pollen storage temperature and duration of pollen viability, germinability and fruit set of date palm (Phoenix dactylifera L.) cv. “Deglet Nour.” Saudi Journal of Biological Sciences 29 (2): 1085–1091. https://doi.org/10.1016/j.sjbs.2021.09.062.
Kadri, K., A. Othmani, S. Makhloufi, M.S. Chebbi, A. Chokmani, and A. Touil. 2019. Contribution to the study of the effect of pollination mode on fruit set rate and yield in the date palm. International Journal of Agricultural Innovations and Research 7 (5): 533–537.
Karim, K., M.A. Awad, A. Manar, J. Monia, A. Karim, and E. Mohammed. 2022. Effect of flowering stage and storage conditions on pollen quality of six male date palm genotypes. Saudi Journal of Biological Sciences 29: 2564–2572. https://doi.org/10.1016/j.scienta.2021.12.038.
Kelen, M., and I. Demirtas. 2003. Pollen viability, germination capability and pollen production level of some grape varieties (Vitis vinifera L.). Acta Physiologiae Plantarum 25 (3): 229–233. https://doi.org/10.1007/s11738-003-0002-7.
Koubouris, G.G., I.T. Metzidakis, and M.D. Vasilakakis. 2009. Impact of temperature on olive (Olea europaea L.) pollen performance about relative humidity and genotype. Environmental and Experimental Botany 67: 209–214. https://doi.org/10.1016/j.envexpbot.2009.06.002.
Kumawat, P.K., R.S. Rathore, S. Kumar, G. Kumawat, and S. Rawar. 2022. Effect of stored pollen grains on quality and yield of date palm in western Rajasthan. The Pharma Innovation SP-11 (4): 1128–1132.
Maryam, M., J. Jaskani, B. Fatima, M.S. Haider, S.A. Naqvi, M. Nafees, R. Ahmad, and I.A. Khan. 2015. Evaluation of pollen viability in date palm cultivars under different storage temperatures. Pakistan Journal of Botany 47 (1): 377–381.
Mendiburu, F. 2019. Agricolae: Statistical procedures for Agricultural Research. R package version 1.3–1. https://CRAN.R-project.org/package=agricolae.
Mesnoua, M., M. Roumani, and A. Salem. 2018. The effect of pollen storage temperatures on pollen viability, fruit set and fruit quality of six date palm cultivars. Scientia Horitculturae 236: 279–283. https://doi.org/10.1016/j.scienta.2018.03.053.
Munir, M. 2019. Influence of liquid pollination technique on fruit yield and physicochemical characteristics of date palm cultivars Khadrawy and Zahidi. Journal of Biodiversity and Environmental Sciences 15: 41–49.
Muralidharan, C.M., C.N. Panchal, D.A. Baidiyavadra, K.M. Sharma, and P. Verma. 2020. Pistillate receptivity of date palm (Phoenix dactylifera L.) cv Barhee. Sugar Tech 22 (6): 1166–1169. https://doi.org/10.1007/s12355-020-00859-2.
Pinillos, V., and J. Cuevas. 2007. Artificial pollination in tree crop production. Horticultural Reviews 34: 239–276. https://doi.org/10.1002/9780470380147.ch4.
R Core Team. 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rezazadeh, R., H. Hassanzadeh, Y. Hosseini, Y. Karami, and R.R. Williams. 2013. Influence of pollen source on fruit production of date palm (Phoenix dactylifera L.) cv. Barhi in humid coastal regions of Southern Iran. Scientia Horticulturae 160: 182–188. https://doi.org/10.1016/j.scienta.2013.05.038.
Salomón-Torres, R., R. Krueger, J.P. García-Vázquez, R. Villa-Angulo, C. Villa-Angulo, N. Ortiz-Uribe, J.A. Sol-Uribe, and L. Samaniego-Sandoval. 2021. Date palm pollen: Features, production, extraction and pollination methods. Agronomy 11 (3): 504. https://doi.org/10.3390/agronomy11030504.
Shabana, H.R., A.A. Saeed, and A.H. Hamoodi. 2001. Determination of the optimal pollination period for Khalas date palm cultivar. In Proceedings of the second international conference on date palms, Al-Ain, United Arab Emirates, ed. A.A. Al-Badawy, 13–20. Al-Ain: UAE University.
Shaheen, M.A. 2004. Evaluation of date palm males using pollen viability and ultrastructure. Acta Horticulturae 632: 37–43.
Sharma, K.M., M. Kumar, C.M. Muralidharan, and R. Salomón-Torres. 2023. Date palm pollination management. In Date palm, ed. J.M. Al-Khayri, S.M. Jain, D.V. Johnson, and R.R. Krueger, 209–240. Wallingford: CABI. https://doi.org/10.1079/9781800620209.0007.
Sharma, K.M., C.M. Muralidharan, D.A. Baidiyavadra, K. Bardhan, and C.N. Panchal. 2021. Evaluation of potentiality of different adjuvants for date palm pollination and fruit set. Sugar Tech 23 (1): 139–145. https://doi.org/10.1007/s12355-020-00885-0.
Thompson, J.D., L.P. Rigney, K.M. Karoly, and B.A. Thomson. 1994. Pollen viability, vigor, and competitive ability in Erythronium grandiflorum (Liliaceae). American Journal of Botany 81: 1257–1266.
Wickam, H. 2017. tidyverse: Easily Install and Load the ‘Tidyverse’. R package version 1.2.1. https://CRAN.R-project.org/package=tidyverse.
Wickam, H. 2016. ggplot2: Elegant graphics for data analysis. New York: Springer.
Yao, S.D.M., J.L.M. Konan, R.S. Sie, A.R. Assa, and K. Allou. 2010. Effet de la duree de conservation sur la qualite du pollen en production de semences chez le cocotier (Cocos nucifera L.). Sciences & Nature 7: 87–96. https://doi.org/10.4314/scinat.v7i1.59938.
Author information
Authors and Affiliations
Contributions
KMS conducted, analyzed and wrote the manuscript; DAB conducted and supported in experimentation; CMM formulated and facilitated the experiment; CNP and PV helped in the formulation of the experiment. All the authors have reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, K.M., Baidiayavadra, D.A., Muralidharan, C.M. et al. Effect of Storage Temperature and Containers on Date Palm (Phoenix dactylifera L.) Pollen Viability and Post-storage Pollination. Sugar Tech (2024). https://doi.org/10.1007/s12355-024-01415-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12355-024-01415-y