Abstract
Sweet sorghum is one of the many types of cultivated sorghums and is a promising multipurpose biofuel feedstock. The objective of this study was to assess phenotypic and genotypic diversity among 75 selected diverse sweet sorghum genotypes available at ICRISAT-Patancheru. Genotyping-by-sequencing generated 135,162 SNPs having minor allele frequencies > 0.20. Cluster analysis grouped the genotypes into three distinct clusters, with some of the sweet sorghum lines falling in distinct clusters. This grouping pattern was in conformity with available race and pedigree information. Mean performance and analysis of variance for sugar yield-related traits shows considerable variability. The results also revealed close genetic relationships between elite genotypes such as ICSV 25316, ICSV 25311, SSV 74 and ICSV 25300 that are superior for various sugar yield-associated traits like plant height, biomass, juice yield and sugar content. Not surprisingly given their pedigrees, these elite materials grouped together tightly in a single sub-cluster.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sorghum (Sorghum bicolor (L.) Moench, 2n = 20) is the fifth most important cereal crop and occupies the second position among the staple food grains in semiarid tropics. Sorghum is an excellent feedstock for bioenergy production because of three distinct features: high photosynthetic efficiency for converting solar energy into biomass; high water use efficiency; and high tolerance to stressed environments for growing in marginal lands.
Sweet sorghums accumulate sugars (10 to 25%) in their stalk juice at physiological maturity. Sweet sorghums are typically characterized by moderate grain yield, but high biomass production. Because of its high sugar content, sweet sorghum is cultivated mainly for fodder, syrup, molasses, sugar and ethanol production (Rao et al. 2009; Wang et al. 2009). The genetic diversity in the germplasm of any breeding program affects the potential for genetic gain through selection. Knowledge of genetic diversity permits the germplasm to be classified into heterotic groups, which is important in hybrid breeding (Menz et al. 2004). Crosses between unrelated and genetically distant adapted parents show greater hybrid vigor than crosses between closely related parents (Stuber 1994; Hallauer 1999). More studies to assess the genetic diversity of sweet sorghum are necessary for crop improvement. Ritter et al., (2007) suggested that sweet sorghum is of polyphyletic origin. As sweet sorghum traits such as sugar rich juicy tall stem, high biomass, high stem/leaf ratio and low grain were found in several races of subspecies bicolor, it raises questions about the origin, selection and genetic and genomic basis of sweet sorghum (Zheng et al. 2011).
Recently, single-nucleotide polymorphisms (SNPs) have become the marker of choice because they can provide accurate and reproducible data along with the potential for high throughput, low-cost genotyping (Yan et al. 2009). Many studies were reported to determine the genetic relationships among sweet sorghum accessions using different types of markers such as AFLPs, (Ritter et al. 2007), SSR markers (Ali et al. 2008; Murray et al. 2009) and SNPs (Murray et al. 2009; Burks et al. 2015; da Silva et al. 2017; Luo et al. 2020; Hao et al. 2021). The objectives of the present study were to assess the agronomic performance of sweet sorghum breeding material and their derived lines and also to assess the genetic variability among them using the SNP markers. This study could be useful to identify the genetically diverse material with superior agronomic characters to use in the future sweet sorghum breeding programs aiming at heterotic hybrids for sugar yield-related traits.
Results
Evaluation for Morpho-Physiological Traits
The two-way analysis of variance (ANOVA) for pooled data showed significant differences for all measured traits at P < 0.001 Table 1. The maximum and minimum mean, standard error, least significant difference (5%) and coefficient of variation (CV, %) for all observed traits for all 75 genotypes are presented in Table 1.
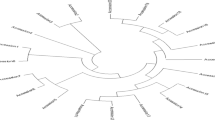
Plant height ranged from 1.3 to 3.4 m. High biomass landraces, specifically most of the durra race accessions, recorded plant heights over 3 m. These landraces are photosensitive lines (Fig. 1, most of clusters IIb and IIc) that grow tall and produce high biomass, under long day length conditions and are induced to flower only when the day length falls below 11 h 45 min. The average plant height for all the high biomass germplasm accessions was 2.7 m. Plant height for most of the remaining sweet sorghum varieties was in the range of 2 to 3.6 m, with the average of 2.6 m, which was almost same as the mean value of germplasm accessions (Fig. 1) clusters I and IIa and for the maintainer lines, range was 1.3 m to 2.1 m (Fig. 1, clusters I and IIb), with the average of 1.6 m. These results indicate, there was significant variability among studied genotypes for plant height. Significant variability was also observed among these germplasm lines, maintainer lines and improved sweet sorghum varieties for stalk yield, brix and sugar content. All the sweet sorghum varieties and breeding lines (Fig. 1) clusters I and II are corded average stalk yield of 37.54 t ha−1, whereas germplasm accessions (Fig. 1) cluster IIb recorded average yields of 54.4 t ha−1. The lowest average yields were observed for maintainer lines, which was 23 t ha−1. Highest brix value (19.4%) was recorded by the breeding line ICSV 12008 while lowest brix value (8.3%) was recorded for Nr 481. All the high biomass germplasm accessions (Fig. 1, most of clusters IIb and III) recorded lower brix values, averaging about 12.7%. Cluster I largely comprised of improved sweet sorghum lines, most of which exhibited higher brix values (> 13%). The average brix value for this group was 15.6%. Cluster II contained breeding lines, most of which were having brix value above 15%, and germplasm accessions, with brix values ranging from 9.2 to 16.1%.
Neighbor-joining tree using SNP data of 75 genotypes. Genetic distances between the sweet sorghum accessions were calculated using the identity-by-state (IBS) coefficient. Ia, Ib, IIa, IIb and III correspond to correspond to the clusters identified through the neighbor-joining method. IS International sorghum, ICSB ICRISAT sorghum female parent, ICSV ICRISAT sorghum variety
Genetic Diversity
Sweet sorghum samples were processed through the GBS pipelines, and a total of 135,162 SNPs were identified after filtering the total SNPs with minor allele frequencies (MAF) at 20 percent. The NJ cluster analysis grouped the 75 genotypes into five distinct major groups primarily based on their biological status (photoperiod-sensitive high biomass germplasm lines or sweet sorghum varieties and derived breeding lines) and also based on their morpho type (race) and pedigree. Grouping of genotypes according to the race is in conformity with previously published reports in diverse germplasm lines (Billot et al. 2013; Ramu et al. 2013). Cluster I consists of two sub-clusters, clusters Ia and Ib. Cluster Ia comprises of 40 genotypes, mainly belonging to bicolor race except for a ICSV 25266 belong to race guinea-caudatum). A majority of them developed at ICRISAT-Patancheru in its sweet sorghum breeding program are parents of that material and grouped based on their common ancestry. Cluster Ib consists of five genotypes all belonging to bicolor race, except IS 18521 belonging to caudatum race. Interestingly, IS 18521 clustered together with SPSSV30, a bicolor race cultivar, indicating latter’s origin from bicolor race.
In the remaining 46 genotypes, 44 genotypes formed cluster II, which has two subgroups (II a and II b) primarily consisting of high biomass and sweet sorghums developed by India’s National Agricultural Research System (NARS), a few ICRISAT-bred lines (some involving NARS parental lines) and 12 high biomass germplasm accessions. Cluster IIa consists of bicolor race breeding products including superior sweet sorghum and high biomass lines (> 2.5 m height). Grouping in this cluster is primarily based on their pedigree Table 1. Two genotypes (viz., SP-08 16427 and SP 08 16421-2) sharing a common ancestry grouped closely together in this cluster, near their stem borer and shootfly resistant parent ICSV 93046, several other of its derivatives, and its progenitor ICSV 700. Cluster IIb mainly consists of non-bicolor race genotypes (including caudatum, durra, guinea and their intermediate races), the majority of them are high biomass germplasm lines and a low biomass maintainer line (ICSB 27) Table 1. Interestingly, IS27206 falling under guinea race, and IS18542, an accession from durra race cluster together. The maintainer line, ICSB 27, was grouped with these germplasm lines and was found to be closely related to durra line IS27314. ICSB 27 although was found to be inferior for all the quantitative traits compared to other genotypes of the group. Surprisingly, two high biomass germplasm accessions IS 16575 and IS 8813 formed into a separate group, cluster III, though they belong to different races durra-caudatum and guinea, respectively. In the rainy season, these two landraces have the same maturity period with the capacity of producing huge biomass (IS 16575: ~ 100 t ha−1 and IS 8813: ~ 75 t ha−1). The pure line variety ICSV 25266 (selected from germplasm accession IS 23526) grouped closely with improved sweet sorghum derivatives of that same germplasm accession, namely 5655-3 and 5651-3, in cluster I along with NTJ2 and other improved sweet sorghum varieties and breeding lines. These two genotypes were showing superiority over their parent ICSV 25266 for plant height, stalk yield, juice yield, brix and sugar yield (Table 1). Also, SPSSV 30 grouped closely with germplasm line IS 18521, probably because of its similar genetic background.
Discussion
Sweet sorghum is one of the potential and promising biofuel feedstocks that has many characteristics such as high stalk sugar content, wide adaptability and tolerance to biotic and abiotic stress. The knowledge on genetic diversity is very important in hybrid breeding program since it permits the germplasm to be classified into heterotic groups (Menz et al. 2004). The genetic diversity in the germplasm of any breeding program affects the potential for genetic gain through selection.
Phenotypic Traits
The seasonal variance and variance for genotype × season interactions showed significant differences for the candidate sugar traits (plant height, juice yield and brix %) at P < 0.001 corroborating with the earlier observations (Rao et al. 2013a, b; Ratnavathi et al. 2010). The landraces are photosensitive lines and clustered in IIa, IIb and III, that grow tall and produce high biomass, under long day length conditions (Fig. 1). They flower only when the day length falls below 11 h 45 min, and it was evident from the mean values (Table 1) that germplasm accessions had lower brix values, and hence, less sugar content (clusters IIb and III) vis a vis improved sweet sorghum varieties and parental lines that recorded superior stalk yield and sugar yield (cluster I). Therefore, based on phenotypic data, entries from cluster Ia and clusters IIb and III could be used as potential parents for developing new heterotic hybrid combinations for sugar yield.
Most of the sweet sorghum genotypes that belong to bicolor race in this study were grouped together although they showed significant differences in terms of observed quantitative agronomic traits. Deu et al. (2006) earlier reported that the bicolor race has high genetic diversity and many rare alleles. This race is considered to be oldest and most widely distributed geographically due to its several uses (fodder, brooms, and sweet stems) (Pecina-Quintero et al. 2012). The three caudatum race genotypes (SSV 84, IS 22868 and IS 18521) were distributed in different clusters, supporting that this race is broadly dispersed (Billot et al. 2013; Ramu et al. 2013). It is evident that these sweet sorghums exhibit wide variability in multiple phenotypic traits such as stalk yield and plant height which corroborates the findings of earlier reports (Balaravi et al. 1997; Bennetzen et al. 2001; Biswas and Sundaralingam 1997) (Table 1). Genotypic differences were also reported by Rao et al. 2013a for extractable juice, stem sugar content and fermentation efficiency. The major roles of nonadditive gene action for plant height, total soluble solids, juice yield and stalk yield indicate the potential of heterosis breeding for improving these traits in sweet sorghum (Rao et al. 2013a, b). Significant genetic differences that were observed for all quantitative traits studied indicate that there is considerable amount of genetic variability existing among genotypes. Cultivar development is based on the exploitation of genetic variability for traits of interest (Makanda et al. 2009). Progress of breeding in such characters is primarily determined by the magnitude and nature of variation and interrelationship among them (Gandhi et al. 1964).
Breeding for Bioenergy
Sweet sorghum has high amounts of total reducing sugars, which prevent sucrose crystallization and has 90% fermentation efficiency (Kumar et al. 2013).Compared to grain sorghum, sweet sorghum grows rapidly producing high biomass and also has wider adaptation. It is important to identify suitable superior genotypes for ethanol production in terms of brix, biomass, juice yield and fermentation efficiency. The genotypes with high sugar yield in contrast to their high biomass and low sugar yield counterparts may possess alleles for increased amount of sugar yield in the stem juice. So, contrasting pairs of these lines that are otherwise most similar for flowering time and plant height can be used as parents to develop mapping populations, targeting genes responsible for sugar accumulation. The segregating population derived from the crosses between these diverse pairs of lines can be used to develop high density linkage maps and to map genes which influence sugar accumulation in the stems (Ali et al. 2008; Zhang et al. 2018; Kong et al. 2020; Hao et al. 2021).
The most elite sweet sorghum genotypes in cluster I in the present study (namely SSV 74 and lines derived from cross DSV 4 × SSV 84), which exhibited superior performance in terms of brix (> 15%), sugar yield (> 2.5 t ha−1), plant height (> 2.8 m) and biomass yield (> 50 t ha−1), can be predicted to show only limited genetic variation, which can be confirmed using SNP data available for them. Also, by performing genome-wide association studies with high density SNP genotyping, genes underlying natural variation in various traits can be identified (Morris et al. 2013a, 2013b). All these genomic studies would be helpful to better understand the diverse pairs of genotypes under study and aid in the incorporation of these lines in future molecular breeding programs.
In conclusion, this study provides detailed analysis of genetic diversity existing among materials under study in the ICRISAT-Patancheru sweet sorghum breeding program. In the present study, NJ analysis based on genetic background revealed that genotypes ICSV 25311, ICSV 25316, ICSV 25300 and SSV 74 show very limited diversity (cluster I), but were found to be superior in terms of sugar yield. Nevertheless, they are genetically distant from germplasm lines (clusters IIb and III) which have potential high biomass yield. Hence, these diverse groups of genotypes could be of valuable material for sweet sorghum breeding programs aiming for heterotic hybrid combinations for sugar yield (Li et al. 2019; Hao et al. 2021). Thus, the results suggest that the information on sugar yield-related traits and genetic relationships will be helpful in guiding future molecular breeding approaches to improve sugar and biomass yield while maintaining and exploiting a broad range of genetic diversity.
Materials and Methods
Phenotyping
Sweet sorghum genotypes used in the present study were selected from the ICRISAT-Patancheru sweet sorghum breeding program in India. A total of 75 sweet sorghum genotypes including germplasm lines with high biomass yield, maintainer lines and sweet sorghum varieties were selected based on their phenotype with an emphasis on panicle type, grain, days to flowering and plant height (Table 2). These genotypes were further tested to assess their sugar yield-related traits. Field studies were performed during October 2010, post-rainy season and October 2013, post-rainy season in vertisols of ICRISAT, Patancheru, India, using a randomized complete block design (RCBD) with three replications. In both the years, fields were fertilized with di-ammonium phosphate (DAP) at the time of sowing and top dressed with urea after 21 days of planting (N-P-K: 90-40-0). Each entry was planted in two rows of four meters each. Spacing between the rows and in between the plants were 60 cm and 15 cm, respectively. Twenty randomly selected plants from each entry were used for observations on agronomic traits. Days to 50% flowering were recorded during crop growth. Plants were harvested at the time of physiological maturity and data were collected following methodology described in (Munirathnam et al. 2013) for the traits: plant height (m), stalk yield (t ha−1), juice yield (t ha−1), brix (%), and sugar yield (t ha−1). The juice was extracted from the clean stalks of sweet sorghum after stripping the leaves harvested from 1 m−2 area at physiological maturity by passing through the three-roller Wiley mill thrice and expressed in per ha basis. The sugar yield (t ha−1) is calculated as brix (%) X juice yield (t ha−1)/100.
Genotyping
DNA was extracted from 10-day-old seedlings of the 75 entries using the CTAB method described in Mace et al. (2003). Samples were genotyped with genotyping-by-sequencing (GBS) technology at the Institute of Genomic Diversity (IGD, Cornell University, USA, (Elshire et al. 2011). Sequences generated were processed through GBS pipelines using TASSEL version 3.0 to identify SNP markers (Glaubitz et al. 2014). BTx623 was used as reference genome (v1.4, (Paterson et al. 2009) in the pipeline to identify SNP markers on all chromosomes.
Data Analysis
The data on the measured quantitative traits were pooled over both the seasons, and analysis of variance (ANOVA), mean, standard error, least significant difference and coefficient of variation for the recorded traits were calculated using Genstat v.14.0 program (VSN International 2011). SNP data generated were used in TASSEL version 3.0 to generate a distance matrix among the entries. The corresponding similarity matrix was used inDARwinv5 software (Perrier et al. 2003; Perrier and Jacquemoud-Collet 2006) to construct a dendrogram using the neighbor-joining (NJ) method.
Conclusions
The study established the genetic relationship among the elite sweet sorghum lines and germplasm accessions with tropical and temperate adaptation. In order to develop heterotic hybrid combinations for sugar yield it would be appropriate to attempt distant crosses between sugar-rich lines from cluster I (ICSV 25311, ICSV 25316, ICSV 25300 and SSV 74) and high biomass yielding lines from clusters IIb and III (IS 8813, IS 16575, IS 16529, IS 18542). Future studies should assess large pool of lines with much larger data points to harness the potential of genomic selection to accelerate genetic gain for sugar yield and associated traits.
Abbreviations
- GBS:
-
Genotyping-by-sequencing
- SNPs:
-
Single-nucleotide polymorphisms
- ANOVA:
-
Analysis of variance
- RCBD:
-
Randomized complete block design
- DAP:
-
Di-ammonium phosphate
- NJ:
-
Neighbor-joining method
References
Ali, M.L., J.F. Rajewski, P.S. Baenziger, K.S. Gill, K.M. Eskridge, and I. Dweikat. 2008. Assessment of genetic diversity and relationship among a collection of US sweet sorghum germplasm by SSR markers. Molecular Breeding 21: 497–509.
Balaravi, S, P K Biswas, and M Elangovan. 1997. Advances in the improvement of the sweet sorghum in India. In Proceedings of first international sweet sorghum conference, China, 14–19.
Bennetzen, Jeffrey L., Vaidyanathan Subramanian, Xu Jichen, Shanmukhaswami S. Salimath, Sujatha Subramanian, Dinakar Bhattramakki, and Gary E. Hart. 2001. A framework genetic map of sorghum containing RFLP, SSR and morphological markers. In DNA-based markers in plants, ed. R.L. Phillips, Indra K. Vasil, and Pp513, 347–355. Springer. https://doi.org/10.1007/978-94-015-9815-6.
Billot, Claire, Punna Ramu, Sophie Bouchet, Jacques Chantereau, Monique Deu, Laetitia Gardes, Jean-Louis. Noyer, Jean-François. Rami, Ronan Rivallan, and Yu. Li. 2013. Massive sorghum collection genotyped with SSR markers to enhance use of global genetic resources. PLoS ONE 8: e59714.
Biswas, Roopa, and Muttaiya Sundaralingam. 1997. Crystal structure of r (GUGUGUA) dC with tandem GU/UG wobble pairs with strand slippage. Journal of Molecular Biology 270: 511–519.
Burks, Payne S., Chris M. Kaiser, Elizabeth M. Hawkins, and Patrick J. Brown. 2015. Genomewide association for sugar yield in sweet sorghum. Crop Science 55: 2138–2148.
da Silva, Michele Jorge, Maria Marta Pastina, Vander Fillipe de Souza, Robert Eugene Schaffert, Pedro Crescêncio Souza Carneiro, Roberto Willians Noda, José Eustáquio de Souza Carneiro, Cynthia Maria Borges Damasceno, and Rafael Augusto da Costa Parrella. 2017. Phenotypic and molecular characterization of sweet sorghum accessions for bioenergy production. PLoS One 12:e0183504.
Deu, Monique, F. Rattunde, and Jacques Chantereau. 2006. A global view of genetic diversity in cultivated sorghums using a core collection. Genome 49: 168–180.
Elshire, Robert J., Jeffrey C. Glaubitz, Qi. Sun, Jesse A. Poland, Ken Kawamoto, Edward S. Buckler, and Sharon E. Mitchell. 2011. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 6: e19379.
Gandhi, S.M., A.K. Sanghi, K.S. Nathawat, and M.P. Bhatnagar. 1964. Genotypic variability and correlation coefficients relating to grain yield and a few other quantitative characters in Indian wheats. Indian Journal of Genetics and Plant Breeding (The) 24: 1–8.
Glaubitz, Jeffrey C., Terry M. Casstevens, Lu. Fei, James Harriman, Robert J. Elshire, Qi. Sun, and Edward S. Buckler. 2014. TASSEL-GBS: A high capacity genotyping by sequencing analysis pipeline. PLoS ONE 9: e90346.
Hallauer, A.R. 1999. Temperate maize and heterosis. American Society of Agronomy, Crop Science Society of America.
Hao, H., Li, Z., Leng, C., Lu C., Luo, H., Liu, Y., Wu, X., Liu, Z., Shang, L., Jing H-C. 2021. Sorghum breeding in the genomic era: Opportunities and challenges. Theoretical and Applied Genetics 134: 1899-1924.
Kong, Wenqian, Huizhe Jin, Valorie H. Goff, Susan A. Auckland, Lisa K. Rainville, and Andrew H. Paterson. 2020. Genetic analysis of stem diameter and water contents to improve sorghum bioenergy efficiency. G3: Genes, Genomes, Genetics 10: 3991–4000.
Kumar, C.G., R.N. Rao, P. Srinivasa Rao, A. Kamal, A. Ashok Kumar, Ch Ravinder Reddy, and B.V.S. Reddy. 2013. Assessing sweet sorghum juice and syrup quality and fermentation efficiency. International Crops Research Institute for the Semi-Arid Tropics.
Li, Yin, Wenqin Wang, Yaping Feng, Tu. Min, Peter E. Wittich, Nicholas J. Bate, and Joachim Messing. 2019. Transcriptome and metabolome reveal distinct carbon allocation patterns during internode sugar accumulation in different sorghum genotypes. Plant Biotechnology Journal 17: 472–487.
Luo, Feng, Zhongyou Pei, Xiongwei Zhao, Huifen Liu, Yiwei Jiang, and Shoujun Sun. 2020. Genome-wide association study for plant architecture and bioenergy traits in diverse sorghum and sudangrass germplasm. Agronomy 10: 1602.
Mace, Emma S., Kutokshi K. Buhariwalla, Hutokshi K. Buhariwalla, and Jonathan H. Crouch. 2003. A high-throughput DNA extraction protocol for tropical molecular breeding programs. Plant Molecular Biology Reporter 21: 459–460.
Makanda, I., P. Tongoona, and J. Derera. 2009. Quantification of genotypic variability for stem sugar accumulation and associated traits in new sweet sorghum varieties. African Crop Science Conference Proceedings 9: 391–398.
Menz, Monica A., Robert R. Klein, Natalie C. Unruh, William L. Rooney, Patricia E. Klein, and John E. Mullet. 2004. Genetic diversity of public inbreds of sorghum determined by mapped AFLP and SSR markers. Crop Science 44: 1236–1244.
Morris, Geoffrey P., Punna Ramu, Santosh P. Deshpande, C. Thomas Hash, Trushar Shah, Hari D. Upadhyaya, Oscar Riera-Lizarazu, Patrick J. Brown, Charlotte B. Acharya, and Sharon E. Mitchell. 2013a. Population genomic and genome-wide association studies of agroclimatic traits in sorghum. Proceedings of the National Academy of Sciences 110: 453–458.
Morris, Geoffrey P., Davina H. Rhodes, Zachary Brenton, Punna Ramu, Vinayan Madhumal Thayil, C. Santosh Deshpande, Thomas Hash, Charlotte Acharya, Sharon E. Mitchell, and Edward S. Buckler. 2013b. Dissecting genome-wide association signals for loss-of-function phenotypes in sorghum flavonoid pigmentation traits. G3: Genes, Genomes, Genetics 3: 2085–2094.
Munirathnam, P., K. Ashok Kumar, and P. Srinivasa Rao. 2013. Performance of sweet sorghum varieties and hybrids during post rainy season (maghi) in vertisols of scarce rainfall zone in Andhra Pradesh. Sugar Tech 15: 271–277.
Murray, Seth C., William L. Rooney, Martha T. Hamblin, Sharon E. Mitchell, and Stephen Kresovich. 2009. Sweet sorghum genetic diversity and association mapping for brix and height. The Plant Genome 2: 48–62.
Paterson, Andrew H., John E. Bowers, Remy Bruggmann, Inna Dubchak, Jane Grimwood, Heidrun Gundlach, Georg Haberer, Uffe Hellsten, Therese Mitros, and Alexander Poliakov. 2009. The Sorghum bicolor genome and the diversification of grasses. Nature 457: 551–556.
Pecina-Quintero, Víctor., José Luis. Anaya-López, Alfredo Zamarripa-Colmenero, Noe Montes-García, Carlos Nuñez-Colín, Jose Luis Solis-Bonilla, María Rocío. Aguilar-Rangel, and Louis Prom. 2012. Genetic diversity of sweet sorghum germplasm in Mexico using AFLP and SSR markers. Pesquisa Agropecuária Brasileira 47: 1095–1102.
Perrier, X., and J. P. Jacquemoud-Collet. 2006. DARwin software.
Perrier, X.A.F.P., A. Flori, and F. Bonnot. 2003. Data analysis methods. Genetic Diversity of Cultivated Tropical Plants 43: 76.
Ramu, Punna, Claire Billot, Jean-François Rami, S Senthilvel, H D Upadhyaya, L Ananda Reddy, and Charles Tom Hash. 2013. Assessment of genetic diversity in the sorghum reference set using EST-SSR markers. Theoretical and Applied Genetics 126:2051–2064.
Rao, P Srinivasa, S.S. Rao, N. Seetharama, A.V. Umakath, P. Sanjana Reddy, B.V.S. Reddy, and C.L.L. Gowda. 2009. Sweet sorghum for biofuel and strategies for its improvement. International Crops Research Institute for the Semi-Arid Tropics. pp. 80 ISBN: 978-92-9066-518-2
Rao, P Srinivasa, Abhishek Rathore, and Belum V S. Reddy. 2013a. Interrelationship among biomass related traits and their role in sweet Sorghum cultivar productivity in main and ratoon crops. Sugar Tech 15: 278–284.
Rao, P. Srinivasa, A.V. Umakanth, Belum V.S. Reddy, Ismail Dweikat, Sujata Bhargava, C. Ganesh Kumar, Serge Braconnier, and J.V. Patil. 2013b. Sweet sorghum: genetics, breeding and commercialization. Biofuel Crops Production, Physiology and Genetic. CABI, Nosworthy Way 172–198.
Ratnavathi, C.V., K. Suresh, B.S. Vijay Kumar, M. Pallavi, V.V. Komala, and N. Seetharama. 2010. Study on genotypic variation for ethanol production from sweet sorghum juice. Biomass and Bioenergy 34: 947–952.
Ritter, Kimberley B., C. Lynne McIntyre, Ian D. Godwin, David R. Jordan, and Scott C. Chapman. 2007. An assessment of the genetic relationship between sweet and grain sorghums, within Sorghum bicolor ssp. bicolor (L.) Moench, using AFLP markers. Euphytica 157: 161–176. https://doi.org/10.1007/s10681-007-9408-4.
Stuber, Charles W. 1994. Heterosis in plant breeding. Plant Breeding Reviews 12: 227–251.
Wang, Ming L., Chengsong Zhu, Noelle A. Barkley, Zhenbang Chen, John E. Erpelding, Seth C. Murray, Mitchell R. Tuinstra, Tesfaye Tesso, Gary A. Pederson, and Yu. Jianming. 2009. Genetic diversity and population structure analysis of accessions in the US historic sweet sorghum collection. Theoretical and Applied Genetics 120: 13–23.
Yan, Jianbing, Trushar Shah, Marilyn L. Warburton, Edward S. Buckler, Michael D. McMullen, and Jonathan Crouch. 2009. Genetic characterization and linkage disequilibrium estimation of a global maize collection using SNP markers. PLoS ONE 4: e8451.
Zhang, Li-Min., Chuan-Yuan. Leng, Hong Luo, Wu. Xiao-Yuan, Zhi-Quan. Liu, Yu-Miao. Zhang, Hong Zhang, Yan Xia, Li. Shang, and Chun-Ming. Liu. 2018. Sweet sorghum originated through selection of Dry, a plant-specific NAC transcription factor gene. The Plant Cell 30: 2286–2307.
Zheng, Lei-Ying., Xiao-Sen. Guo, Bing He, Lian-Jun. Sun, Yao Peng, Shan-Shan. Dong, Teng-Fei. Liu, et al. 2011. Genome-wide patterns of genetic variation in sweet and grain sorghum (Sorghum bicolor). Genome Biology 12: R114. https://doi.org/10.1186/gb-2011-12-11-r114.
Acknowledgements
The authors acknowledge the financial support received from European Commission through sweet-fuel Project and CGIAR Research Program on Dryland Cereals.
Funding
Funding was provided by Seventh Framework Programme through “Sweetfuel” project (Grant No. 227422).
Author information
Authors and Affiliations
Contributions
SRP and PR conceived and designed research. AK and SRP conducted experiments. PR contributed new reagents or analytical tools. PR and GS analyzed data. AK wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study established the genetic relationship between elite temperate and tropical sweet sorghum germplasm useful for sweet sorghum breeding programs.
Rights and permissions
About this article
Cite this article
Pinnamaneni, S.R., Somanna, A.K.G., Ramu, P. et al. Assessment of Phenotypic and Genotypic Diversity in Elite Temperate and Tropical Sweet Sorghum Cultivars. Sugar Tech 24, 1670–1679 (2022). https://doi.org/10.1007/s12355-022-01117-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-022-01117-3