Abstract
As the wild relative genera of sugarcane, Narenga porphyrocoma and Erianthus rockii are becoming more potential germplasm sources due to valuable traits in sugarcane breeding. Many previous studies were performed to integrate the desirable characters from wild species into modern sugarcane cultivars. Until now, the lack of fertility in hybrids is still a thorny problem in most cases. In our present study, a rare hybrid between E. rockii (2n = 30) and N. porphyrocoma (2n = 30) had been developed for the first time. The molecular primers MSSCIR66, SMC720BS and SMC597CS were successfully applied to identify the putative F1 hybrids. Besides, the chromosome composition and transmission have also been reported to screen the true F1 hybrids via genomic in situ hybridization. From the breeding point of view, the implications of gene introgression from N. porphyrocoma and E. rockii are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane is categorized as the poaceae C4 plant with high photosynthesis efficiency and is one of the most efficient renewable energy crops. Recent years, due to the limited original parents, the hybridization and directional selection of modern sugarcane varieties, it is difficult to make breakthroughs in breeding new sugarcane varieties (D’Hont et al. 1995; Piperidis et al. 2010a, b). At present, there are many problems in sugarcane production in China, such as the single variation, the low heterogeneity among varieties, the poor adaptability, the reduced resistance ability to biotic and abiotic stress factors and so on. To gain more and better agronomic traits, excellent genes of wild species and related genera are introgressed into sugarcane by distant hybridization, which also enhance the genetic base of sugarcane. Sugarcane breeders believe that it is an effective way to solve the problems of similar genetic background and reduce resistance of varieties.
The genera Saccharum, Erianthus, Miscantnus, Narenga and Sclerostachya are important germplasm resources for breakthrough breeding (Singh et al. 2011). Due to its high biomass, strong tillering ability, tolerance to both waterlogging and drought and resistance to diseases and insects, some Erianthus species have become indispensable genetic resources to modern sugarcane cultivars (D’Hont et al. 1995; Piperidis et al. 2000). Erianthus rockii is classified as one of eight species in the Erianthus genus (Cai et al. 2005b) and is a drought and cold tolerant wild relative of sugarcane from China. Narenga porphyrocoma (Hance) Bor is also a wild species of sugarcane, which has many excellent characters, such as precocity, stocky stem, high tillering ability, drought tolerance and mosaic disease resistance (Liu et al. 2018). These advantages are currently being used in sugarcane introgression programs.
Over the past several decades, molecular markers detection and genomic in situ hybridization (GISH) were applied to specifically identify intergeneric hybrids. Since 1999, inter-Alu-like sequences MsCIR2 and EaCIR6 in sugarcane and related species have been developed to identify intergeneric hybrids of Saccharum × Miscanthus and Saccharum × Erianthus (Alix et al. 1999). In addition, the 5S rDNA PCR markers have been used to characterize sugarcane intergeneric hybrids between E. arundinaceus and S. officinarum (D’Hont et al. 1995; Piperidis et al. 2000; Pan et al. 2001). Afterward, more genus-specific microsatellites and AFLP markers have also proved to be efficient in evaluating hybrids from various sugarcane species (Cai et al. 2005a, b; Pan 2006; Liu et al. 2016).
Recent years, genomic in situ hybridization (GISH) is becoming a powerful molecular cytogenetic tool to detect the chromosome composition in interspecific hybrids derived from two or more distinct species, as well as to test genomic recombinant segments and track down the chromosome introgression in allopolyploids. Genomic in situ hybridization was first used to identify hybrids of S. officinarum × S. spontaneum (D’Hont et al. 1996). Using GISH, the chromosome transmission in intergeneric hybrids and backcross progeny has been reported (Piperidis et al. 2010a, b, 2013; Pachakkil et al. 2019). Similarly, chromosome composition and transmission mechanisms were assessed in various cross and backcross generation originated from S. officinarum and E. arundinaceus (Wu et al. 2014; Huang et al. 2015). In other species, GISH analysis was applied effectively to identify the genome constitution, such as tomato, potato and colchicaceous ornamentals (Ji et al. 2004; Pendinen et al. 2012; Kishimoto et al. 2014). Thus, as a widely used molecular cytogenetic technology, GISH will make it possible to provide a reference for efficient utilization in sugarcane breeding strategies.
In this study, F1 intergeneric hybrids were obtained by the hybridization of Erianthus rockii (2n = 30) and Narenga porphyrocoma (2n = 30). The hybrids were characterized by both molecular and cytological methods. In addition, chromosome number identification was performed in order to determine the chromosome transmission in F1 generation during the hybridization process, which can provide a reasonable basis for breeding strategies for further deployment of genes and traits from E. rockii and N. porphyrocoma.
Materials and Methods
Plant Materials and Growth Conditions
In this study, when pollen shedding of Narenga porphyrocoma was about to begin, treated with emasculation technology by hot water at 50 °C for 5 min, nurtured for 2 days in greenhouses. Then fresh pollen of Erianthus rockii was collected at 8:30 every morning and granted artificially to the treated tassels of Narenga porphyrocoma for 5 days. Hybrid tassels were transplanted to the greenhouse until hybrid seeds were produced.
92 F1 hybrids were produced from an intergeneric wild cross between E. rockii (2n = 30, male) and N. porphyrocoma (2n = 30, female). All the F1 plants and their wild parents were grown at the Hainan Sugarcane Breeding Station of Guangdong Bioengineering Institute under natural growth conditions.
Molecular Characterization Using SSR Markers
According to CTAB protocol, genomic DNA was extracted from fresh and young leaves. UV–Vis Spectrophotometer Q5000 (Q105K200, USA) was applied to calculate the absorbance of samples to get high-quality DNA. The 260 nm (A260) and 280 nm (A280) were taken as an important index to judge the DNA quantity; samples with a value of A260/280 between 1.8 and 2.1 should be used to perform the PCR amplification.
The PCR reaction was carried out in a 10 μL mix containing 0.2 mM dNTPs, 10 × TransTaq® HiFi Buffer I, 0.04-0.08 μM primer, 1 unit TransTaq® HiFi DNA Polymerase, 5 μL deionized water and 5 ng DNA extract. According to published primer sequences, the following SSR primers were used: MSSCIR66-F: 5′-AGGTGATTTAGCAGCATA-3′, MSSCIR66-R: 5′-CACAAATAAACCCAATGA-3′, SMC720BS-F: 5′-CGCACCGACGCACGTCT-3′, SMC720BS-R: 5′-GCCAATGGAACGGGTCTA-3′, SMC597CS-F: 5′-GCACACCACTCGAATAACGGAT-3′, SMC597CS-R: 5′- AGTATATCGTCCCTGGCATTCA-3′.
Tprofessional Standard Thermocycler (PE9700, Germany) were used to perform thermal cycling. The PCR amplification procedure was as follows: 5 min at 94 °C; 35 cycles of 30 s at 94 °C, 30 s at 52–64 °C and 1–2 kb/min at 72 °C; 10 min at 72 °C.
By electrophoresis at 100 V, the amplification products were analyzed on 6% agarose gels and then visualized by Goldview™ staining. SSR fragments were photographed using a photo-documentation system WD-9413B (1301320, Beijing, China). Gel-Pro Analyzer Imaging System (Media Cybernetics, USA) was employed to analyze all the photographs. All the clear and reproducible bands were selected to conduct the analysis.
Genomic In Situ Hybridization (GISH) Procedure
According to the method described by D’Hont et al. (1995), GISH experiments were uniformly performed. Genomic DNA from Erianthus rockii was labeled with biotin-16-dUTP (Roche, UK); Avidin D, Rhodamine 600 (XRITC) and biotinylated anti-avidin antibody (Vector Laboratories, CA) were used to detect the signal of biotin-labeled probes. Meanwhile, genomic DNA from Narenga porphyrocoma was labeled with digoxigenin-11-dUTP (Roche, UK), sheep-anti-Digoxin-FITC (Roche, UK) and rabbit-anti-sheep-FITC secondary antibody (Roche, UK) which were used to detect the signal of digoxigenin-labeled probes. Then 4′,6-diamidino-2-phenylindole (DAPI) in a Vectashield anti-fade solution (Vector Laboratories, CA) was applied to counterstain chromosomes. By Axio Scope A1 Imager fluorescent microscope (Carl Zeiss, Germany), the signals from F1 progeny plants were observed. Images were captured assisted by Axio Cam MRc5 and Axio Vision v.4.7 imaging software (Carl Zeiss, Germany).
Results
Main Agronomic Traits of F1 Hybrids
F1 hybrid seedlings were obtained from the Narenga porphyrocoma florets pollinated with Erianthus rockii pollen. In general, the hybrids lacked vigor and were slow in growth and establishment. However, a rare hybrid between E. rockii × N. porphyrocoma survived easily because of the intergeneric vigor of sugarcane in this study.
Characteristics of F1 hybrid seedlings are presented in Table 1. The hybrids resembled the intergeneric vigor in gross morphology, though some of the Narenga porphyrocoma characters were noticeable. Compared with the male and female parents, these hybrid plants were relatively more vigorous, such as plant height, stem diameter, primary panicle length and biomass. The average height of F1 plants was 418.70 cm, while the plant height of N. porphyrocoma and E. rockii was 273.43 cm and 306.80 cm, respectively. The stem diameter of F1 progeny was 0.34 cm on an average; however, the stem diameter of N. porphyrocoma and E. rockii was 0.20 cm and 0.25 cm, respectively. In primary panicle length, the F1 progeny showed significant longer than N. porphyrocoma and E. rockii. The average biomass of F1 progeny was 11.29 kg, while the biomass of N. porphyrocoma and E. rockii was only 2.93 kg and 3.12 kg. In addition, the tiller and internode number of F1 plants were 93.10 and 10.70, compared to N. porphyrocoma which had 169.43 tillers and 5.14 stems, E. rockii which had 45.00 tillers and 12.80 stems. Taken together, these results showed the intergeneric vigor of F1 progeny, and plant height, stem diameter, primary panicle length of F1 plants may influence the biomass.
Anther Characteristics of F1 Hybrids
Anther characteristics of F1 hybrids are presented in Fig. 1. Mature anther was collected from tassels of Narenga porphyrocoma, Erianthus rockii and F1 plants (Fig. 1a, b). The anther length of E. rockii and F1 progeny was significantly larger than that of N. porphyrocoma (Fig. 1d). However, the anther width of F1 progeny was significantly smaller than that of N. porphyrocoma; there was no difference in anther width of N. porphyrocoma and E. rockii (Fig. 1e). In addition, the stigmas were dissected from tassels of N. porphyrocoma, E. rockii and F1 plants (Fig. 1c).
Anther characteristics of Narenga porphyrocoma, Erianthus rockii and F1 progeny. Mature tassels (a), mature anthers (b) and mature anther stigmas (c) of Narenga porphyrocoma, Erianthus rockii and F1 progeny. d The average anther length of Narenga porphyrocoma (n = 68), Erianthus rockii (n = 82) and F1 plants (n = 68). e The average anther width of Narenga porphyrocoma (n = 68), Erianthus rockii (n = 82) and F1 plants (n = 68). Values (d–e) are given as the mean ± SD. **P < 0.01 compared with the wild type by Student’s t test. Bars: 5 mm in (a); 1 mm in (b–c)
The Pollen of F1 Progeny was Sterile
The pollen of Narenga porphyrocoma, Erianthus rockii and F1 progeny were stained with potassium iodide. It has been found that N. porphyrocoma and E. rockii mature pollen were stained to the dark blue. However, F1 pollen was failed in dyeing due to the abortion of pollen (Fig. 2a). To calculate the pollen germination, all the pollen were treated with the culture medium (200 g L−1 sucrose, 400 mg L−1 boric acid, 100 mg L−1 calcium nitrate, 400 mg L−1 magnesium sulfate and 1 g L−1 agar). As a result, N. porphyrocoma and E. rockii mature pollen were almost germinated completely; on the contrary, F1 pollen had no germination rate (Fig. 2b).
Molecular Identification of F1 Hybrids
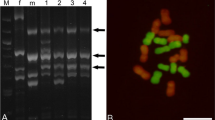
To identify F1 hybrids clearly, 81 simple sequence repeats (SSR) markers were utilized to perform series of molecular experiments. Molecular primers MSSCIR66, SMC720BS and SMC597CS (Fig. 3a–c) were applied to identify F1 generation plants originated from N. porphyrocoma × E. rockii. The hybrids 1–10 represented a unique combination of N. porphyrocoma and E. rockii. Some genus-specific SSR markers were screened to confirm the hybridity of this F1 progeny. As Fig. 3a shows, DNA fragments of F1 hybrids and their wild parents were amplified by MSSCIR66; four SSR fragments were observed in F1 hybrids, which included two fragments from N. porphyrocoma, two fragments from E. rockii. The amplification assay by SMC720BS indicated that there were five SSR fragments in F1 hybrids, including two fragments from N. porphyrocoma and three fragments from E. rockii (Fig. 3b). Similarly, F1 hybrids were distinctly identified by SMC597CS. In F1 hybrids, two SSR fragments were observed, one fragment from N. porphyrocoma, the other one from E. rockii (Fig. 3c). These results indicated that E. rockii and N. porphyrocoma genomes were transmitted to the F1 generation. By the identification of hybridity, F1 hybrids were confirmed as the true hybrids due to sharing the marker profiles of N. porphyrocoma × E. rockii.
Identification of F1 plants between N. porphyrocoma and E. rockii using different molecular primers. Simple sequence repeats (SSR) primers MSSCIR66 (a), SMC720BS (b) and SMC597CS (c) were applied to identify N. porphyrocoma and E. rockii F1 generation plants. M indicated DL2000 DNA Marker, 1–10 indicated N. porphyrocoma and E. rockii F1 generation plants, 11 indicated N. porphyrocoma plants, 12 indicated E. rockii plants in (a–c)
Chromosome Composition of F1 Hybrids by GISH
The character of F1 progeny was analyzed and confirmed by GISH (Fig. 4a–c). It has been reported that there are 2n = 30 chromosomes in N. porphyrocoma and E. rockii. Similar to their parents, hybrids should be diploid individuals possessing 2n = 30 chromosomes. In mitotic metaphase stage, genomic in situ hybridization (GISH) was conducted to analyze the chromosome composition of F1 progeny. As Fig. 4c shows, in F1 generation, 15 chromosomes from N. porphyrocoma were labeled with FITC, while the remaining 15 chromosomes of E. rockii were labeled with Texas red. However, due to the cross hybridization, weak signals were presented.
Genomic in situ hybridization (GISH) in F1 progeny. Genomic in situ hybridization (GISH) analysis was conducted to identify interspecific hybrids of E. rockii × N. porphyrocoma. The transmission of chromosomes were revealed by GISH using total genomic DNA from E. rockii (detected with Texas red, red) and N. porphyrocoma (detected with FITC, green). The white arrowheads showed chromosomes originated from Narenga porphyrocoma. Bars: 5 μm in (a–c) (color figure online)
Discussion
For all these years, Erianthus rockii and Narenga porphyrocoma have always been regarded as potential sources for various important traits, such as multi-ratoon ability, tolerance to environmental stresses, vigor and high biomass production (Piperidis et al. 2000; Jackson and Henry 2011; Liu et al. 2018). Previous studies indicated that the crosses between Saccharum and Erianthus had been limited due to the high genetic distance and consequent low compatibility of the two genera. In addition, the past experience showed that hybrid identification based on morphological traits could not satisfy the development of modern breeding. Along with the increasingly difficulty in identifying true hybrids among the sugarcane progenies, various types of molecular markers were developed and applied for the identification of intergeneric hybrids. As Erianthus-specific markers, Inter-Alu sequences, 5S ribosomal RNA gene markers, RAPD markers and SSR markers have been reported and successfully utilized to identify the hybrids of Saccharum × Erianthus (Piperidis et al. 2000; Pan 2006; Nair et al. 2006; Aitken et al. 2007; Liu et al. 2016). Especially SSR markers, as Erianthus-specific molecular primers, MSSCIR66, SMC720BS and SMC597CS (Fig. 3a–c), were applied to identify F1 progeny of N. porphyrocoma and E. rockii in our study. Considering the process of sugarcane breeding in future, we confirmed that hybrids originated from F1 progeny of N. porphyrocoma × E. rockii would be efficiently identified by the applications of different molecular markers.
Along with the development of genomic DNA in situ hybridization, the distinction between chromosomes of E. rockii and N. porphyrocoma has become possible, which provides a detailed description of the genomic composition of the hybrids. Various types of chromosome transmission were reported in sugarcane, such as n + n, n + 2n, 2n + n and 2n + 2n (Burner and Legendre 1993; Piperidis et al. 2010a; Deng et al. 2010; Hermann et al. 2012). As the common type of chromosome transmission, n + n transmission at F1 stage had been generally reported (D’Hont et al. 1995; Piperidis et al. 2000, 2010b). It was reported that the chromosome number of E. rockii and N. porphyrocoma were both 2n = 30. In our research, genomic in situ hybridization was performed; the chromosome number of the F1 progeny was found to be 30 (Fig. 4a–c), which largely conformed to n + n transmission. Then we found that 15 chromosomes were originated from E. rockii, and the other 15 chromosomes were from N. porphyrocoma (Fig. 4c); the hybrid already had the genome of E. rockii and N. porphyrocoma following n + n transmission at F1 stage, which could possibly keep the genomic balance. However, as Fig. 4c shows, the red color from F1 hybrids is not so red comparing to E. rockii; the green color from F1 hybrids is not so green as N. porphyrocoma. As we know, Erianthus rockii and Narenga porphyrocoma both belong to related genus of sugarcane; we assume that presented weak signals may be caused by the close genetic relationship. Additionally, the mutual influence effect of both colors may also weaken the fluorescence signals.
As different subtribes of Andropogoneae, intergeneric hybrids crossed by sorghum and sugarcane were morphologically similar to the sugarcane parent, but lacked vegetative vigor (Nair 1999). According to our present results, F1 hybrids showed more significant vigor, such as plant height, stem diameter, primary panicle length and biomass (Table 1). Considering its high yield potential, hybrids derived from both E. rockii and N. porphyrocoma sources revealed the growing importance as a potential genetic stock in modern hybridized breeding. It has been reported that intergeneric hybrids between E. arundinaceus and S. spontaneum were produced and utilized as the potential genetic germplasm. Previous studies showed that F1 and BC1 progenies of sugarcane (Saccharum spp.) and intergeneric hybrid complex (E. arundinaceus × S. spontaneum) were superior to their parents (Gao et al. 2012; Liu et al. 2012), which provided theoretical bases to improve sugarcane cultivars by superior genes in hybrid complex in introgression breeding program. In our subsequent studies, due to the sterility of F1 pollen, F1 hybrids were regarded as female parent and had been crossed with ROC22, Liucheng 05-136 to develop new varieties with better adaptability and productivity.
References
Aitken, K., J. Li, L. Wang, C. Cai, Y.H. Fan, and P. Jackson. 2007. Characterization of intergeneric hybrids of Erianthus rockii and Saccharum using molecular markers. Genetic Resources and Crop Evolution 54: 1395–1405.
Alix, K., F. Paulet, J. Glaszmann, and A. D’Hont. 1999. Inter-Alu-like species-specific sequences in the Saccharum complex. Theoretical and Applied Genetics 99 (6): 962–968.
Burner, D.M., and B.L. Legendre. 1993. Chromosome transmission and meiotic stability of sugarcane (Saccharum spp.) Hybrid Derivatives. Crop Science 33 (3): 600–606.
Cai, Q., K.S. Aitken, H.H. Deng, X.W. Chen, C. Fu, P. Jackson, and C.L. McIntyre. 2005a. Verification of the introgression of Erianthus arundinaceus germplasm into sugarcane using molecular markers. Plant Breeding 124: 322–328.
Cai, Q., K.S. Aitken, Y.H. Fan, G. Piperidis, P. Jackson, and C.L. McIntyre. 2005b. A preliminary assessment of the genetic relationship between Erianthus rockii and the ‘Saccharum Complex’ using microsatellite and AFLP markers. Plant Science 169: 976–984.
Deng, Z.H., M.Q. Zhang, W.L. Lin, F. Cheng, C.M. Zhang, Y.C. Li, L.P. Lai, Y.Q. Lin, and R.K. Chen. 2010. Analysis of disequilibrium hybridization in hybrid and backcross progenies of Saccharum officinarum × Erianthus arundinaceus. Agricultural Sciences in China 9 (9): 1271–1277.
Dhont, A., L. Grivet, P. Feldmann, S. Rao, N. Berding, and J.C. Glaszmann. 1996. Characterisation of the double genome structure of modern sugarcane cultivars (Saccharum spp.) by molecular cytogenetics. Molecular and General Genetics 250 (4): 405–413.
D’Hont, A., P.S. Rao, P. Feldmann, N. Berding, and J.C. Glaszmann. 1995. Identification and characterization of sugarcane intergeneric hybrids, Saccharum officinarum × Erianthus arundinaceus, with molecular markers and DNA in situ hybridization. Theoretical and Applied Genetics 91: 320–326.
Gao, Y.J., F.X. Fang, X.H. Liu, R.H. Zhang, H.Z. Song, R.Z. Yang, T. Luo, W.X. Duan, J.H. You, and G.M. Zhang. 2012. Identification of progeny from crosses between sugarcane (Saccharum spp.) and intergeneric hybrid complex (Erianthus arundinaceus × Saccharum spontaneum) with molecular markers. Journal of Plant Genetic Resources 13 (5): 912–916.
Hermann, S.R., K.S. Aitken, P.A. Jackson, A.W. George, N. Piperidis, X. Wei, A. Kilian, and F. Detering. 2012. Evidence for second division restitution as the basis for 2n + n maternal chromosome transmission in a sugarcane cross. Euphytica 187 (3): 359–368.
Huang, Y.J., H.Y. Wu, P. Wang, Y.Q. Lin, C. Fu, Z.H. Deng, Q.N. Wang, Q.W. Li, R.K. Chen, and M.Q. Zhang. 2015. Characterization of chromosome inheritance of the intergeneric BC2 and BC3 Progeny between Saccharum spp. and Erianthus arundinaceus. PLoS ONE 10 (7): e0133722.
Jackson, P., and R.J. Henry. 2011. Erianthus. In Kole C (ed), Wild crop relatives: genomic and breeding resources, industrial crops. Berlin: Springer 97–107.
Ji, Y., R. Pertuze, and R.T. Chetelat. 2004. Genome differenciation by GISH in interspecific and intergeneric hybrids of tomato and related nightshades. Chromosome Research 12: 107–116.
Kishimoto, T., M. Yamakakawa, D. Nakazawa, J. Amano, S. Kuwayama, and M. Nakano. 2014. Meiotic chromosome pairing in intergeneric hybrids of colchicaceous ornamentals revealed by genomic in situ hybridization (GISH). Euphytica 200 (2): 251–257.
Liu, X.H., F.X. Fang, Y.J. Gao, R.H. Zhang, H.Z. Song, R.Z. Yang, W.K. Fang, W.X. Duan, T. Luo, G.M. Zhang, and Y.R. Li. 2012. Identification and genetic analysis of hybrid from cross between Erianthus arundinaceus and Saccharum spontaneum. Acta Agronomica Sinica 38 (05): 914–920.
Liu, X.H., R.H. Zhang, H.P. Ou, Y.Y. Gui, J.J. Wei, H. Zhou, H.W. Tan, and Y.R. Li. 2018. Comprehensive transcriptome analysis reveals genes in response to water deficit in the leaves of Saccharum narenga (Nees ex Steud.) hack. BMC Plant Biology 18 (1): 250.
Liu, X.L., X.J. Li, C.H. Xu, X.Q. Lin, and Z.H. Deng. 2016. Genetic diversity of populations of Saccharum spontaneum with different ploidy levels using SSR molecular markers. Sugar Tech 18 (4): 365–372.
Nair, N.V. 1999. Production and cyto-morphological analysis of intergeneric hybrids of Sorghum × Saccharum. Euphytica 108: 187–191.
Nair, N.V., A. Selvi, T.V. Sreenivasan, K.N. Pushpalatha, and S. Mary. 2006. Characterization of intergeneric hybrids of Saccharum using molecular markers. Genetic Resources and Crop Evolution 53 (1): 163–169.
Pachakkil, B., Y. Terajima, N. Ohmido, M. Ebina, S. Irei, H. Hayashi, and H. Takagi. 2019. Cytogenetic and agronomic characterization of intergeneric hybrids between Saccharum spp. hybrid and Erianthus arundinaceus. Scientific Reports 9 (1): 1748.
Pan, Y.B. 2006. Highly polymorphic microsatellite DNA markers for sugarcane germplasm evaluation and variety identity testing. Sugar Tech 8 (4): 246–256.
Pan, Y.B., D.M. Burner, and Q. Wei. 2001. Developing species-specific DNA markers to assist in sugarcane breeding. Proceedings International Society of Sugar Cane Technologists 24 (II): 337–342.
Pendinen, G., D.M. Spooner, J. Jiang, and T. Gavrilenko. 2012. Genomic in situ hybridization reveals both auto- and allopolyploid origins of different North and Central American hexaploid potato (Solanum sect. Petota) species. Genome 55: 407–415.
Piperidis, G., M.J. Christopher, B.J. Carroll, N. Berding, and A. D’Hont. 2000. Molecular contribution to selection of intergeneric hybrids between sugarcane and the wild species Erianthus arundinaceus. Genome 43 (6): 1033–1037.
Piperidis, G., N. Piperidis, and A. D’Hont. 2010a. Molecular cytogenetic investigation of chromosome composition and transmission in sugarcane. Molecular Genetics and Genomics 284: 65–73.
Piperidis, N., K. Aitken, and S. Hermann. 2013. Towards a reliable method to select potentially high value Erianthus hybrids. International Sugar Journal 115 (1379): 794–799.
Piperidis, N., J. Chen, H. Deng, L. Wang, P. Jackson, and G. Piperidis. 2010b. GISH characterization of Erianthus arundinaceus chromosomes in three generations of sugarcane intergeneric hybrids. Genome 53 (5): 331–336.
Singh, R.K., R.B. Singh, S.P. Singh, and M.L. Sharma. 2011. Identification of sugarcane microsatellites associated to sugar content in sugarcane and transferability to other cereal genomes. Euphytica 182: 335–354.
Wu, J.Y., Y.J. Huang, Y.Q. Lin, C. Fu, S.M. Liu, Z.H. Deng, Q.W. Li, Z.X. Huang, R.K. Chen, and M.Q. Zhang. 2014. Unexpected inheritance pattern of Erianthus arundinaceus chromosomes in the intergeneric progeny between Saccharum spp. and Erianthus arundinaceus. PLoS ONE 9 (10): e110390.
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (31701488), Guangdong Provincial Team of Technical System Innovation for Sugarcane Sisal Industry (2019KJ104-02), Guangdong Provincial Key Lab of Sugarcane Improvement & Biorefinery (2017B030314123) and Province and Ministry Co-sponsored Collaborative Innovation Center of Sugar Industry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chang, H., Wang, Q., Qiu, Y. et al. Production, Identification and Characterization of Erianthus rockii × Narenga porphyrocoma Intergeneric Hybrids as a New Germplasm for Sugarcane Breeding and Genetic Research. Sugar Tech 22, 389–395 (2020). https://doi.org/10.1007/s12355-020-00804-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-020-00804-3