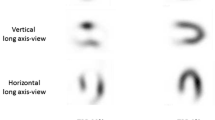Abstract
Purpose
Semi-quantitative scores can be used as an adjunct to visual assessment in rubidium-82 positron emission tomography (82Rb PET). The semi-quantitative cut-off values used in 82Rb PET are derived from single-photon emission computed tomography (SPECT). It is unknown whether these cut-off values can be extrapolated to 82Rb PET. We compared the semi-quantitative with the visual assessment of ischemia and determined which summed difference score (SDS) score predicts ischemia best.
Methods
We included 108 patients who underwent 82Rb PET imaging and performed visual and semi-quantitative assessment. A scan with a SDS ≥ 2 and a summed stress score (SSS) ≥ 4 was considered to demonstrate ischemia. We compared the semi-quantitative with the visual assessment.
Results
41 (38%) Normal scans, and 67 (62%) scans with ischemia and/or an irreversible defect were included. The semi-quantitative assessment showed ischemia more often than the visual assessment (51% vs 29%, P < .001). Patients with a low or intermediate pre-test probability of coronary artery disease (CAD) and a SDS < 4 did not demonstrate ischemia by visual assessment.
Conclusion
Semi-quantitative assessment in 82Rb PET imaging clearly demonstrates the presence of ischemia. Ischemia is unlikely in patients with low and intermediate pre-test probability of CAD and a SDS < 4.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-invasive imaging with rubidium-82 positron emission tomography (82Rb PET) is increasingly used for the functional assessment of coronary artery disease (CAD).1 PET myocardial perfusion imaging has a high sensitivity and specificity for the detection of CAD using both qualitative (visual) as well as semi-quantitative assessment (summed rest score, SRS summed stress score, SSS and summed difference scores, SDS).2,3,4 Current guidelines recommend that automated semi-quantitative analysis can be used as an adjunct to visual assessment.5,6 In 82Rb PET imaging, a SSS ≥ 4 and a SDS ≥ 2 are considered abnormal and indicative for reversible and fixed perfusion defects. These cut-off values for abnormality are primarily derived from previous single-photon emission computed tomography (SPECT) studies.7,8,9,10 Previous studies showed that semi-quantitative SPECT analysis is reproducible and provides similar diagnostic accuracy to visual assessment.11,12,13 However, since 82Rb PET has higher spatial resolution and a superior diagnostic performance when compared to SPECT imaging, it is unknown whether the cut-off values of semi-quantitative SPECT scanning can be extrapolated to 82Rb PET imaging.2,3,4,10 If the semi-quantitative approach has a high sensitivity for the assessment of ischemia and does not assess an abnormal scan as normal incorrectly, the semi-quantitative approach may be useful in the initial interpretation of 82Rb PET scans. The semi-quantitative approach may possibly contribute to automatic scan assessment in the future.
Our aim was to determine the value of semi-quantitative scores in 82Rb PET imaging. We compared semi-quantitative assessment of ischemia using a SDS ≥ 2 and SSS ≥ 4 as cut-off value, with visual assessment of ischemia in 82Rb PET imaging. We investigated whether semi-quantitative assessment of ischemia differentiates between the presence or absence of ischemia. Furthermore, we tried to determine a semi-quantitative cut-off score that predicts ischemia.
Methods
Study Population
We retrospectively included 108 patients with suspected stable CAD who underwent 82Rb PET imaging in Isala Hospital, Zwolle, The Netherlands. All patients underwent 82Rb PET between August 2017 and February 2018. We wanted to analyze the accuracy of the semi-quantitative scoring in both scans with and without perfusion defects. Hence, we included 41 (38%) consecutive patients with a normal scan and 67 (62%) consecutive patients with an abnormal scan. The abnormal scans showed either ischemia (36%), an irreversible defect (42%) or both ischemia and an irreversible defect (22%) (Table 1). All patients provided written consent for the use of their data for research purposes. Because the patients neither were subject to research procedures nor were required to follow rules of behavior, the Accredited Committee on Research Ethics of our Hospital (Isala, Zwolle) decided that the study did not fall under the scope of the Medical Research Involving Human Subjects Act in Dutch law.
Rubidium-82 PET Acquisition
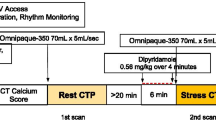
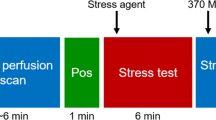
All patients were asked to discontinue dipyridamole containing medications for 48 hours before the scan. They were asked to abstain from caffeine containing medication, food and drinks 24 hours before imaging. Prior to PET imaging a single low-dose computed tomography (CT) (120 kV and 20 mA) was acquired during free-breathing to provide an attenuation map of the chest. Next, 740 MBq 82Rb was administered with a flow of 50 mL·min−1 using a 82Sr/82Rb generator (CardioGen-82, Bracco Diagnostics, Inc.).14 A 7 minute acquisition was started immediately after the administration of 82Rb using a Discovery 690 PET/CT scanner (GE Healthcare). Ten minutes after the start of the rest scan, stress was pharmacologically induced after injecting 400 µg regadenoson in 5 mL, which was administered over 15 seconds. After a flush with saline (5 ml NaCl 0.9%) over 10 seconds, again 740 MBq was administered for the stress scan which took 7 minutes. Attenuation correction was applied to all data on the PET system after co-registration of the CT and PET data.
Data Processing
We reconstructed the relative perfusion images using the data acquired between 2:30 and 7:00 minutes for both the rest and stress scan. The default settings as recommended by the manufacturer were used for the reconstruction: 3D iterative reconstruction with 2 iterations and 24 subsets. The reconstructed images were post-processed using Corridor4DM (INVIA Medical Imaging Solutions). After alignment of the PET images with the myocardium contours, the software automatically calculated the SSS, the SRS and the SDS, using a 17-segment bull’s eye model and enabled correction of these scores by manual adjustment if required. The semi-quantitative scores were related to the Philips 4DM-PET normalcy database (INVIA Medical Imaging Solutions).8,15 Scans were displayed in the traditional short, vertical long, and horizontal long axes and the corresponding bulls eyes using the 17-segment model and reviewed using the ‘Cool’ color scale.16
Reproducibility Visual and Semi-quantitative Assessment
We assessed the intra- and inter-observer variability of the visual analysis. One pair of expert readers (a cardiologist and a nuclear medicine physician) visually interpreted the 82Rb PET results of all patients twice to determine the intra-observer variability. For determination of inter-observer variability, another pair of expert readers assessed the same 82Rb PET scans.
We also wanted to assess the reproducibility of the semi-quantitative 82Rb PET analysis. Therefore, we performed an interim analysis of the first 51 scans that were included in the study. To evaluate the intra-technologist variability, one experienced technologist processed the scans twice (‘Semi-quantitative A’). Another technologist processed the data as well in order to assess the inter-technologist variability (‘Semi-quantitative B’). The technologists were blinded to the clinical condition of the patient and data sets were presented in a random order.
Visual vs Semi-quantitative Assessment
Semi-quantitative scores were compared to the visual assessment. Visual assessment was performed by consensus of one experienced nuclear medicine physician and one experienced cardiologist. All scans were visually interpreted using a 17-segment model, all segments were interpreted using a 5 point scoring system (0 = visually normal, 5 = visually no tracer uptake). The scans were categorized as normal or abnormal. An abnormal scan could demonstrate ischemia, an irreversible defect or both, irrespective of the segment. The semi-quantitative scores were categorized as well. For the distinction between ‘normal’ and ‘abnormal scans’, scans with a SSS ≥ 4 were all considered abnormal. An abnormal scan could demonstrate either ischemia, an irreversible defect, or both a reversible and irreversible defect. We considered a SDS ≥ 2 with a SSS ≥ 4 as cut-off values for the assessment of ischemia.8,9,10 In this way, all scans were categorized as normal or abnormal. Abnormal scans were categorized as demonstrating ischemia or an irreversible defect or both (Table 2).
We then compared the visual assessment to the semi-quantitative assessment of ischemia. Furthermore, we determined the lowest SDS score that predicted ischemia by visual assessment.
Since most patients undergoing 82Rb PET imaging are patients with a low or intermediate pre-test probability of CAD, we performed a subgroup analysis of all patients with a low or intermediate pre-test probability of CAD. We compared visual assessment with semi-quantitative assessment in this subgroup and determined what SDS score was related to a scan demonstrating ischemia by visual assessment.
Statistics
All statistical analyses were performed using IBM SPSS Statistics 24.0. Patient characteristics were computed as mean ± standard deviation or as percentage. Continuous variables were compared using an unpaired Student’s t-test or a non-parametric Mann-Whitney U-test. Normal distribution was verified using the test of normality. Categorical variables were compared using Pearson’s χ2 test. Intra- and inter-observer variability were determined with Cohen’s κ. A κ of 0.6-0.8 was categorized as good, a κ > 0.8 as excellent.17 The differences in outcome between the visual assessment and semi-quantitative scores were compared using McNemar test. The level of statistical significance was set at 0.05.
Results
Study Population
Of all 108 included patients, 74 (69%) were male. Mean age was 68 ± 11 years. Table 1 demonstrates the baseline characteristics and scan outcomes of all 108 patients. 56 (52%) Patients had a cardiac history of myocardial infarction, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Some patients had a history of both a PCI and CABG or myocardial infarction.
Reproducibility Visual and Semi-quantitative Assessment
Intra- and inter-observer reproducibility of the visual assessment of a normal scan demonstrated a κ of 0.93 and 0.91 respectively. The intra- and inter-observer agreement for the qualitative assessment of a scan with ischemia (ischemia: SSS ≥ 4 and SDS ≥ 2) was 0.84 and 0.76 respectively.
The intra- and inter-technologist reproducibility of the semi-quantitative assessment of ischemia demonstrated a κ of 0.80 and 0.84, respectively. The intra- and inter-technologist reproducibility of the semi-quantitative assessment of a normal scan demonstrated a κ of 0.94 and a kappa of 0.88, respectively. As these interim analyses of the first 51 scans showed that intra- and inter-technologist reproducibility was excellent (κ ≥ 0.80), an additional analysis of the reproducibility with the remaining 57 scans was considered unnecessary. One technologist (Semi-quantitative A) therefore processed the remaining 57 scans.
Visual vs Semi-quantitative Assessment
The semi-quantitative outcomes more often demonstrated ischemia, n = 55 (51%) than the visual assessment, n = 31 (29%), as shown in Table 3 (P < .001). Of the 55 scans that demonstrated ischemia by semi-quantitative assessment, 25 (45%) did not demonstrate ischemia by visual assessment. Of the 53 scans that demonstrated no ischemia by semi-quantitative assessment, 1 (1.9%) scan demonstrated ischemia by visual assessment. This scan demonstrated a large irreversible defect both by visual and by semi-quantitative assessment (SSS = 8, SRS = 10, SDS = 1). In the visual assessment, the interpreters found an additional small reversible defect on this scan. Figure 1 demonstrates two examples of discordant scans.
Mean SDS was 11 ± 5.8 in all scans with ischemia by visual assessment, whereas mean SDS was 1.7 ± 2.3 in all scans without ischemia by visual assessment (P < .001). Figure 2A demonstrates the SDS scores in scans with and without ischemia by visual assessment. The maximum SDS score found in a normal scan (e.g., without ischemia) was 11. The minimum SDS score that was found in a scan with ischemia by visual assessment was a SDS of 1. This scan with a SDS value of 1, was a scan with both ischemia and a large irreversible defect.
We performed a subgroup analysis of all patients with low or intermediate pre-test probability of CAD. In this population, the minimum SDS score found in a scan demonstrating ischemia by visual assessment was a SDS score of 4. This is demonstrated in Figure 2B.
Follow-up
We assessed 1-year follow-up of death due to a possible cardiac event, invasive coronary angiography, PCI or CABG. A total of 24 patients (22%) underwent invasive coronary angiography within 1 year after PET. Of these 24 patients, 2 patients did not have obstructive CAD by invasive angiography, 3 patients underwent changes in their medication, 12 patients underwent PCI and 7 patients underwent CABG. Of all 24 patients who underwent invasive angiography within 1 year, consensus was found between visual and semi-quantitative assessment in 19 patients (79%). The 5 patients with a discrepancy in visual and semi-quantitative assessment all demonstrated ischemia by semi-quantitative assessment but were considered normal by visual assessment.
Furthermore, 4 patients (3.7%) deceased because of a possible cardiac event within 1 year of follow-up. These patients all demonstrated ischemia by semi-quantitative and visual assessment of the PET scan.
Discussion
82Rb PET perfusion imaging is a highly sensitive and specific non-invasive method to assess myocardial ischemia in patients with suspected significant CAD. When interpreting PET perfusion scans, it is advised to use both visual assessment of myocardial perfusion as well as semi-quantitative perfusion measures such as SSS, SRS and SDS scores. Our study showed that the use of semi-quantitative measures clearly differentiates between either the presence and the absence of ischemia. In patients with a low of intermediate pre-test probability of CAD, ischemia was not found in scans with a SDS < 4. Especially in this population, semi-quantitative scoring with 82Rb PET perfusion imaging could be very valuable in the initial assessment of CAD.
Furthermore, the intra- and inter-observer variation of SDS scores in 82Rb PET is excellent. This latter finding is in agreement with previous studies after the repeatability of semi-quantitative assessment of ischemia with myocardial perfusion imaging.10,18
Previous 82Rb PET studies used cut-off values for ischemia (SSS ≥ 4 and SDS ≥ 2), which are derived from previous SPECT studies.7,8,9,10 82Rb PET has a higher spatial resolution compared to SPECT which enables the detection of smaller and more subtle perfusion defects.2,4,10 We reasoned that the higher spatial resolution of 82Rb PET imaging might result in a lower specificity for the detection of ischemia by semi-quantitative scoring when using the same cut-off values for ischemia as in SPECT imaging. We compared the semi-quantitative assessment of ischemia with the visual assessment of ischemia in 82Rb PET imaging. We demonstrated a big discrepancy when comparing our visual assessment of ischemia with our semi-quantitative assessment of ischemia using SSS ≥ 4 and SDS ≥ 2 as cut-off values. Our results show an overestimation of ischemia using these semi-quantitative cut-off values. Suggesting that semi-quantitative scoring has a low specificity for the detection of ischemia using these cut-off values. Specificity for the detection of ischemia might be higher using different semi-quantitative cut-off values. Sensitivity for the detection of ischemia with these semi-quantitative cut-off values was high, with only one scan demonstrating ischemia by semi-quantitative assessment without ischemia by visual assessment.
The consequence of selecting a different semi-quantitative cut-off value for the assessment of ischemia influences the sensitivity and the specificity. We analyzed the minimum SDS score associated with ischemia by visual assessment. In the whole cohort, a SDS of 1 was associated with ischemia by visual assessment.
In our subgroup analysis of patients with a low or intermediate pre-test probability of CAD the minimum SDS score associated with ischemia by visual assessment was a SDS of 4. Possibly, in patients with a low or intermediate pre-test probability of CAD, a SSS ≥ 4 and SDS ≥ 4 can be used as cut-off values for the detection of ischemia. This subgroup analysis is relevant, since the majority of patients referred for 82Rb PET imaging are patients without a cardiac history of CAD and with a low or intermediate likelihood of obstructive CAD. It is interesting considering the possibility to assess 82Rb PET scans of a relatively low risk population only by semi-quantitative assessment without further visual assessment.
We performed 1-year follow-up of death due to a possibly cardiac event, invasive angiography and PCI or CABG. A total of 24 patients underwent invasive angiography and 4 patients deceased because of a possible cardiac event. None of these patients demonstrated ischemia visually but demonstrated no ischemia by semi-quantitative assessment. In the whole population, one scan without ischemia by semi-quantitative assessment using SSS ≥ 4 and SDS ≥ 2 as cut-off value, demonstrated ischemia visually. This was a scan with a large irreversible defect (SSS 8, SDS 1, SRS 10). The ischemia found in this patient visually was clinically not relevant as no additional invasive angiography was performed nor did myocardial death occur within 1 year of follow-up. These findings suggest that the semi-quantitative assessment does not consider a scan non-ischemic incorrectly with clinical consequences.
Limitations
We wanted to assess whether the semi-quantitative scoring does not assess an abnormal scan as normal incorrectly, therefore, we selected a population with normal and abnormal scans (62%). In daily practice, mostly patients a low or intermediate pre-test probability of CAD are referred for 82Rb PET imaging and we expect the percentage of patients with an abnormal scan to be much lower in comparison to our study population. Making semi-quantitative scoring even more applicable, especially in patients with a low-intermediate pre-test likelihood.
The study focused on the comparison of semi-quantitative and visual assessment of ischemia and we did not use invasive angiography as reference. Even though visual assessment has lower validity compared to invasive angiography, most laboratories use visual analysis for the assessment of ischemia. We believe that the semi-quantitative approach may be very useful in this initial assessment of ischemia by non-invasive imaging. Therefore, we compared the semi-quantitative assessment with the frequently used visual assessment. To increase the power of the results we assessed the intra- and inter-observer reproducibility of the visual assessment of ischemia, which was good and in line with previous studies.11,13 Furthermore, we performed 1-year follow-up. In the patients with invasive angiography or death due to a possible cardiac event within 1 year of follow-up, the semi-quantitative assessment did not assess the scans as normal incorrectly.
We also did not compare semi-quantitative measurements with absolute myocardial blood flow measurements. Previous research demonstrated that myocardial blood flow analysis has an additional prognostic factor to visual assessment of myocardial perfusion imaging.19 However, even though myocardial blood flow quantification is feasible with 82Rb PET, due to intrinsic limitations of the tracer it is less reliable and not often used in clinical practice.10
Conclusion
Semi-quantitative assessment of ischemia in 82Rb PET imaging differentiates between the presence and absence of ischemia in 82Rb PET imaging. Overestimation of ischemia assessed by semi-quantitative assessment was found using SSS ≥ 4 and SDS ≥ 2 as cut-off values. However, our study demonstrates a high sensitivity of the semi-quantitative assessment of ischemia using SSS ≥ 4 and SDS ≥ 2 as cut-off values. In particular, in patients with a low or intermediate pre-test probability of CAD semi-quantitative scoring could be very valuable in the initial assessment of ischemia by 82Rb PET. Ischemia is unlikely in this population when SSS and SDS are < 4. This knowledge may be useful for trainees or inexperienced readers and may possibly contribute to automatic scan assessment in future.
New Knowledge Gained
The frequently used cut-off values for the assessment of ischemia by semi-quantitative assessment with 82Rb PET are a SSS ≥ 4 and SDS ≥ 2. Using these cut-off values, semi-quantitative scoring has a high sensitivity for the assessment of ischemia and a low specificity for the detection of ischemia. Overestimation of ischemia using these semi-quantitative scores is related to the cut-off values used. Especially in patients with a low or intermediate pre-test probability of CAD overestimation was demonstrated. Possibly, we should determine new-semi-quantitative cut-off values for the assessment of ischemia by 82Rb PET imaging. A SDS < 4 was not associated with ischemia by visual assessment in patients with a low or intermediate pre-test probability of CAD. Maybe the cut-off value SSS ≥ 4 and SDS ≥ 4 predicts ischemia better in this population.
Abbreviations
- 82Rb PET:
-
Rubidium-82 positron emission tomography
- SPECT:
-
Single-photon emission tomography
- CAD:
-
Coronary artery disease
- SDS:
-
Summed difference score
- SSS:
-
Summed stress score
- SRS:
-
Summed rest score
- CT:
-
Computed tomography
- PCI:
-
Percutaneous coronary intervention
- CABG:
-
Coronary artery bypass grafting
References
Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C. ESC guidelines on the management of stable coronary artery disease: The Task Force on the Management of Stable Coronary Artery Disease of the European Society of Cardiology. Eur Heart J 2013;2013:2949‐3003.
McArdle BA, Dowsley TF, deKemp RA, Wells GA, Beanlands RS. Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease? A systematic review and meta-analysis. J Am Coll Cardiol 2012;60:1828‐37.
Takx RA, Blomberg BA, El Aidi H, Habets J, de Jong PA, Nagel E, et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging 2015;8:e002666.
Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: A meta-analysis. J Am Coll Cardiol 2012;59:1719‐28.
Dorbala S, Ananthasubramaniam K, Armstrong IS, Chareonthaitawee P, DePuey EG, Einstein AJ, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: Instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol 2018. https://doi.org/10.1007/s12350-018-1283-y.
Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol 2016. https://doi.org/10.1007/s12350-016-0522-3.
Slomka PJ, Nishina H, Berman DS, Akincioglu C, Abidov A, Friedman JD, et al. Automated quantification of myocardial perfusion SPECT using simplified normal limits. J Nucl Cardiol 2005;12:66‐77.
Kaster T, Mylonas I, Renaud JM, Wells GA, Beanlands RSB, deKemp RA. Accuracy of low-dose rubidium-82 myocardial perfusion imaging for detection of coronary artery disease using 3D PET and normal database interpretation. J Nucl Cardiol 2012;19:1135‐45.
Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740‐8. https://doi.org/10.1016/j.jacc.2011.01.065.
Driessen RS, Raijmakers PG, Stuijfzand WJ, Knaapen P. Myocardial perfusion imaging with PET. Int J Cardiovasc Imaging 2017;33:1021‐31.
Driessen RS, Raijmakers PG, Danad I, Stuijfzand WJ, Schumacher SP, Leipsic JA, et al. Automated SPECT analysis compared with expert visual scoring for the detection of FFR-defined coronary artery disease. Eur J Nucl Med Mol Imaging 2018;45:1091‐100.
Lane Duvall W, Slomka PJ, Gerlach JR, Sweeny JM, Baber U, Croft LB, et al. High-efficiency SPECT MPI: Comparison of automated quantification, visual interpretation, and coronary angiography. J Nucl Cardiol 2013;20:763‐73.
Xu Y, Hayes S, Ali I, Ruddy TD, Wells RG, Berman DS, et al. Automatic and visual reproducibility of perfusion and function measures for myocardial perfusion SPECT. J Nucl Cardiol 2010;17:1050‐7.
Huizing ED, van Dijk JD, van Dalen JA, Timmer JR, Arkies H, Slump CH, et al. Minimizing rubidium-82 tracer activity for relative PET myocardial perfusion imaging. Nucl Med Commun 2017;38:708‐14.
Kaster T, Mylonas I, Renaud JM, et al. Clinical interpretation standards and quality assurance for the multicenter PET/CT trial rubidium-ARMI. J Nucl Med 2014;55:58‐64.
Berman DS, Abidov A, Kang X, Hayes SW, Friedman JD, Sciammarella MG, et al. Prognostic validation of a 17-segment score derived from a 20-segment score for myocardial perfusion SPECT interpretation. J Nucl Cardiol 2004;11:414‐23.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159‐74.
Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol 2007;49:1052‐8.
Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215‐24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
N.B. Borren, T.J. Gerritse, J.P. Ottervanger, M. Mouden, J.R. Timmer, J.A. van Dalen, P.L. Jager and J.D. van Dijk has no conflicts of interest and/or funding to declare that are relevant to the contents of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borren, N.M., Gerritse, T.J., Ottervanger, J.P. et al. Semi-quantitative assessment of ischemia with rubidium-82 PET myocardial perfusion imaging. J. Nucl. Cardiol. 29, 3155–3162 (2022). https://doi.org/10.1007/s12350-021-02884-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-021-02884-4