Abstract
Purpose
Sarcoidosis is a multi-systemic inflammatory disease of unknown etiology. Cardiac sarcoidosis (CS) has been reported in as much as 25% of patients with systemic involvement. 18Fluorodeoxyglucose (FDG) positron emission tomography (PET) has a high diagnostic sensitivity/specificity in the diagnosis of CS. The aim of this review is to summarize evidence on the prognostic role of FDG PET.
Methods
Studies were identified by searching MEDLINE from inception to October 2020. Medical subject headings (MeSH) terms for sarcoidosis; cardiac and FDG PET imaging were used. Studies of any design assessing the prognostic role of FDG PET in patients with either suspected or confirmed cardiac sarcoidosis imaging done at baseline were included. Abnormal PET was defined as abnormal metabolism (presence of focal or focal-on-diffuse uptake of FDG) OR abnormal metabolism and a perfusion defect. Studies reporting any outcome measure were included. Pooled risk ratio for the composite outcome of MACE was done.
Results
A total of 6 studies were selected for final inclusion (515 patients, 53.4% women, 19.8% racial minorities.) Studies were institution based, retrospective in design and enrolled consecutive patients. All were observational in nature and published in English. All studies used a qualitative assessment of PET scans (abnormal FDG uptake with or without abnormal perfusion). Two studies assessed quantitative metrics (summed stress score in segments with abnormal FDG uptake, standardized uptake value and cardiac metabolic activity.) All studies reported major adverse cardiovascular events (MACE) as a composite outcome. After a mean follow up ranging from 1.4 to 4.1 years, there were a total of 105 MACE. All studies included death (either all-cause death or sudden cardiac death) and ventricular arrhythmia (ventricular tachycardia or ventricular fibrillation) as a component of MACE. Four of the six studies adjusted for several characteristics in their analysis. All four studies used left ventricular ejection fraction (LVEF). However, other adjustment variables were not consistent across studies. Five studies found a positive prognostic association with the primary outcome, two of which assessing right ventricular uptake.
Conclusion
Although available evidence indicates FDG PET can be used in the risk stratification of patients with CS, our findings show further studies are needed to quantify the effect in this patient group.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is a multi-systemic inflammatory disease of unknown etiology characterized by non-necrotizing sarcoid granulomas often located in the lungs and mediastinal lymph nodes.1 Cardiac sarcoidosis (CS) can occur alone or along with involvement of other organ-systems. CS has been reported in as much as 25% of patients with systemic involvement.2,3 The most important clinical sequelae of CS are heart failure, conduction abnormalities, ventricular arrhythmia, and sudden cardiac death.4,5 Identification of cardiac involvement is important as prior studies have shown significant morbidity and mortality in patients with CS.5,6,7
Diagnosis of CS is challenging considering the lack of pathognomonic signs and symptoms. Diagnostic criteria, such as the Japanese Ministry of Health and Welfare (JMHW) Heart Rhythm Society (HRS) and the World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG) have not been validated and have shown poor sensitivity and concordance.8,4,9,10 Endomyocardial biopsy, considered the gold standard test, is not commonly used due to its inherent risks.11 Furthermore, sarcoid inflammation is patchy and has a predilection to sub-endocardial layers leading to a poor diagnostic yield (test sensitivity of 20%.)12,13 Consequently, the diagnosis of CS is currently established utilizing a combination of cardiac imaging and clinical criteria.
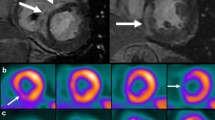
18Fluorodeoxyglucose (FDG) positron emission tomography (PET) is an important imaging modality that utilizes radionuclide labeled glucose to detect areas of inflammation through its uptake by inflammatory cells (Figure 1).14,15 FDG PET is one of the imaging modalities included in the current guidelines for the diagnosis of CS and several prior studies have shown its high sensitivity and specificity.16
Most prior studies focused on the diagnostic accuracy of FDG-PET in CS while few small-center studies examined its prognostic capability. The aim of this review is to summarize findings of the prognostic role of FDG PET.
Methods
Source
Figure 2 shows the PRISMA flow diagram. Studies were identified by searching MEDLINE from inception to present. The last search was run on Oct 26, 2020. We used Medical Subject Headings (MeSH) terms, including all subheadings and entry terms, for sarcoidosis; cardiac and FDG PET imaging.
Studies
Studies of any design assessing the prognostic role of FDG PET imaging done at baseline were included. Studies with patients of any age and gender with either suspected or confirmed cardiac sarcoidosis were included. If multiple publications from the same cohort were found, the most recent publication with the larger sample size was included.
Imaging
Abnormal PET was defined as abnormal metabolism (presence of focal or focal-on-diffuse uptake of FDG) OR abnormal metabolism and a perfusion defect. Studies reporting any outcome measure were considered.
Study Selection and Data Collection
Eligibility assessment was performed independently in an unblinded standardized manner by 2 reviewers. Disagreements between reviewers were resolved by consensus.
A data extraction sheet was developed and refined after pilot testing. Data on publication year, study size, main predictors and variables, follow up duration and outcome measures were collected. One review author extracted the data from included studies and the second author checked the extracted data. Disagreements were resolved by discussion between the two review authors; if no agreement could be reached, a third author assessed the evidence for final decision.
Analysis
A meta-analysis was performed by computing the pooled risk ratio (RR) using random-effects model. Abnormal PET was defined as abnormal metabolism (presence of focal or focal-on-diffuse uptake of FDG) OR abnormal metabolism and a perfusion defect. Subgroups of patients with abnormal metabolism or perfusion defect were excluded from the analysis if reported in the study.
All studies reporting abnormal PET findings in the left ventricle were pooled for the primary analysis. A separate analysis was done for studies reporting right ventricular findings. Finally, results were analyzed on sensitivity analysis restricting to studies with patients who only had PET imaging. We tested for heterogeneity with the Breslow-Day test, and used the method proposed by Higgins et al to measure inconsistency (the percentage of total variation across studies due to heterogeneity).17 All analysis was done using the meta suite of commands in Stata 16.0 (StataCorp, College Station, Texas) and a p-value of 0.05 was considered the threshold for statistical significance.
Results
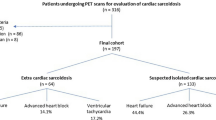
A total of 464 studies were identified using the search strategy. Of these, 439 studies were excluded after reviewing abstracts because the papers did not meet the criteria or were duplicates. The full text of the remaining 25 citations were examined in more detail and a total of 6 studies were selected for final inclusion. No unpublished relevant studies were obtained (Figure 2).
Table 1 summarizes study characteristics.18,19,20,21,22,-23 All included studies were observational in nature and published in English. There were a total of 515 patients with 53.4% females and 19.8% racial minorities. The proportion of patients meeting established diagnostic criteria ranged from 29 to 100%. Only one study reported the number of patients with biopsy proven extracardiac sarcoidosis (83%).22
All studies used a qualitative assessment of PET scans (abnormal FDG uptake with or without abnormal perfusion). In addition, two studies assessed quantitative metrics (summed stress score in segments with abnormal FDG uptake, standardized uptake value and cardiac metabolic activity).19,23 Three studies assessed Late Gadolinium Enhancement (LGE) on Cardiac Magnetic Resonance Imaging (CMR) in addition to PET.20,21,-22 Three studies (n = 269) also reported right ventricular PET abnormalities.18,22,23
All studies reported Major Adverse Cardiovascular Events (MACE) as a composite outcome. After a mean follow up ranging from 1.4 to 4.1 years, there were a total of 105 MACE. All studies included death (either all-cause death or sudden cardiac death) and ventricular arrhythmia (ventricular tachycardia or ventricular fibrillation) as a component of MACE. However, other components of outcomes were not consistent and ranged from all-cause mortality to hospitalization for heart failure and heart transplantation.
Four of the six studies adjusted for several characteristics in their analysis.18,19,-20,22 All four studies used Left Ventricular Ejection Fraction (LVEF). However, other adjustment variables were not consistent across studies. Diagnostic criteria were used to adjust for effect measures in three studies (two used the Japanese Ministry of Health and Welfare criteria and one used the Heart Rhythm Society criteria).18,19,-20 One study used sequential model building to generate risk scores that parsimoniously adjusted for variables without overfitting the final model.19
Figures 3 and 4 and online resource 1 summarize findings of the meta-analysis. Analysis pooling all studies included in this review showed abnormal PET was significantly associated with patient outcomes (RR = 2.08, 95% CI 1.48-2.92, p < 0.001). Sensitivity analysis restricting to studies with patients who only had PET imaging showed a similar statistically significant association (RR = 2.30, 95% CI 1.53-3.47, p < 0.001). Lastly, a statistically significant association was observed in the subgroup of studies reporting abnormal PET in RV (RR 2.96, 95% CI 1.12-7.78, p = 0.03).
Discussion
In the current review, we have shown the role of PET imaging in prognosticating patients with known or suspected CS. Our analysis found a lack of studies in general, and a lack of consistency in the definition of outcome measure. Our pooled effect analysis, albeit limited by the inconsistency in design and outcome measures, showed how an abnormal PET was significantly associated with patient outcomes.
Although sarcoidosis primarily affects the lungs, the importance of identifying cardiac involvement lies in its associated degree of morbidity and mortality. Even though endomyocardial biopsy is the gold standard for diagnosis of CS, it has a sensitivity of 20-30% largely because of the patchy nature of the disease. Thus, diagnosis is challenging and relies on integrating clinical presentation and imaging findings.1
FDG-PET is the gold standard for evaluation of inflammation in the myocardium because it is known to be taken up by the activated macrophages, epithelioid cells, and Langerhans giant cells found in sarcoid granulomas.24 It can also be used in patients with implanted pacemakers or defibrillators, which are common devices in patients with CS. Furthermore, studies have shown how FDG-PET can help to assess disease activity and guide response to therapy.15 Considering the heterogenous cardiac complications and adverse events of CS patients, FDG-PET’s potential role in prognostication lies in its ability to help tailor therapy for each individual.25
The prognostic value of CMR in CS has been previously demonstrated. Pooled analysis from 10 studies involving 760 patients with known or suspected CS showed that patients with LGE had a higher odds of all-cause mortality and arrhythmogenic events compared to patients without LGE. The odds ratio for the association between LGE and outcomes was markedly higher in patients with a higher LVEF (> 50%). In studies where the LVEF was < 50%, the prevalence of LGE was higher and it no longer predicted the composite endpoint.18,26,27
Our finding of the strong association between FDG uptake in the RV and patient outcomes is an important consideration for clinical practice. However, most of the studies included in this review were from single centers, the patient population lacked racial diversity, and outcome measures were not adjudicated. Furthermore, the small number of studies included in our analysis and the high heterogeneity in unadjusted estimates is a limitation. Despite this, we have reported summary effect estimates for the presence of RV uptake quantitatively or qualitatively in patients undergoing FDG-PET for CS. This is a ripe area for future research and given the scarcity of the disease should ideally include a collaborative effort from multiple centers to get a large sample size and adequate number of events.
Limitations
This review is not without limitation. The variation in definition of the primary outcome in the included studies is a significant limitation in the interpretation of our pooled outcome. However, it is reported here not as a true pooled measure but rather as summary of the best available evidence from published studies. Most of the studies included in this review were of patients who had both PET and CMR. Selection bias is a possibility as these are unlikely to be consecutive patients. However, in clinical practice a majority of patients with suspected cardiac sarcoidosis are undergoing both tests. A further cause of selection bias is the fact that cardiac PET isn’t routinely done for all patients with sarcoidosis but reserved in cases of unclear diagnosis or suboptimal treatment response. Lastly, as with all systematic reviews our analysis inherits all the shortcomings of the individual studies it is based on.
Conclusion
To our knowledge, this is the first systematic review of the prognostic role of cardiac PET imaging in sarcoidosis. We have shown that an abnormal FDG-PET in patients with known or suspected CS, particularly significant RV uptake, identifies patients at the highest risk for adverse cardiac events. However, we have also shown that the few published studies are from single institutions, involve a small number of patients, have heterogenous endpoints and short follow-up. Thus, there is a need for well-designed large-scale registries and cohort studies to address these limitations.
New Knowledge Gained
Cardiac Sarcoidosis is reported in as much as a quarter of cases of systemic sarcoid and can cause major complications if it is not detected early. Several aspects of FDG-PET make for a test uniquely suited for patients with Cardiac Sarcoidosis. In this systematic review, we have shown that FDG-PET can be used to risk-stratify and ultimately guide the management of patients with Cardiac Sarcoidosis.
Abbreviations
- CMR:
-
Cardiac Magnetic Resonance Imaging
- CS:
-
Cardiac sarcoidosis
- FDG:
-
18Fluorodeoxyglucose
- HRS:
-
Heart Rhythm Society
- JMHW:
-
Japanese Ministry of Health and Welfare
- LGE:
-
Late Gadolinium Enhancement
- PET:
-
Positron Emission Tomography
- WASOG:
-
World Association of Sarcoidosis and Other Granulomatous Diseases
References
Trivieri MG, Spagnolo P, Birnie D, et al. Challenges in cardiac and pulmonary sarcoidosis: JACC state-of-the-art review. J Am Coll Cardiol 2020;76:1878-901.
Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009;120:1969-77.
Dubrey SW, Falk RH. Diagnosis and management of cardiac sarcoidosis. Prog Cardiovasc Dis 2010;52:336-46.
Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1305-23.
Birnie DH, Nery PB, Ha AC, et al. Cardiac sarcoidosis. J Am Coll Cardiol 2016;68:411-21.
Osborne MT, Hulten EA, Singh A, et al. Reduction in 18F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol 2014;21:166-74.
Kron J, Sauer W, Schuller J, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace 2013;15:347-54.
Hiraga H, Yuwai K, Hiroe M. Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord 2007;27:89-102.
Ribeiro Neto ML, Jellis C, Hachamovitch R, et al. Performance of diagnostic criteria in patients clinically judged to have cardiac sarcoidosis: Is it time to regroup? Am Heart J 2020;223:106-9.
Judson MA, Costabel U, Drent M, et al. The WASOG sarcoidosis organ assessment instrument: An update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19-27.
Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: A seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol 1992;19:43-7.
Leone O, Veinot JP, Angelini A, et al. 2011 consensus statement on endomyocardial biopsy from the association for european cardiovascular pathology and the society for cardiovascular pathology. Cardiovasc Pathol 2012;21:245-74.
Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007;357:2153-65.
El-Tallawi KC, Parikh R, Nabi F, et al. A positive tc-99 m PYP scan in a patient with cardiac sarcoidosis. J Nucl Cardiol 2020. https://doi.org/10.1007/s12350-020-02158-5.
Khalaf S, Al-Mallah MH. Fluorodeoxyglucose applications in cardiac PET: Viability, inflammation, infection, and beyond Methodist Debakey. Cardiovasc J 2020;16:122-9.
Kim S, Pak K, Kim K. Diagnostic performance of F-18 FDG PET for detection of cardiac sarcoidosis: A systematic review and meta-analysis. J Nucl Cardiol 2019;27:2103-15.
Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60.
Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329-36.
Sperry BW, Tamarappoo BK, Oldan JD, et al. Prognostic impact of extent, severity, and heterogeneity of abnormalities on 18F-FDG PET scans for suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 2018;11:336-45.
Bravo PE, Raghu G, Rosenthal DG, et al. Risk assessment of patients with clinical manifestations of cardiac sarcoidosis with positron emission tomography and magnetic resonance imaging. Int J Cardiol 2017;241:457-62.
Gowani Z, Habibi M, Okada DR, et al. Utility of cardiac magnetic resonance imaging versus cardiac positron emission tomography for risk stratification for ventricular arrhythmias in patients with cardiac sarcoidosis. Am J Cardiol 2020;134:123-9.
Wicks EC, Menezes LJ, Barnes A, et al. Diagnostic accuracy and prognostic value of simultaneous hybrid 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging 2018;19:757-67.
Tuominen H, Haarala A, Tikkakoski A, et al. FDG-PET in possible cardiac sarcoidosis: Right ventricular uptake and high total cardiac metabolic activity predict cardiovascular events. J Nucl Cardiol 2019;28:199-205.
Treglia G, Annunziata S, Sobic-Saranovic D, et al. The role of 18F-FDG-PET and PET/CT in patients with sarcoidosis: An updated evidence-based review. Acad Radiol 2014;21:675-84.
Tavora F, Cresswell N, Li L, et al. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol 2009;104:571-7.
Pöyhönen P, Holmström M, Kivistö S, Hänninen H. Late gadolinium enhancement on CMR and sustained ventricular tachycardia predict severe cardiac inflammation. Acta Cardiol 2014;69:637-47.
Watanabe E, Kimura F, Nakajima T, et al. Late gadolinium enhancement in cardiac sarcoidosis: Characteristic magnetic resonance findings and relationship with left ventricular function. J Thorac Imaging 2013;28:60-6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Al-Mallah receives support from the Houston Methodist Research Institute and Siemens. No other potential conflicts of interest relevant to this article exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahmed, A.I., Abebe, A.T., Han, Y. et al. The prognostic role of cardiac positron emission tomography imaging in patients with sarcoidosis: A systematic review. J. Nucl. Cardiol. 28, 1545–1552 (2021). https://doi.org/10.1007/s12350-021-02681-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-021-02681-z