Abstract
Purpose
We aimed to assess normal values for quantified myocardial blood flow (MBF) on a hybrid PET/coronary-CT scanner and to test their diagnostic performance in patients with suspected CAD.
Materials and Methods
Patients underwent 82Rb-PET/CT and integrated CT-based coronary angiography (CCTA) and were classified as normal (no stenosis), with non-obstructive stenosis (< 50%) and with CAD (≥ 50%). Global and regional stress MBF (sMBF), rest MBF and myocardial flow reserve (MFR) were calculated. Ischemia was defined as SDS ≥ 2, severe ischemia as SDS ≥ 7.
Results
357 consecutive patients were included. Global sMBF and MFR were higher in normal patients than in patients with CAD (3.61 ± 0.71 vs 3.04 ± 0.77, P < 0.0001; 3.08 ± 0.84 vs 2.68 ± 0.79, P = 0.0001), but not different compared to patients with non-obstructive stenosis (3.61 ± 0.71 vs 3.43 ± 0.69, P = 0.052; 3.08 ± 0.84 vs 2.99 ± 0.82, P = 0.45). sMBF yielded superior accuracy over MFR in identifying both ischemia (AUC 0.74 vs 0.62, P = 0.003) and severe ischemia (AUC 0.88 vs 0.78, P = 0.012). Optimal threshold for global sMBF to rule out myocardial ischemia was 3.5 mL g−1 min−1.
Conclusions
Normal quantitative values are provided. Global sMBF provided higher diagnostic accuracy than MFR. Using sMBF-threshold of 3.5 mL·g−1·min−1 on 82Rb-PET/CT yielded similar NPV (96%) as CCTA to rule out CAD. Hence, resting scan could be omitted in patients with sMBF values above reference.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Myocardial perfusion positron emission tomography/computed tomography with Rubidium-82 (82Rb-PET/CT) is increasingly used in the diagnostic and prognostic assessment of patients with suspected or known coronary artery disease (CAD), due to significant advantages over single photon emission tomography (SPECT), including lower radiation exposure, higher resolution and sensitivity, robust attenuation correction and absolute quantification of stress and rest myocardial blood flow (sMBF and rMBF, respectively).1,2
To date, two questions remain unanswered for quantitative 82Rb-PET/CT: 1) which are the normal values of sMBF and rMBF? and 2) which quantitative parameter is the most accurate to allow for a precise diagnosis of CAD (sMBF or myocardial flow reserve [MFR])?
Although 82Rb-PET was introduced in the 80´s, normal values of absolute MBF have not been yet validated in large cohorts based on a robust reference standard. Some investigators report reference MFR values for 82Rb PET in normal populations based on low-probability with small patient number (n = 22) and a comparison between stress MBF and MFR within the same patients with regard to diagnostic performance and to rule out CAD has not been explored yet.3,4 Rather, reference values for stress MBF are currently derived from studies on cardiac perfusion PET using other tracers, with different pharmacodynamics.5 Furthermore, previous studies using 82Rb PET/CT suffered from the lack of a validated and robust gold standard capable to identify flow-limiting coronary stenosis. In this regard, coronary computed tomography angiography (CCTA) has been reported to have high diagnostic accuracy and an excellent negative predictive value (NPV) when compared to invasive coronary angiography (ICA).6 This favorable feature makes this technique ideal to identify patients without CAD.
While many papers demonstrated the impact of MBF quantification using various PET perfusion tracers in clinical practice,7 the choice of the most accurate quantitative parameter is still open to debate. The role of MFR has been elucidated both for 82Rb-8,9 and 13NH-Ammonia10 PET/CT, whilst a superiority of sMBF over MFR has been recently demonstrated for 15O-water PET/CT.5 Other parameters like relative flow reserve (RFR), defined as the ratio of hyperemic MBF in a stenotic area to hyperemic MBF in a normal perfused area have been investigated, but failed to show an improvement in diagnostic accuracy.11
Summarized, current evidence of normal flow values using 82Rb-PET is either based on very small patient number, on clinical grouping based on SSS where smaller stenosis could still be present4 or on the adaption of different tracers such as 15OH2O that harbor different kinetics.5 To close this gap, we therefore aimed to determine reference values of 82Rb-PET/CT compared to CCTA and to assess for the most accurate parameter able to identify patients with CAD. Thresholds in quantitative values able to detect a myocardial ischemia were also investigated.
Materials and Methods
Study Design
This single-center observational study was conducted in accordance with the declaration of Helsinki. The study was accepted by the local ethics committee (Req-2019-00447).
In this study, only patients without prior history of known CAD were included. All patients were referred as part of clinical care for signs or symptoms suggestive for CAD (typical or atypical chest pain, exertional dyspnea, electrocardiogram (ECG) changes during ergometric test). Exclusion criteria were age under 18, pregnancy, breast feeding, previous allergic reaction to contrast medium, hyperthyroidism and impaired renal function.
All consecutive patients underwent both 82Rb-PET/CT and CCTA on the same hybrid scanner between 1st January 2016 and 1st May 2019.
Imaging Protocol
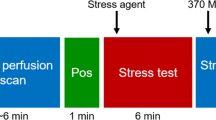
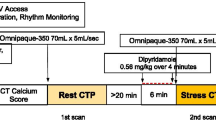
All patients underwent sequential cardiac 82Rb-PET/CT with rest/stress protocol (Figure 1) and 128-slice CT-based coronary angiography (CCTA) on an integrated hybrid PET/CT system (Biograph mCT, Siemens Healthineers, Erlangen, Germany). First, a non-enhanced low-dose CT scan was performed for attenuation correction purpose (increment 0.6 mm, soft-tissue reconstruction kernel, 120 keV, CAREDOSE 4D), followed by a non-enhanced calcium scoring CT with breath commands (increment 0.6 mm, slice thickness 3 mm, soft-tissue reconstruction kernel, 120 keV, CAREDOSE4D).
Stress was induced by weight-adapted dose of Adenosine (140 μg·kg−1·min−1) using a perfusor i.V. over 6 minutes or by a single i.V. application of 400 μg Regadenoson, if Adenosine was contra-indicated.
Rest images were always performed first, followed by stress. For both rest and stress, a weight-adjusted dose of 82Rb-chloride was intravenously injected as bolus (30 mCi—1110 MBq if weight < 100 Kg, 40 mCi—1480 MBq if ≥ 100 Kg and < 140 kg and 50 mCi—1850 MBq if ≥ 140 kg). Electrocardiogram-gated PET images were acquired for both rest and stress over 7 minutes in list mode, starting simultaneously with 82Rb-chloride injection.
Static and dynamic images were reconstructed using an ordered subset expectation maximization algorithm (OSEM, matrix 512 × 512, 3 iterations, 21 subsets, gauss-filtering, 8 mm full-width half-maximum). Random, scatter, attenuation and decay corrections were automatically applied to the emission data. Alignment between PET and CT images was visually inspected by experienced technologists and corrected if necessary. Automatic motion correction was also performed. Dynamic images were reconstructed with the following framesets: 1 second delay, 12 × 10 seconds, 2 × 30 seconds, 2 × 60 seconds, 1 × 120 seconds. Static and ECG-gated images were reconstructed using the last four minutes of acquisition, both with and without attenuation correction
At last, CCTA (increment 0.6 mm, soft-tissue reconstruction kernel, 120 keV, dynamic mAs using CAREDOSE4D, pitch 0.8) was performed after PET/CT scan by administering 80 ml of iodine contrast (Ultravist 370). To enable best image quality, application of metoprolol i.v. (5-20 mg) and nitroglycerin sublingual (2 sprays) was performed after PET and immediately before CCTA acquisition with a target heart rate of ≤ 70 bpm. Scans were prospectively gated.
Image Evaluation and Quantification
All images were read in consensus by a board-certified nuclear medicine physician, a board-certified radiologist and a board-certified cardiologist, not blind to clinical information.
CCTA was visually rated by two independent readers and stenosis were graded according to their severity extent using a 5-point scale (grade 0 = none, grade 1 = 1 to 50%, grade 2 = 50 to 74% grade 3 = 75 to 90%, grade 4 = 90 to 100%). Patients were classified as normal (group 1, no stenosis on CCTA), with non-obstructive stenosis (group 2, < 50% stenosis on CCTA in at least one vessel) and with CAD (group 3, ≥ 50% stenosis on CCTA in at least one vessel).
sMBF, rMBF and myocardial flow reserve (MFR) were automatically calculated both globally and in each vascular territory using a proprietary software (Syngo MBF) included in SyngoVia Package (Siemens Healthineers, Erlangen, Germany). Individual assignment of ventricular segmentation to its correlating vascular territory was performed based on hybrid PET/CTCA data. The arterial input function was derived from the dynamic PET data. A single tissue compartment model was used to calculate myocardial perfusion in mL·g−1·min−1, as previously described.12 As in previous studies, rMBF was corrected for rate-pressure product to compensate for the bias resulting from cardiac preload by high heart rate and systolic blood pressure in the resting state,13 thus generating two datasets of rMBF and MFR, each with and without correction. The corrected rMBF (rMBFcorrected) was calculated with the following formula:5,13
Static PET/CT perfusion images were visually assessed on a 17-segment model using individual known coronary anatomy both at rest and during stress and scored according to American Society of Nuclear Cardiology guidelines (0 normal perfusion, 1 mild reduction in counts but not definitively abnormal, 2 moderate reduction in counts and definitively abnormal, 3 severe reduction in uptake, and 4 absent uptake).14,15 Summed scores were calculated for the global myocardium. SSS equals the sum of the scores for all segments in the stress scan, SRS equals the sum of the scores in the rest scan, and the summed difference score (SDS) equals the sum of the differences between SSS and SRS in each segment. Ischemia was defined as SDS ≥ 2 while severe ischemia was defined as SDS ≥ 7, corresponding to ≥ 10% of ischemic myocardium.
Statistical Analysis
For statistical analyses, MedCalc Software Version 18 (MedCalc Software, Ostend, Belgium) was used employing an alpha-level of 0.05 to indicate statistical significance. Normal distribution was assessed using Shapiro-Wilk test. Mann-Whitney-U test and Kruskal-Wallis test were used for comparison of non-parametric data across groups; ROC-analysis was performed to determine the diagnostic accuracy of quantified blood flows in identifying ischemia. Youden’s index with optimal cut-off values was determined. DeLongs method was used to compare between ROC curves.
To correlate heart rate with rMBF, Spearman’s rank correlation was used. To correct for alpha error accumulation by multiple testing, Bonferroni method was used for Mann-Whitney-U and for Kruskal-Wallis, Dunn’s post-hoc test was used. Data are given as mean with standard deviation unless otherwise specified.
Results
Patient Demographics
Overall, 357 consecutive patients were included (male 196/357 (54.9%), age 61.2 ± 10.3 years, BMI 28.9 ± 6.4 kg·m−2, 20.4% with diabetes mellitus, 57.4% with arterial hypertension). 344/357 (96.4%) patients were stressed using Adenosine, 13/357 (3.6%) using Regadenoson due to contra-indications against Adenosine. Detailed demographics are shown in Table 1.
PET/CT Imaging
On a global level, 153 patients were classified as normal (i.e., without detectable stenoses of any degree on CCTA, group 1), 99 as with non-obstructive stenosis (i.e., with < 50% stenosis on CCTA, group 2) and 105 as with CAD (i.e., with ≥ 50% stenosis on CCTA, group 3). Normal perfusion values and those in patients with obstructive stenosis are given in Table 2.
Global sMBF and MFR were significantly higher in normal patients than in patients with CAD (3.61 ± 0.71 vs 3.04 ± 0.77, P < 0.0001; 3.08 ± 0.84 vs 2.68 ± 0.79, P = 0.0001) but were not significantly different compared to patients with non-obstructive stenosis (3.61 ± 0.71 vs 3.43 ± 0.69, P = 0.052; 3.08 ± 0.84 vs 2.99 ± 0.82, P = 0.45). rMBF in normal patients was not significantly different compared to patients with non-obstructive stenosis (1.27 ± 0.40 vs 1.24 ± 0.36, P = 0.70) and CAD (1.27 ± 0.40 vs 1.21 ± 0.35, P = 0.26). Comparing the distribution of all three groups, global sMBF and MFR were significantly different (P < 0.0001 and P = 0.0003) but rMBF was not (P = 0.52). Statistical comparison of global perfusion values is given in Figure 2.
In the right coronary artery (RCA), sMBF[RCA] in normal vessels was significantly higher than in those with obstructive stenosis (3.84 ± 0.89 vs 3.36 ± 1.11, P = 0.02) and those with non-obstructive stenosis (3.84 ± 0.89 vs 3.45 ± 1.03, P = 0.006). Both rMBF[RCA] and MFR[RCA] were not different compared to patients with CAD (1.27 ± 0.44 vs 1.20 ± 0.49, P = 0.17; 3.29 ± 1.03 vs 3.10 ± 1.22, P = 0.20) and those with non-obstructive stenosis (1.27 ± 0.44 vs 1.17 ± 0.39, P = 0.15; 3.29 ± 1.03 vs 3.24 ± 1.25, P = 0.50). Comparing the distribution of all three groups, sMBF[RCA] was significantly different (P = 0.002) but rMBF[RCA] and MFR[RCA] were not (P = 0.17 and P = 0.39). Statistical comparison of RCA perfusion values is given in Figure 2.
In the circumflex artery (RCX), sMBF[RCX] and MFR[RCX] in normal vessels were significantly higher than in stenotic ones, (3.26 ± 0.76 vs 2.53 ± 0.86, P < 0.0001; 2.84 ± 0.83 vs 2.19 ± 0.68, P < 0.0001) but not compared to those with non-obstructive stenosis (3.26 ± 0.76 vs 3.18 ± 0.66, P = 0.37; 2.84 ± 0.83 vs 2.76 ± 0.80, P = 0.39). rMBF[RCX] was similar between normal and stenotic vessels (1.24 ± 0.38 vs 1.19 ± 0.25, P = 0.93) and between normal vessels and those with non-obstructive stenosis (1.24 ± 0.38 vs 1.25 ± 0.39, P = 0.85). Comparing the distribution of all three groups, sMBF[RCX] and MFR[RCX] were significantly different (P = 0.0002 and P = 0.0002) but rMBF[RCX] was not (P = 0.98). Statistical comparison of RCX perfusion values is given in Figure 2.
In the left anterior descending artery (LAD) sMBF[LAD] and MFR[LAD] in normal vessels were higher than in stenotic ones (3.54 ± 0.72 vs 2.91 ± 0.78, P < 0.0001; 3.00 ± 0.83 vs 2.48 ± 0.74, P < 0.0001) but not compared to those with non-obstructive stenosis (3.54 ± 0.72 vs 3.36 ± 0.77, P = 0.07; 3.00 ± 0.83 vs 2.92 ± 0.86, P = 0.30). rMBF[LAD] was similar between normal and stenotic vessels (1.26 ± 0.38 vs 1.25 ± 0.36, P = 0.95) and between normal vessels and those with non-obstructive stenosis (1.26 ± 0.38 vs 1.24 ± 0.35, P = 0.94). Comparing the distribution of all three groups, sMBF[LAD] and MFR[LAD] were significantly different (P < 0.0001 and P < 0.0001) but rMBF[LAD] was not (P = 0.99). Statistical comparison of LAD perfusion values is given in Figure 2.
Using SDS ≥ 2 (ischemia) as separator, global sMBF yielded superior accuracy in identifying myocardial ischemia over MFR (AUC 0.74 vs 0.62, P = 0.003 [0.0425-0.204], Table 3). Optimal threshold for sMBF defined by Youden’s index was 3.5 mL·g−1·min−1 (sensitivity 87.5%, specificity 48.7%, positive predictive value 22% (PPV), NPV 96%, Figure 3).
Using SDS ≥ 7 (severe ischemia) as separator, again global sMBF yielded superior accuracy in identifying myocardial ischemia over MFR (AUC 0.88 vs 0.78, P = 0.012 [0.0223-0.178]). Optimal threshold for sMBF defined by Youden’s index was 2.6 mL·g−1·min−1 (sensitivity 75%, specificity 85.5%, PPV 46%, NPV 96%, Figure 4).
After correcting the MFR by using the rate-pressure product (MFRcorrected) to compensate from bias by cardiac preload in the resting condition, there was still a significant difference between the AUC of sMBF and MFRcorrected for both patients with ischemia (SDS ≥ 2, AUC 0.74 vs 0.67, P = 0.03 [0.006-0.150]) and those with severe ischemia (SDS ≥ 7, AUC 0.88 vs 0.81, P = 0.04 [0.005-0.142]). Furthermore, a significant correlation between the heart rate and global rMBF was found (ρ = 0.49, P < 0.0001).
Discussion
Absolute coronary flow quantification is known to increase diagnostic accuracy of myocardial perfusion PET imaging owing to its capability to effectively identify balanced 3-vessels disease or microvascular dysfunction. Yet, for a correct interpretation in clinical practice, optimal thresholds of absolute perfusion should be defined. There is currently no consensus on normal perfusion values for MBF during stress condition on 82Rb-PET imaging. Ziadi et al. proposed a threshold of < 2.0 for MFR to distinguish between patients with and without significant CAD,9 but the threshold was retrieved using only the SSS for grouping patients and thus, the presence of stenosis cannot be excluded in this cohort and thus the values of perfusion are not reflecting a normal population. Furthermore, no accepted threshold has been provided so far for sMBF. In fact, in clinical routine, normal values for sMBF on 82Rb-PET are adapted from other tracers, which have however different kinetics due to highly different molecular profiles.5,16
In a single instance, normal values of sMBF were investigated and validated against 15O-H2O PET/CT,4 showing reference values in our range with regard to sMBF (3.82 ± 1.21 mL·min−1·g−1) but a reference MFR higher than currently accepted as threshold (3.88 ± 0.91), due to a very small normalcy population (n = 22). To note, the normalcy group in their study was selected only on the basis of a very low likelihood of CAD and not by using a test such as CCTA or ICA. To date, a validation in a larger cohort was not pursued and in this regard, we here provide for the first time normal reference values, able to assist nuclear medicine physicians in clinical practice.
Our work provides novelty also in another important aspect. To date, the choice of the most accurate quantitative parameter to detect a CAD is a matter of debate. In 15O-H2O myocardial PET, sMBF has been reported to yield the highest diagnostic accuracy, clearly outperforming MFR.5 This appears to be more consistent than other derived parameters such as the relative flow reserve (RFR), defined as the ratio of sMBF in a stenotic area to sMBF in a normally perfused area, which failed to show an improvement in diagnostic accuracy compared to MFR.11
Our paper confirms that the same concept also pertains to 82Rb PET/CT Imaging. This is not completely surprising, as both global and regional MFR can be affected by relatively high flow rates at rest condition.17 This was the main reason why previous studies corrected the calculation of rMBF for rate-pressure product,5,13 thus taking into account possible confounders due to patient’s nervousness and anxiety during the examination. This is also consistent with the results of our study, wherein the rest heart rate correlated to rMBF (P < 0.0001, ρ = 0.49), thus potentially hampering MFR calculation. But to note, in our paper the superiority of sMBF was demonstrated also over MFRcorrected, both for ischemia (P < 0.03) and severe ischemia (P < 0.04).
In our study, we used CCTA as gold standard to rule out obstructive stenosis and our choice deserves further clarification. Among non-invasive techniques, CCTA yields highest performance in ruling out significant CAD and hence, the use of CCTA as ground truth to select the normal population is acceptable with minimal error rate.6
However, despite the excellent negative predictive value of CCTA in ruling out obstructive coronary artery stenoses, there is a poor correlation between anatomical data (e.g., coronary stenosis severity) and the functional impact of coronary stenoses (e.g., myocardial ischaemia). In a sub-study of the FAME study,18 Tonino et al. demonstrated that around 65% of coronary stenoses graded between 50% and 70% on invasive coronary angiography were not associated with myocardial ischemia as established by fractional flow reserve (FFR). On the other hand, Gaur et al. showed that 17% of coronary stenoses < 50% was associated with FFR ≤ 0.80.19 Similar results have been demonstrated also in a manuscript by Toth et al.,20 wherein the correlation between invasive quantitative coronary angiography (QCA) and FFR values were evaluated.
In regard to our paper, it should be noted that, although invasive coronary angiography with determination of FFR is still the currently accepted gold standard in the assessment of CAD, an invasive coronary angiography is not recommended in patients with normal CCTA.21 In fact, while FFR can provide important information on the correlation between the extent of a stenosis and quantified MBF, it has no impact in patients with normal coronary anatomy, as an FFR measurement is per definition impossible if no stenoses are detected.
As the main aim of the present study was to assess still unpublished normal values of MBF in 82Rb PET, it can be maintained that CTCA represents a robust gold standard to identify patients without CAD and this reliability cannot be improved by FFR measurement. The only limitation of CTCA in this regard is the incapability to reveal a microvascular dysfunction, which would be however missed also by FFR.
Of note, the normal population for the assessment of normal values in our paper included only those patients without stenoses of any degree. Rather, patients with < 50% stenoses were assigned to another group, in order to account for possible differences in measured flows. To note, we also demonstrated a statistical equivalence between patients without stenoses and those with < 50% stenosis, which gives even more reliance on the applicability of the present results to the clinical practice.
In clinical practice, another potential advantage of a simultaneous acquisition of CCTA and PET is the correct assignment of ischemia in any ventricular segment to its correlating vascular territory, with evident impact on clinical management of patients with suspected CAD.22
From a direct comparison between CCTA and PET, we also identified optimal thresholds of sMBF able to effectively rule out a significant ischemia. In fact, sMBF ≥ 2.6 mL·g−1·min−1 yields a high NPV (96%) to rule out severe ischemia, and sMBF ≥ 3.5 mL·g−1·min−1 achieves equal NPV (96%) to rule out ischemia, similar to that of CCTA itself. Although the results of our study may indicate that sMBF may be used alone to diagnose an ischemia, in clinical practice such reliance may be excessive. Indeed, in borderline situations a diagnosis should be based on a “puzzle” of many parameters, such as SDS, calcium score and the presence of obstructive stenosis on CCTA. A comprehensive evaluation of such parameters is expected to increase the readers’ confidence and consequently the diagnostic accuracy. Nevertheless, it should be noted that a sMBF ≥ 3.5 mL·g−1·min−1 would virtually exclude the need for a subsequent rest test, with reduced radiation exposure for the patients and optimization of department’s workload. Furthermore, such a high NPV, coupled with a very low radiation dose (on average, 1.8 mSv) may render 82Rb-PET suitable as “first step” in the assessment of CAD, hence competing with CCTA itself. It may be hypothesized that each examination starts with a stress scan, followed by rest and CCTA. While the additional implementation of hybrid CCTA may give significant incremental value to localize potential stenosis,22 there is a clear rationale to omit further scans of the diagnostic cascade if stress PET/CT shows sMBF values higher than 3.5.
Some limitations should be acknowledged. First, we used only one software package to calculate MBF and MFR. While Slomka et al. showed that quantitative values do not differ significantly among three different software packages using 13N-Ammonia PET,23 Nesterov et al. showed significant differences among 10 software solutions with 8 different compartment models in 82Rb PET imaging, if different compartment models were used. Of note, software implementing the Ottawa Heart Institute 1-tissue compartment model (OHI-1-TCM) were proven to have consistent inter-software results.24 The same OHI-1-TCM was also implemented in the software tested in the work of Slomka et al.23 as well as in the majority of software solutions available on the market for 82Rb myocardial PET.24 As such, although our results may not fit those obtained with software capitalizing on other tissue compartment models, still they should be widely applicable.
Second, due to the inclusion of patients without known CAD, the number of patients with multivessel disease is very low in our population. As such, a direct correlation between the extent of coronary disease and reduction in blood flow could not be properly assessed. While this aspect was beyond the scope of the present paper, still the identification of different thresholds able to identify different degrees of CAD is highly anticipated in clinical practice.
Finally, due to the retrospective nature of the present paper, we cannot rule out that some clinical conditions other than CAD or ongoing therapies may have impacted the calculated flow rates. In this regard, the fact that no patients were on beta-blocker therapy gives reliance on the translation of the present results into clinical practice.
New Knowledge Gained
Normal quantitative values for 82Rb PET/CT were reported for the first time. We also demonstrated that the superiority of stress MBF over MFR pertains also to 82Rb PET/CT. This underlines the opportunity to perform stress-only protocols in patients with stress flow values above reference.
Conclusion
For the first time, we define cut-off values for absolute sMBF in the assessment of CAD with 82Rb PET/CT in patients without detectable stenoses, using CCTA as standard of reference. A value of sMBF ≥ 3.5 mL·g−1·min−1 effectively rules out CAD similarly to CCTA with NPV of 96%. Absolute sMBF measurements were superior to MFR for diagnosing hemodynamically significant CAD, meaning that stress-only protocols would be robust enough in patients with sMBF values above reference.
Abbreviations
- CCTA:
-
CT-based coronary angiography
- LAD:
-
Left anterior descending artery
- RCA:
-
Right coronary artery
- RCX:
-
Ramus circumflexus (left circumflex artery)
- sMBF:
-
Stress myocardial blood flow
- rMBF:
-
Rest myocardial blood flow
- MBF:
-
Myocardial blood flow
- MFR:
-
Myocardial flow reserve
- PPV/NPV:
-
Positive/negative predictive value
- SRS/SSS/SDS:
-
Summed rest/stress/difference score
References
Flotats A, Bravo PE, Fukushima K, Chaudhry MA, Merrill J, Bengel FM. 82Rb PET myocardial perfusion imaging is superior to 99mTc-labelled agent SPECT in patients with known or suspected coronary artery disease. Eur J Nucl Med Mol Imaging. 2012;39:1233-9.
Bengel FM, Higuchi T, Javadi MS, Lautamäki R. Cardiac positron emission tomography. J Am Coll Cardiol. 2009;54:1-15.
Sunderland JJ, Pan X-B, Declerck J, Menda Y. Dependency of cardiac rubidium-82 imaging quantitative measures on age, gender, vascular territory, and software in a cardiovascular normal population. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2015;22:72-84.
Prior JO, Allenbach G, Valenta I, et al. Quantification of myocardial blood flow with 82Rb positron emission tomography: Clinical validation with 15O-water. Eur J Nucl Med Mol Imaging. 2012;39:1037-47.
Danad I, Uusitalo V, Kero T, et al. Quantitative assessment of myocardial perfusion in the detection of significant coronary artery disease. J Am Coll Cardiol. 2014;64:1464-75.
Arbab-Zadeh A, Di Carli MF, Cerci R, et al. Accuracy of computed tomographic angiography and single-photon emission computed tomography-acquired myocardial perfusion imaging for the diagnosis of coronary artery disease. Circ Cardiovasc Imaging. 2015;8:e003533.
Tahari AK, Lee A, Rajaram M, et al. Absolute myocardial flow quantification with (82)Rb PET/CT: Comparison of different software packages and methods. Eur J Nucl Med Mol Imaging. 2014;41:126-35.
Fukushima K, Javadi MS, Higuchi T, et al. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med Off Publ Soc Nucl Med. 2011;52:726-32.
Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58:740-8.
Herzog BA, Husmann L, Valenta I, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150-6.
Stuijfzand WJ, Uusitalo V, Kero T, et al. Relative flow reserve derived from quantitative perfusion imaging may not outperform stress myocardial blood flow for identification of hemodynamically significant coronary artery disease. Circ Cardiovasc Imaging. 2015. https://doi.org/10.1161/CIRCIMAGING.114.002400.
Lortie M, Beanlands RSB, Yoshinaga K, Klein R, DaSilva JN, deKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging. 2007;34:1765-74.
Czernin J, Muller P, Chan S, et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation. 1993;88:62-9.
Hansen CL, Goldstein RA, Akinboboye OO, et al. Myocardial perfusion and function: Single photon emission computed tomography. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2007;14:e39-60.
American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging, Cerqueira MD, Weissman NJ, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539-542.
Knuuti J, Kajander S, Mäki M, Ukkonen H. Quantification of myocardial blood flow will reform the detection of CAD. J Nucl Cardiol. 2009;16:497-506.
Caobelli F. Anatomical and functional assessment of coronary artery disease in patients with stable angina: Which is the gold standard? J Nucl Cardiol. 2019;9:S26-35.
Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study: fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816-21.
Gaur S, Øvrehus KA, Dey D, et al. Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. Eur Heart J. 2016;37:1220-7.
Toth G, Hamilos M, Pyxaras S, et al. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J. 2014;35:2831-8.
Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain. J Am Coll Cardiol. 2009;53:1642-50.
Caobelli F. Left ventricular segmentation in myocardial perfusion positron emission tomography: Tailor-made or prêt-à-porter? Eur Heart J Cardiovasc Imaging. 2019;20:502-3.
Slomka PJ, Alexanderson E, Jácome R, et al. Comparison of clinical tools for measurements of regional stress and rest myocardial blood flow assessed with 13N-ammonia PET/CT. J Nucl Med Off Publ Soc Nucl Med. 2012;53:171-81.
Nesterov SV, Deshayes E, Sciagrà R, et al. Quantification of myocardial blood flow in absolute terms using 82Rb PET imaging. JACC Cardiovasc Imaging. 2014;7:1119-27.
Acknowledgements
We greatly appreciate the help and assistance of our excellent technicians.
Disclosure
Martin T. Freitag, Jens Bremerich, Damian Wild, Philip Haaf and Michael Zellweger report no disclosures relevant to the manuscript. Federico Caobelli received academic grant support from GE Healthcare and Tillots AG and speaker honoraria from Siemens, for matters not related to the current study. All the authors declared no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. A formal approval from the local Ethical Board was obtained for the present study (Req-2019-00447).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
All editorial decisions for this article, including selection of reviewers and the final decision, were made by guest editor Henry Gewirtz, MD, PhD.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Freitag, M.T., Bremerich, J., Wild, D. et al. Quantitative myocardial perfusion 82Rb-PET assessed by hybrid PET/coronary-CT: Normal values and diagnostic performance. J. Nucl. Cardiol. 29, 464–473 (2022). https://doi.org/10.1007/s12350-020-02264-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-020-02264-4