Abstract
Hypereosinophilic syndrome (HES) is characterized by blood and tissue hypereosinophilia leading to organ damage. Gastrointestinal involvement is the third most common manifestation. We present a patient with idiopathic HES with secondary eosinophilic esophagitis (EoE), gastritis, and enteritis, corticosteroids-dependent, azathioprine- and mepolizumab-refractory. The patient achieved clinical and histopathologic remission following dupilumab treatment. A 28 year-old female presented with chronic episodic nausea and emesis since childhood and initial diagnosis of primary eosinophilic gastrointestinal disease (EGID), improved with corticosteroids, refractory to azathioprine. She was found to have peripheral eosinophilia and multifactorial anemia, with iron, B12, and folate deficiencies. Esophageal, gastric, duodenal, and terminal ileum biopsies showed significant eosinophilic infiltrate. Bone marrow biopsy at age 31 confirmed HES diagnosis. By age 32, she became total parental nutrition (TPN)-dependent. She failed trials of benralizumab and mepolizumab [anti-interleukin (IL)-5 inhibitors], and cromolyn (mast-cell stabilizer). After developing new esophageal stricture, we initiated dupilumab (IL-4/13 inhibitor), recently FDA-approved for EoE. After 9 weeks, esophageal stricture, gut tissue eosinophilia, and prior intestinal ulcerations resolved. She ceased TPN and is tolerating a non-restricted diet, with complete symptom resolution. Our patient’s complete remission with dupilumab shows promise for broadening its use in treating GI involvement in HES, along with primary EGIDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypereosinophilic syndrome (HES) is a rare, multisystem group of disorders characterized by tissue hypereosinophilia or persistent elevated eosinophil counts, defined as peripheral blood eosinophil count greater than 1,500 cells/microliter (uL), with associated eosinophil-mediated organ damage [1]. In idiopathic HES, all known primary and secondary causes of hypereosinophilia must be excluded. First-line treatment for idiopathic hypereosinophilic syndrome is systemic steroids.
Gastrointestinal (GI) involvement is the third most common clinical manifestation of HES, after dermatologic and pulmonary manifestations, affecting nearly 40% of patients [2]. The pathophysiology of GI injury is thought to be secondary to the activation of eosinophils, which leads to degranulation of preformed toxic granules (including major basic protein and eosinophil cationic protein), the release of enzymatic mediators (including lysosomal hydrolases and eosinophil peroxides), cytokine upregulation and production, and local inflammation – all leading to tissue injury [3].
We present a case of idiopathic HES involving the GI tract, which we call “secondary eosinophilic gastrointestinal disease (EGID)”, with eosinophilic esophagitis (EoE), eosinophilic gastritis (EoG), and eosinophilic enteritis (EoN), refractory to corticosteroids, benralizumab (Fasenra) and mepolizumab (Nucala). Dupilumab (Dupixent) has previously been reported to improve symptoms in patients with cutaneous manifestations of HES [4, 5], but to the authors’ knowledge, this is the first report of complete clinical and histopathologic remission of HES-associated GI involvement following dupilumab treatment.
Case report
A 28 year-old female presented to our EGID Clinic with chronic episodic nausea and emesis. She endorsed recurrent infections in childhood, daily emesis at age 7 for 1 month, and 15 years of episodic nausea and bimonthly vomiting. Past medical history was notable for food allergies and asthma. At age 22, she underwent her first esophagogastroduodenoscopy (EGD) and was diagnosed with primary EGID, and specifically EoG (Fig. 1). Her symptoms persisted despite dietary elimination of wheat, dairy, corn, or eggs. She saw some temporary improvement with prednisone but relapsed on steroid-sparing azathioprine. At age 24, EGD showed an esophageal nodule, multiple medium-sized gastric papules, and normal duodenum. Biopsies showed EoG and EoE. Colonoscopy was unremarkable. At age 27, a course of 9 mg daily of budesonide improved her symptoms, but her dysphagia to solids persisted. She was started on swallowed fluticasone for EoE. At that time, she was taking azathioprine 100 mg daily but with subtherapeutic metabolites for optimal therapy for inflammatory bowel disease (IBD) [6]. Due to poor efficacy, azathioprine was discontinued. Serologic celiac evaluation was unremarkable, and she tested negative for celiac genes (HLA DQ2 and DQ8). She was found to have peripheral eosinophilia, 3,940 cells/uL, and multifactorial anemia associated with iron, B12, and folate deficiencies.
EGD at age 30 showed Los Angeles Grade B esophagitis and gastritis, with significant eosinophilic infiltrate in biopsies from the esophagus, stomach, and duodenum. Colonoscopy showed moderate eosinophilic infiltrate in the terminal ileum. Colonic biopsies were normal. Bone marrow biopsy at age 31 confirmed diagnosis of HES. Given her protein-losing enteropathy, vitamin and mineral deficiencies, and oral intake intolerance, she was started on total parenteral nutrition (TPN) at age 32. Shortly after, and while still on TPN 7 days per week, she was started on benralizumab, an interleukin (IL)-5 inhibitor, and 9 mg daily of budesonide. To avoid long-term high-dose corticosteroids, the budesonide dose was ultimately tapered to 3 mg daily within 2 months. EGD at age 33 showed active EoE with 75 eosinophils per high power field (HPF). Repeat endoscopic evaluation that year showed HES involvement of the stomach and terminal ileum, with gastric eosinophils > 100/HPF. Mild duodenal villous blunting was also noted, although without intraepithelial lymphocytosis or eosinophils. Patchy mild-to-moderately active pancolitis was noted, presumed to be infectious or drug-induced, without evidence of eosinophilic or IBD. She subsequently underwent wireless capsule endoscopy (WCE), which showed multiple nodules and ulcers affecting the jejunum and ileum, attributed to EoN.
At this point, having seen limited clinical or histological improvement on benralizumab, in discussion with her hematologist, she switched from benralizumab to mepolizumab, a humanized anti-IL-5 antibody (300 mg subcutaneously monthly), and started prednisone 30 mg daily, which was tapered by about 10 mg each month until reaching 5 mg daily. At this time, she remained on TPN between 3 and 5 days per week. Her dysphagia, nausea, and vomiting notably improved, and peripheral eosinophils were low, between 40 and 200 cells/uL, highly correlated with degree of steroid suppression. However, push enteroscopy still showed mild esophageal rings, edema, and exudates (EREFS score E1R1E1F0S0) along with gastric body erythema, antral nodularity (EG-REFS score E1G0R1E1F0S0), and duodenal erythema and edema. Biopsies were consistent with GI involvement of HES, or secondary EGID, with the proximal esophagus showing 7 eosinophils/HPF, distal esophagus 25 eosinophils/HPF, and moderate inactive non-Heliobacter pylori gastritis with improved gastric eosinophilia (30 eosinophils/HPF). Biopsies also showed duodenal mucosa with moderate villous blunting and crypt hyperplasia, and mixed inflammation in the lamina propria, compatible with moderate non-celiac enteropathy, with 30 eosinophils/HPF. The jejunum did not meet EoN criteria.
She was started on cromolyn, a mast-cell stabilizer, in addition to mepolizumab and prednisone 5 mg daily. She endorsed ongoing intermittent emesis. Repeat push enteroscopy showed esophageal rings and a mid-esophageal stricture (Fig. 2A, B). Findings were also consistent with EoG, including gastric erythema (Fig. 2C) and antral nodularity. The duodenal bulb and postbulbar duodenum demonstrated erythema and edema (Fig. 2D). Pathology showed improved EoE, now in histologic remission (proximal and distal eosinophils of 2 and 7 per HPF, respectively) but with new esophageal stricture, worsening EoG (> 50/HPF) and EoN (> 75/HPF) (Fig. 3A, B). The patient continued to report intermittent emesis and solid dysphagia. Cromolyn was discontinued, and IL-4/13 inhibitor dupilumab, which was FDA-approved for treating EoE in 2022, was added, via 300 mg injection weekly, along with prednisone 40 mg daily, which was tapered to 10 mg daily within 1 month. Follow-up EGD after 9 weeks of therapy showed resolution of her esophageal stricture (Fig. 2D) and of gut eosinophilia (Fig. 2F). On biopsies, EoG and EoN were also resolved (Fig. 3C, D). WCE showed resolution of prior endoscopic pathology. The patient has since been able to wean off TPN and has been tolerating a non-restricted diet with complete resolution of her GI symptoms. At 1 year follow-up, she remains in remission and continues treatment with dupilumab and mepolizumab and has slowly tapered prednisone to a very low dose of 3 mg daily.
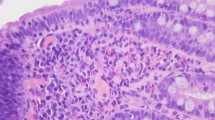
Representative histologic images of pre-treatment biopsies from A the duodenum and B gastric antrum showing increased eosinophils within the lamina propria, including degranulating forms and patchy infiltration of crypt and surface epithelium by eosinophils. A reduction in eosinophils was observed in post-treatment biopsies from C the duodenum and D gastric antrum
Discussion
We report a rare case of HES with secondary EGID, including EoE and non-esophageal involvement (EoG and EoN) that responded to high-dose corticosteroids but failed multiple steroid-sparing agents including immunomodulator, mast-cell stabilizer, and biologics (IL-5 inhibitors). The novel addition of dupilumab, an IL-4/13 inhibitor, remarkably promoted clinical and histopathologic remission in addition to resolution of esophageal stricture. The underlying complex pathophysiology of EGID in general, and more specifically GI involvement in HES, has not been entirely elucidated. Non-esophageal EGIDs are less frequent and more challenging to treat than isolated EoE. Most patients require dietary elimination and/or pharmaceutical interventions, mainly corticosteroids, but also biologics (monoclonal antibodies against IL-4, IL-5, TNFα, integrin α4β7, and IgE), mast-cell stabilizers, leukotriene (LT)-receptor antagonists, and antihistamines [7]. Our patient was started on benralizumab, an anti-IL-5 inhibitor, without success. In a clinical trial, benralizumab was shown to completely deplete both blood and GI tract eosinophils in HES patients with GI involvement [8], but unfortunately our patient did not achieve clinical or histopathologic improvement. Our patient was next started on anti-IL-5 inhibitor mepolizumab, with concomitant prednisone, also with minimal benefit. While mepolizumab can improve esophageal eosinophilia, it failed to improve duodenal eosinophilia in patients with comorbid EoE [9, 10]. The patient’s peripheral eosinophilia was sensitive to steroid suppression, but improved peripheral eosinophilia did not correlate with improved symptoms nor improved histologic GI findings.
GI involvement of HES, or secondary EGID, can be potentially severe and debilitating, leading to TPN-dependence, and vitamin and nutrient deficiencies as observed in our patient. In a patient who has HES and any GI symptoms, providers should have a high index of suspicion for GI involvement of HES. If first-line treatments are refractory, they should consider treatment with dupilumab to obtain clinical and histopathologic remission and prevent further complications. Although there have been studies demonstrating minimal differences in efficacy and safety of various IL-5 inhibitors on eosinophilic airway disease [11], little research has been done comparing the effect of various IL-5 inhibitors on eosinophilic tissue infiltration in patients with GI involvement. Our case report shows promise of dupilumab over other IL-5 inhibitors. One potential limitation is that the patient achieved remission while on a combination of mepolizumab and dupilumab. However, our patient was highly symptomatic with active disease and persistence of her secondary EGID on mepolizumab and prednisone alone, but we noted significant symptomatic improvement following the initiation of dupilumab ultimately leading to total remission. This strongly suggests that dupilumab was the key addition that induced remission, as it has been able to achieve in cases of primary EGIDs [12], not necessarily the co-administration of both medications. To our knowledge, this is the first report of dupilumab demonstrating benefit in a patient with GI involvement of HES. In addition to dupilumab being documented as an effective treatment for various conditions, such as asthma, atopy, EoE, and chronic rhinosinusitis [13], our report shows promise for broadening its use for the treatment of HES-associated secondary EGID, along with primary EGIDs.
References
Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129–48.
Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124:1319-1325.e3.
Nanagas VC, Kovalszki A. Gastrointestinal manifestations of hypereosinophilic syndromes and mast cell disorders: a comprehensive review. Clinic Rev Allerg Immunol. 2019;57:194–212.
Wieser JK, Kuehn GJ, Prezzano JC, et al. Improvement in a patient with hypereosinophilic syndrome after initiation of dupilumab treatment. JAAD Case Reports. 2020;6:292–5.
Jiang X, Ye J, Wu X, et al. A case of complete recovery in a hypereosinophilic dermatitis patient with dupilumab. Inflamm Res. 2023;72:875–8.
Cuffari C, Hunt S, Bayless T. Utilisation of erythrocyte 6-thioguanine metabolite levels to optimise azathioprine therapy in patients with inflammatory bowel disease. Gut. 2001;48:642–6.
Chen PH, Anderson L, Zhang K, et al. Eosinophilic gastritis/gastroenteritis. Curr Gastroenterol Rep. 2021;23:13.
Kuang FL, De Melo MS, Makiya M, et al. Benralizumab completely depletes gastrointestinal tissue eosinophils and improves symptoms in eosinophilic gastrointestinal disease. J Allergy Clin Immunol Pract. 2022;10:1598-1605.e2.
Straumann A, Conus S, Grzonka P, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21–30.
Conus S, Straumann A, Bettler E, et al. Mepolizumab does not alter levels of eosinophils, T cells, and mast cells in the duodenal mucosa in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:175–7.
Akenroye A, Lassiter G, Jackson JW, et al. Comparative efficacy of mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: a bayesian network meta-analysis. J Allergy Clin Immunol. 2022;150:1097-1105.e12.
Greenhawt M. Biologics in eosinophilic gastrointestinal disease treatment: a new frontier. Ann Allergy Asthma Immunol. 2023;130:155–6.
Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82:377–88.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest
Informed consent
Informed patient consent was obtained for publication of the case details.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moffatt, C., Soriano, C., Dawson, D.W. et al. Successful novel use of dupilumab for gastrointestinal involvement of idiopathic hypereosinophilic syndrome: case report and review of the literature. Clin J Gastroenterol (2024). https://doi.org/10.1007/s12328-024-02036-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12328-024-02036-4






