Abstract
Eosinophilic enteritis (EoN) is associated with an eosinophilic infiltrate confined to the small intestine, but treatment options other than diet and corticosteroid therapy are scarce. There is only one report of the use of dupilumab for eosinophilic gastrointestinal disease, involving three pediatric patients. We report a case of successful induction of remission with dupilumab in a 53 year-old female patient with steroid-dependent EoN. The patient presented to the emergency room with uncontrollable abdominal pain and CT revealed a thickened ileal wall and small amount of ascites. Despite no abnormalities on endoscopy, histological examination revealed numerous eosinophilic infiltrates (> 100/HPF) and degranulation in the ileal lamina propria, diagnosing the patient with EoN. The patient achieved clinical remission with prednisolone, but EoN relapsed during tapering. Long-term steroid therapy was inappropriate due to mandibular osteomyelitis and osteoporosis, and she was switched to 9 mg budesonide, an intestine-soluble topical steroid without effect. Dupilumab administration resulted in resolution of abdominal pain, and remission was maintained after discontinuation of budesonide. Histological remission was confirmed 2 months after dupilumab administration. This is the first report of remission induced and maintained with dupilumab in an adult patient with EoN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eosinophilic gastrointestinal disease (EGID) is a chronic allergic disease with eosinophilic inflammation of the gastrointestinal tract, and EGID confined to the small intestine is defined as eosinophilic enteritis (EoN) [1, 2]. The prevalence of eosinophilic gastroenteritis in adults is rare at 5.1/100.000 [3] and unlike eosinophilic esophagitis (EoE), for which guidelines have been established, the clinical management of distal esophageal EGID (non-EoE EGID) is not well established. Natural history of EGID progression was reported to be classified into 3 groups: single flare, recurring course, and continuous course [4]. Thus far, only diet and corticosteroid therapy have been the first choice, and a long-term strategy for EGID with recurring or continuous course has not yet been established [1, 5]. There are only a few case reports of treatment with budesonide and some biologics, and so far they are only one of the treatment options [1, 4, 5]. In addition, there is only one report to date on dupilumab for EGID in three pediatric patients [6]. We report here a case of an adult EoN patient with severe asthma who was treated with dupilumab in addition to budesonide and was able to induce and maintain remission.
Case presentation
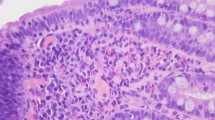
A 53 year-old woman who had been experiencing abdominal pain several times a year for the past 10 years and had been diagnosed with irritable bowel syndrome was brought to the emergency room with uncontrolled abdominal pain with bloating. She had refractory asthma, was a regular inhaled corticosteroids user, and had a history of mepolizumab use and anaphylaxis with non-steroidal anti-inflammatory drugs (NSAIDs). According to our history interview, the patient had a long history of refractory asthma and abdominal symptoms, but there was no apparent association between exacerbations of both diseases. On arrival at the hospital, blood samples revealed increased eosinophils and elevated thymus- and activation-regulated chemokine (TARC) (Table 1), and CT showed thickening of the ileum wall and pelvic ascites (Fig. 1A, B). The patient developed anaphylactic shock due to acetaminophen administered in the emergency room, and pentazocine was used as an analgesic under inpatient management. Although upper and lower gastrointestinal endoscopy showed no abnormality including in the ileum (Fig. 1C), histological examination revealed massive infiltration (> 100/HPF) and degranulation of eosinophils mainly in the lamina propria of the ileum (Fig. 1D, E, F), secondary hypereosinophilia including infection was ruled out, and a patient was diagnosed as EoN. While neither the double-balloon endoscope nor the capsule endoscope was available, 50 cm from the terminal ileum to the oral side was observed, showing normal endoscopic findings and numerous eosinophils infiltrating the tissue. There was no or only small amounts of eosinophil infiltrates of the esophagus, stomach, duodenum, and colon. Prednisolone 1 mg/kg and montelukast resulted in clinical remission with resolution of abdominal pain within 1 week. In addition, eosinophil count and TARC normalized, and ileal wall thickening and ascites disappeared on CT. Thereafter, prednisolone was then tapered over 8 weeks, but when tapered from 7 to 6 mg, uncontrolled pain returned. CT showed edema of the ileum wall and a small amount of ascites, and while the endoscopic findings were normal, numerous eosinophils infiltrated ileum, leading to the diagnosis of EoN recurrence. Since long-term steroid administration was inappropriate due to the history of mandibular osteomyelitis and osteoporosis, prednisolone was discontinued, and 9 mg budesonide, an intestinal local steroid, was used to induce remission (Fig. 2, day 1). The patient kept taking montelukast after relapsing. Nevertheless, the abdominal pain did not improve after starting budesonide, and the eosinophils increased further (Fig. 2, day 10). Because of poor therapeutic response to budesonide and refractory asthma, dupilumab was additionally administered (Fig. 2, day13). We also offered the patient mepolizumab as a treatment option, but the patient preferred dupilumab because of a history of discontinuation without symptom improvement by mepolizumab for asthma. Abdominal pain resolved 1 week after dupilumab administration (Fig. 2, day20), and the patient achieved clinical resolution thereafter. Eosinophils and TARC gradually decreased to normal values (Fig. 2). The patient then self-injected dupilumab 300 mg every 2 weeks, and budesonide was continued at 9 mg for 8 weeks, then tapered to 6 mg, 3 mg every 2 weeks, and discontinued (Fig. 2). Two months after treatment with dupilumab (Fig. 2, day83), endoscopy was performed and histological remission was confirmed (Fig. 3). The patient continued to receive dupilumab and had no relapse until 6 months after initiation.
Images at the time of EoN diagnosis. a, b CT shows ileal wall edema (red arrow) and small amount of ascites (white arrow). c Endoscopy shows no erosions or ulcers in the small intestine. d–f Hematoxylin–eosin (HE) staining shows numerous eosinophils infiltrate mainly in mucosal intrinsic layer. d Low magnification. e High magnification (eosinophil count > 100/HPF). f Degranulation of eosinophils is observed. Abbreviations: EoN eosinophilic enteritis, HPF high-power field
Progress chart of symptoms and laboratory data from EoN treatment. On day 1, prednisolone 6 mg was discontinued and budesonide 9 mg was started. The patient had a history of anaphylaxis to acetaminophen and NSAIDs and was on frequent intravenous pentazocine administration, but pain control was poor. The pain did not improve until day 10, and the eosinophil count increased. 600 mg of dupilumab was administered for the first time on day 13, and the abdominal pain slightly improved from day 15 and completely disappeared on day 20. Eosinophil counts and TARC levels also peaked around the time of dupilumab administration and then declined and normalized. The patient has been receiving subcutaneous injections of 300 mg of dupilumab every 2 weeks, and her symptoms have completely resolved since then. Abbreviations: EoN eosinophilic enteritis, NSAIDs non-steroidal anti-inflammatory drugs, TARC thymus- and activation-regulated chemokine, NRS numerical rating scale
Discussion
The symptoms of EGID are often non-specific and vary depending on the intestinal tract involved and the extent of eosinophilic inflammation (mucosal, muscular, serosal pattern, etc.) [1], making the diagnosis difficult and easily overlooked. Therefore, an accurate diagnosis is usually made several years after the initial symptoms, and this patient also had 10 years elapsed between the first symptoms and diagnosis, although the exact timing of onset is unknown. It is agreed that a definitive diagnosis requires 1. recurrent gastrointestinal symptoms, 2. increased eosinophils for high-power field in biopsy specimens, and 3. absence of secondary causes of gastrointestinal eosinophilia [7], and this patient met all criteria. CT findings of EGID have been reported to include wall thickening, polyps, ulcers, stenosis, ascites, and lymphadenopathy, all of which are non-specific changes [8]. Endoscopic findings have been reported to include erythema, edema, white spots, localized erosions, ulcers, thickened folds, polyps, nodules, and friability, which may appear normal or non-specific [9]. The patient presented with abdominal pain and bloating, numerous eosinophils infiltrated from the lamina propria to the submucosa, despite lack of specific findings on endoscopy, and a thickened wall of the ileum and a small amount of ascites accumulation on CT, suggesting a serosal type with eosinophil infiltration of the entire intestinal wall [1, 9]. Patients with functional gastrointestinal disorder were reported to display duodenal mild inflammation including eosinophils associated with the abdominal symptoms [10, 11]. In this case, there were numerous eosinophilic infiltrates in the ileum, but almost no eosinophils in other regions, including the duodenum.
While no validated guidelines exist for the clinical management of patients with non-EoE EGID, first-line treatment is diet and corticosteroid therapy [1]. Corticosteroids are indicated in severe cases and in cases that are refractory to dietary therapy, and clinical remissions of 50% to 90% have been reported [7]. Approximately 20% of EGID patients are corticosteroid dependent [1], in which case the efficacy of low-dose prednisolone (5–10 mg daily) and budesonide has been reported from a few case reports [4, 7, 12, 13]. Budesonide has high local glucocorticoid activity but minimal systemic side effects due to hepatic initial metabolism, and the recommended dose is 9 mg/day, then tapering to 6 mg/day and finally to 3 mg/day as maintenance therapy [12, 13]. Montelukast has also been suggested to be useful in adults and children with eosinophilic gastroenteritis and duodenal eosinophils [14, 15]. In addition to montelukast, antihistamines and suplatast tosilate have been reported to be effective in reducing or discontinuing systemic steroids [16, 17]. The patient was steroid dependent, but already on montelukast and levocetirizine hydrochloride, suggesting no steroid withdrawal effect of these drugs in the present case. In addition, the patient declined to perform food elimination. Considering the risks of long-term systemic administration of steroids (mandibular osteomyelitis and osteoporosis), budesonide 9 mg was administered at the time of relapse. However, the severe abdominal pain did not improve at all after 10 days of budesonide administration, and the patient requested intensification of treatment, so another treatment was considered.
While the pathogenesis of EGID is only partially understood, recent studies have demonstrated that Th2 cytokines (IL-4, IL-5, IL-13) and chemokines (eotaxin1, eotaxin3, α4β7 integrin) are involved in the pathogenesis of EGID [1]. TARC is a Th2 chemokine and is often elevated in patients with active atopic dermatitis and asthma. In a recent study, TARC has been reported to be useful for the diagnosis of eosinophilic gastritis and help monitor disease activity [18]. Despite the lack of reports on the usefulness of TARC in diagnosing EoN and assessing disease activity, in the present case, TARC reflected the disease activity of EoN. Dupilumab is a monoclonal antibody that blocks the downstream signals of IL-4 and IL-13 and is FDA approved for the treatment of moderate to severe atopic dermatitis and asthma or chronic rhinosinusitis with nasal polyps [19]. Dupilumab was shown in a recent phase III randomized controlled trial to effectively reduce disease activity and symptoms in EoE compared to placebo [20]. In addition, a recent case series reported on the therapeutic efficacy of dupilumab in three pediatric Non-EoE EGID patients with severe atopic dermatitis and severe asthma [6]. All patients were steroid dependent or refractory to existing therapy, including budesonide and systemic steroids, dupilumab was selected as the other treatment option and showed improvement in symptoms and histological eosinophilic infiltration [6]. The present adult patient also had similarly severe asthma, and dupilumab was effective in induction and long-term maintenance of remission in steroid-dependent EoN. In the present case, the patient had a long history of refractory asthma and abdominal symptoms, but there was no apparent association between exacerbations of both diseases. Therefore, we obtain ethics review board approval before prescribing dupilumab for EGID. The TRAVERSE study suggests safety and efficacy of dupilumab in adult patients with moderate to severe asthma for 148 weeks [21]. The patient has had no adverse events of note to date and will continue treatment with caution regarding efficacy and adverse events. A phase II clinical trial (NCT03678545) evaluating the use of dupilumab in eosinophilic gastritis patients aged 12–70 years is ongoing and is expected to be reported in the future.
This is the first report of an adult patient with EoN who was steroid dependent and unable to achieve induction of remission with budesonide, but who achieved induction of remission and long-term maintenance with dupilumab. This report is very valuable in considering treatment options for patients with EGID who are refractory to existing dietary and steroid therapies. Accumulation of knowledge on efficacy and safety of biologics, including dupilumab, for refractory EGID is necessary for the future.
Abbreviations
- EGID:
-
Eosinophilic gastrointestinal disease
- EoE:
-
Eosinophilic esophagitis
- EoN:
-
Eosinophilic enteritis
- Non-EoE EGID:
-
EGID distal to the esophagus
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- TARC:
-
Thymus- and activation-regulated chemokine
References
Licari A, Votto M, D’Auria E, et al. Eosinophilic gastrointestinal diseases in children: a practical review. Curr Pediatr Rev. 2020;16:106–14.
Dellon ES, Gonsalves N, Abonia JP, et al. International consensus recommendations for eosinophilic gastrointestinal disease nomenclature. Clin Gastroenterol Hepatol. 2022;20:2474-84.e3.
Mansoor E, Saleh MA, Cooper GS. Prevalence of eosinophilic gastroenteritis and colitis in a population-based study, from 2012 to 2017. Clin Gastroenterol Hepatol. 2017;15:1733–41.
Pineton de Chambrun G, Gonzalez F, Canva JY, et al. Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2011;9:950-6.e1.
Chen PH, Anderson L, Zhang K, et al. Eosinophilic gastritis/gastroenteritis. Curr Gastroenterol Rep. 2021;23:13.
Patel N, Goyal A, Thaker A, et al. A case series on the use of dupilumab for treatment of refractory eosinophilic gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2022;75:192–5.
Zhang M, Li Y. Eosinophilic gastroenteritis: a state-of-the-art review. J Gastroenterol Hepatol. 2017;32:64–72.
Anuradha C, Mittal R, Yacob M, et al. Eosinophilic disorders of the gastrointestinal tract: imaging features. Diagn Interv Radiol. 2012;18:183–8.
Zhang L, Duan L, Ding S, et al. Eosinophilic gastroenteritis: clinical manifestations and morphological characteristics, a retrospective study of 42 patients. Scand J Gastroenterol. 2011;46:1074–80.
Walker MM, Talley NJ, Prabhakar M, et al. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2009;29:765–73.
Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut. 2014;63:262–71.
Tan AC, Kruimel JW, Naber TH. Eosinophilic gastroenteritis treated with non-enteric-coated budesonide tablets. Eur J Gastroenterol Hepatol. 2001;13:425–7.
Siewert E, Lammert F, Koppitz P, et al. Eosinophilic gastroenteritis with severe protein-losing enteropathy: successful treatment with budesonide. Dig Liver Dis. 2006;38:55–9.
Friesen CA, Kearns GL, Andre L, et al. Clinical efficacy and pharmacokinetics of montelukast in dyspeptic children with duodenal eosinophilia. J Pediatr Gastroenterol Nutr. 2004;38:343–51.
Schwartz DA, Pardi DS, Murray JA. Use of montelukast as steroid-sparing agent for recurrent eosinophilic gastroenteritis. Dig Dis Sci. 2001;46:1787–90.
Sheikh RA, Prindiville TP, Pecha RE, et al. Unusual presentations of eosinophilic gastroenteritis: case series and review of literature. World J Gastroenterol. 2009;15:2156–61.
Gonsalves N. Eosinophilic gastrointestinal disorders. Clin Rev Allergy Immunol. 2019;57:272–85.
Shoda T, Wen T, Caldwell JM, et al. Molecular, endoscopic, histologic, and circulating biomarker-based diagnosis of eosinophilic gastritis: Multi-site study. J Allergy Clin Immunol. 2020;145:255–69.
Pesek RD, Gupta SK. Future therapies for eosinophilic gastrointestinal disorders. Ann Allergy Asthma Immunol. 2020;124:219–26.
Dellon ES, Rothenberg ME, Collins MH, et al. Dupilumab in adults and adolescents with eosinophilic esophagitis. N Engl J Med. 2022;387:2317–30.
Wechsler ME, Ford LB, Maspero JF, et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med. 2022;10:11–25.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no conflict of interest.
Ethical approval
The administration of budesonide and dupilumab to this EoN patient with severe asthma was done after obtaining the approval of the ethics review committee of Soka municipal hospital and the informed consent of the patient.
Informed consent
Written informed consent was given by the patient for the publication of this manuscript. Identifying information, aside from age and sex, was removed and the images provided were anonymized to protect patient confidentiality.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Watanabe, S., Uchida, H., Fujii, R. et al. The efficacy of dupilumab in induction and maintenance of remission in an adult patient with steroid-dependent eosinophilic enteritis (EoN). Clin J Gastroenterol 16, 527–531 (2023). https://doi.org/10.1007/s12328-023-01799-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-023-01799-6






