Abstract
This is a case of a 67-year-old woman diagnosed with a 35-mm pancreatic body cancer with a chief complaint of epigastric discomfort. Computed tomography demonstrated invasion of the common hepatic artery, portal vein, and stomach, and chemotherapy was initiated for locally advanced pancreatic cancer. After 9 months of chemotherapy, the tumor remained stable on imaging, and the tumor markers were within the normal range. After additional chemoradiotherapy, the patient underwent a conversion surgery, a pancreaticoduodenectomy. Magnetic resonance cholangiopancreatography (MRCP) at the time of diagnosis demonstrated main pancreatic duct (MPD) dilatation on the tail side of the tumor; however, most of the MPD signal disappeared on MRCP after chemotherapy. Surgical findings failed to identify MPD on the first pancreatic resection plane, and additional resection was conducted; however, no MPD was found. As a pancreatic duct anastomosis was not available, pancreatic reconstruction was selected for pancreaticogastric anastomosis using the invagination method. Pathologically, the pancreatic tissue on the tail side of the tumor was replaced by fibrotic tissue, and MPD could not be identified. To the best of our knowledge, this is the first case report of the disappearance of a dilated pancreatic duct on the tail side accompanied by exocrine tissue loss during preoperative treatment for pancreatic cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is frequently diagnosed at an advanced stage [1, 2]. Imaging findings are frequently accompanied by the dilation of the main pancreatic duct (MPD) on the tail side of the tumor. Gross and histological findings are characterized by chronic concomitant pancreatitis with fibrosis referred to as “hard pancreas”.
Recently, with advancements in chemotherapy and radiotherapy for pancreatic cancer, preoperative treatment has become more prevalent [2]. A decline in the postoperative recurrence rate of resectable pancreatic cancer and the availability of conversion resection for unresectable pancreatic cancer are expected to enhance the overall prognosis of pancreatic cancer [3,4,5,6,7].
The primary focus is on the evaluation of the primary lesion and the presence of distant metastases in the preoperative imaging, with minimal attention paid to the tail of the pancreas. In this report, an unusual case is elucidated in which the dilated MPD on the tail side disappeared radiographically and histologically during preoperative treatment of locally advanced pancreatic cancer (LAPC).
Case report
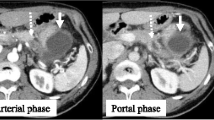
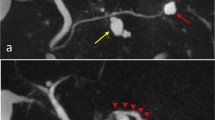
A 67-year-old woman with epigastric discomfort was diagnosed with a pancreatic tumor. A 35-mm diameter tumor with poor contrast enhancement mainly located in the pancreatic body was revealed on contrast-enhanced computed tomography (CT) (Fig. 1a). The tumor was pathologically diagnosed as pancreatic ductal adenocarcinoma (PDAC) via endoscopic ultrasound needle biopsy. The tumor involved the common hepatic artery, portal vein, and stomach on CT and a diagnosis of LAPC was established (Fig. 1b). Magnetic resonance cholangiopancreatography (MRCP) demonstrated that the MPD was stenosed at the tumor site and dilated on the tail side (Fig. 2a). The serum levels of the tumor markers CEA and CA19-9 were both within normal limits. Subsequently, chemotherapy for LAPC was then initiated. A change in chemotherapy regimen was also instituted, from FOLFIRINOX to gemcitabine and nab-paclitaxel due to tumor growth on CT findings, but the patient was able to maintain stable disease (SD) according to the Response Evaluation Criteria in Solid Tumors criteria for 9 months (Fig. 1d, e). During this period, CA19-9 remained within the normal range and maintained a good performance status. According to these findings, conversion surgery was planned, and the patient underwent radical pancreaticoduodenectomy (PD) after carbon-ion radiotherapy. Furthermore, MRCP after chemotherapy and before radiotherapy showed a signal disappearance in most parts of the MPD on the tail side of the tumor, which should have been dilated (Fig. 2b). The pancreatic parenchyma on the tail of the tumor was not changed on CT before and after chemotherapy (Fig. 1c, f). Surgical findings demonstrated no common hepatic artery invasion, and PD with portal vein resection was performed. Findings were suggestive of a “hard pancreas”. Initial pancreatic transection was performed at the level of the left margin of the superior mesenteric artery; however, the MPD could not be grossly identified on the pancreatic dissection section (Fig. 3a, b). Intraoperative frozen section did not show evidence of the MPD in the pancreatic section, with no findings of malignancy. An additional 2-cm-wide pancreas was resected to identify the MPD in preparation for anastomosis to the jejunal mucosal layer (Fig. 3c). As the MPD could not be identified, pancreatic reconstruction was performed via pancreaticogastric anastomosis using the invagination method (Fig. 3d). Postoperatively, pancreatic leakage was uncomplicated, and the patient’s course was uneventful. Gross examination revealed a 23-mm diameter, hard, whitish irregular mass with indistinct borders (Fig. 4a). Histological findings in the tumor area revealed well-to-moderately differentiated adenocarcinoma with tubular invasive growth against a background of preoperative treatment-related changes such as fibrosis, edema, and mucous degeneration (Fig. 4b). The percentage of viable tumor residuals was approximately 40%. Extrapancreatic invasion was noted, but no portal vein, artery, or plexus invasion was observed. In the noncancerous areas, abundant pancreatic acinar cells were observed on the pancreatic head side of the tumor (Fig. 4d). Conversely, on the pancreatic tail side of the tumor, there were islets of Langerhans but few acinar cells, the MPD disappeared, and the pancreatic parenchyma was replaced with fibrotic tissue (Fig. 4c). No cancer cell infiltration was observed within the fibrosis.
CT images at the time of pancreatic cancer diagnosis (a–c) and before surgery (d–f). The pancreatic body cancer with portal vein invasion and gastric wall invasion before treatment (a) showed tumor reduction with chemotherapy (d). Before the treatment, the pancreatic body cancer had invaded the common hepatic artery (b), and the soft shadows around the hepatic artery remained after chemotherapy (e). There is no change in the parenchymal findings of the pancreatic tail on the tail side of the tumor before (c) and after (f) chemotherapy
(a) MRCP at the time of diagnosis of pancreatic cancer showed dilatation of the main pancreatic duct (yellow allows) on the tail side of the tumor and multiple cysts or dilated branching ducts (yellow–green arrowheads). (b) On MRCP after chemotherapy, the signal from the main pancreatic duct on the tail side of the tumor was disappeared except for a short portion (yellow allows). The multiple cyst findings in the pancreatic tail were unchanged at diagnosis
(a) Schema showing the location of the pancreatic tumor (black arrowheads) and the first (black solid line) and second (black dotted line) pancreatic transection lines. (b) Intraoperative picture showing the first pancreatic transection line (black arrowheads). The pancreas was transected on the drain. (c) Intraoperative picture showing additional pancreas sharply resected with scalpel (black arrowheads). (d) Intraoperative picture before pancreatic reconstruction. The black arrows showed the pancreatic transection plane, but the main pancreatic duct could not be identified. Panc; pancreas, SpA; splenic artery
(a) In the cross-section of the pancreatic resection specimen, pancreatic cancer was observed as a white irregular mass with indistinct boundaries (black arrowheads). (b) Histological findings in the tumor area showed well-to-moderately differentiated adenocarcinoma with tubular invasive growth against a background of preoperative treatment-related changes such as fibrosis, edema, and mucous degeneration. The percentage of viable tumor residuals was about 40%. (c) Histological findings on the pancreatic tail side of the tumor. While the islets of Langerhans remain, the acinar cells, conduits, and main pancreatic ducts have disappeared and been replaced by fibrotic tissue. (d) Histological findings on the pancreatic head side of the tumor. The acinar cells and conduits are abundantly preserved
Discussion
We experienced an unusual case in which the MPD on the tail side of the tumor, which was initially dilated at the time of diagnosis, disappeared due to fibrosis during preoperative chemotherapy for pancreatic cancer.
Preoperative chemotherapy has become the standard treatment for advanced pancreatic cancer in recent years. Particularly, the indication for conversion surgery is determined after prolonged preoperative treatment lasting 6 months or longer for LAPC [7, 8]. In the present case, because hepatic artery invasion was also observed, conversion resection was planned after confirming SD status after 9 months of chemotherapy and additional chemoradiotherapy [9]. Conversion surgery usually requires vascular combined resection and can be more complicated than conventional pancreatectomy, but morbidity and mortality rates have been reported to be comparable to those of conventional surgery [8]. This may be because the indication criteria for conversion surgery include not only good tumor control, but also good maintenance of performance status and nutritional status despite long-term multidisciplinary treatment [7].
Usually, at the time of diagnosis, PDAC has already invaded the MPD, and the upstream duct on the pancreatic tail side is dilated. In this case, MRCP performed before treatment confirmed MPD dilatation. In the PD procedure for pancreatic head region cancer, the familiar intraoperative findings are a “hard pancreas” and a dilated MPD on the pancreatic resection surface. However, no case reports of disappearance of the dilated pancreatic duct during preoperative therapy were found in the literature, and this is the first report of such a case. In this case, the signal from the MPD on the tail side of the tumor, which would have been dilated before chemotherapy on MRCP, was almost disappeared after chemotherapy. At the time of surgery 3 months later, the MPD had completely disappeared. Histopathologically, the MPD was not identified as well. The islets of Langerhans remained in the pancreatic parenchyma; however, the acinus and conduits had almost disappeared and were replaced by fibrotic tissue. Therefore, in this case, all of the exocrine structures, from the acinus to the intercalated duct, and large pancreatic ducts had disappeared. Reconstruction with the gastrointestinal tract was not possible due to the disappearance of the MPD, but the risk of pancreatic fistula was rather low due to the reduced pancreatic exocrine function.
How did this dramatic change in the pancreatic duct image occur? One reason could be attributed to the changes in the sites where inflammation and fibrosis occur. Pancreatic cancer is associated with concomitant chronic obstructive pancreatitis and dilatation of the MPD on the tail side, also referred to as the “hard pancreas” [10]. Chronic pancreatitis, which is typically caused by alcohol, is similarly characterized by fibrosis and sclerosis of the pancreas and irregular duct dilation. Fibrosis of the pancreas is observed mainly between the lobes and does not involve the large conduits [11]. Conversely, autoimmune pancreatitis also demonstrates chronic inflammation and fibrosis in the pancreatic parenchyma; however, unlike chronic pancreatitis, it presents with diffuse stenosis of the pancreatic duct. Histopathologically, there is a marked cellular infiltrate consisting mainly of lymphocytes and plasma cells in the fibrosis, and the cellular infiltrate is more severe around the pancreatic duct than in the lobule. The pancreatic duct narrowing is attributed to the periductal cellular infiltration. This difference in the primary site of inflammation and fibrosis can be reflected in the imaging findings, such as the pancreatic duct stenosis or dilation. In this case, the patient initially presented with findings of a dilated MPD with obstructive pancreatitis; however, the MPD may have disappeared during preoperative chemotherapy as the main site of inflammation and fibrosis shifted to the periductal area. There were no previous reports on chemotherapy and pancreatic duct injury, and the disappearance of the MPD was not directly considered a complication of chemotherapy.
Pancreatic cancer has a strong desmoplastic stroma reaction [12], and another possibility is that fibrosis spreads with cancer invasion. However, the changes were limited to one side of the main lesion, that is, the pancreatic tail, and histological examination demonstrated no evidence of cancer cell invasion beyond the primary lesion, suggesting that ductal loss associated with cancer invasion was excluded.
This is the first case report of the disappearance of a dilated pancreatic duct on the tail side of the tumor during preoperative treatment. As preoperative treatment for pancreatic cancer has become the standard of care, this case indicates the need to evaluate the status of the pancreas on the tail side of the tumor, especially when PD is planned.
References
Saad AM, Turk T, Al-Husseini MJ, et al. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer. 2018;18:688.
Park W, Chawla A, O’Reilly EM. Pancreatic cancer: a review. JAMA. 2021;326:851–62.
Versteijne E, van Dam JL, Suker M, et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch Randomized PREOPANC trial. J Clin Oncol. 2022;40:1220–30.
Jang JY, Han Y, Lee H, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg. 2018;268:215–22.
Nagakawa Y, Sahara Y, Hosokawa Y, et al. Clinical impact of neoadjuvant chemotherapy and chemoradiotherapy in borderline resectable pancreatic cancer: analysis of 884 patients at facilities specializing in pancreatic surgery. Ann Surg Oncol. 2019;26:1629–36.
Satoi S, Yamaue H, Kato K, et al. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2013;20:590–600.
Ushida Y, Inoue Y, Oba A, et al. Optimizing indications for conversion surgery based on analysis of 454 consecutive Japanese cases with unresectable pancreatic cancer who received modified FOLFIRINOX or gemcitabine plus nab-paclitaxel: a single-center retrospective study. Ann Surg Oncol. 2022;29:5038–50.
Satoi S, Yamamoto T, Yamaki S, et al. Surgical indication for and desirable outcomes of conversion surgery in patients with initially unresectable pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 2020;4:6–13.
Igarashi T, Yamada S, Hoshino Y, et al. Prognostic factors in conversion surgery following nab-paclitaxel with gemcitabine and subsequent chemoradiotherapy for unresectable locally advanced pancreatic cancer: Results of a dual-center study. Ann Gastroenterol Surg. 2023;7:157–66.
Fukumura Y, Suda K, Mitani K, et al. Expression of transforming growth factor beta by small duct epithelium in chronic, cancer-associated, obstructive pancreatitis: an in situ hybridization study and review of the literature. Pancreas. 2007;35:353–7.
Masamune A, Kikuta K, Kume K, et al. Nationwide epidemiological survey of chronic pancreatitis in Japan: introduction and validation of the new Japanese diagnostic criteria 2019. J Gastroenterol. 2020;55:1062–71.
Beyer G, Habtezion A, Werner J, et al. Chronic pancreatitis. Lancet. 2020;396:499–512.
Acknowledgements
We would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yusuke Okamura, Ken Fukumitsu, Tatsuya Okishio, Yuri Kanaya, Yasuhiro Saito, Ryo Kudo, Michina Morioka, Shinsuke Shibuya, Toshihide Yamaoka, and Dai Manaka declare that they have no confict of interest.
Human/animal rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Okamura, Y., Fukumitsu, K., Okishio, T. et al. A case of pancreatic body cancer with disappearance of the dilated pancreatic duct on the tail side during preoperative treatment. Clin J Gastroenterol (2024). https://doi.org/10.1007/s12328-024-02005-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12328-024-02005-x