Abstract
Here, we report a rare case of intrahepatic sarcomatoid cholangiocarcinoma that expressed granulocyte colony-stimulating factor (G-CSF). An 87-year-old man who presented with a continuous high-grade fever and cough over two weeks, and increased inflammatory response was admitted to our hospital. Laboratory tests revealed marked granulocytosis and high serum levels of G-CSF and interlukin-6. Imaging studies showed a huge liver mass in his right lobe and intrahepatic metastasis. He died of progressive disease. Pathological findings of the primary liver tumor at autopsy showed sarcomatous change; the specimen was positive for cytokeratins (AE1/AE3, cytokeratin-7, cytokeratin-19) and G-CSF. Few cases of G-CSF-producing intrahepatic sarcomatoid cholangiocarcinoma have been reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Granulocyte colony-stimulating factor (G-CSF) is a naturally occurring glycoprotein that stimulates proliferation and maturation of precursor cells in bone marrow into fully differentiated neutrophils [1]. Since 1977, when Asano et al. first reported a case of G-CSF-producing lung cancer, several studies have reported G-CSF production by malignant cells in lung, thyroid gland, urinary bladder, gall bladder, uterine cervix and liver [2,3,4,5,6,7,8]. G-CSF-producing tumors generally grow rapidly and have poor prognoses.
Sarcomatoid cholangiocarcinoma (CCA) is an extremely rare primary liver tumor, and its pathogenesis is unclear. Several hypotheses have been proposed to explain the intermingling of epithelial and mesenchymal malignancies, including biphasic differentiation from pluripotent stem cells, and re-differentiation of immature multipotent carcinoma cells transformed from carcinoma cells. About 4.5% of surgical and autopsied cases of intrahepatic CCA show sarcomatoid transformation [9].
Few cases of G-CSF-producing intrahepatic CCA have been reported [10,11,12,13,14,15,16,17,18]. Here, we report such a case.
Case report
An 87-year-old man was admitted to our hospital due to cough and continuous high-grade fever of two weeks’ duration. He had a history of dementia and hypertension. He did not have hepatitis B or C. He was a non-drinker.
He had a persistent cough and temperature above 38 °C. Hematological laboratory data upon admission were white blood cell count (WBC): 35,560/μl (neutrophils 93.8%), C-reactive protein (CRP): 30.0 mg/dl, α-fetoprotein (AFP): 2.3 ng/ml, protein induced by absence of vitamin K (PIVKA)-II: 26 mAU/ml, carcinoembryonic antigen (CEA): 2.7 ng/ml, and carbohydrate antigen 19–9 (CA19-9): 122.2 U/ml (Table1).
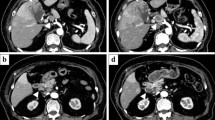
Computed tomography (CT) showed a huge mass, 8 cm in diameter, located in the right lobe of his liver. It was enhanced in early-phase contrast-enhanced CT, accompanied with diffuse enhancement in the surrounding area, and finally washed out in the late phase with delayed hyper-enhancement in the surrounding area (Fig. 1a). Magnetic resonance imaging (MRI) showed low intensity in T1-weighted imaging and high intensity nodules in T2-weighted imaging and diffusion-weighted imaging (Fig. 1b). MRI revealed intrahepatic metastases in the left lobe of his liver (Fig. 1b).
Computed tomography (CT) findings. a The main tumor measured 8 cm in diameter, and was located in the right posterior inferior segment of the liver (S6). The surrounding area of the tumor was enhanced in the early phase (white arrowheads). Magnetic resonance imaging (MRI). b MRI revealed low intensity in T1-weighted imaging and high intensity nodules in T2-weighted imaging and diffusion-weighted imaging (white arrowheads). MRI showed the intrahepatic metastasis in the left lobe of the liver (black arrowheads)
We initially considered the possibility of hepatocellular carcinoma or intrahepatic CCA with abscess, as the patient had fever, extreme leukocytosis, and high serum CRP levels. We intravenously administered sulbactam/ampicillin (9 g/day) for 5 days. No effects were seen, nor did radiography or CT show any infective foci in other organ sites. Blood cultures were also tested after admission but were all negative for bacteria and fungus. Finally, a liver tumor biopsy performed five days after admission led to a diagnosis of sarcomatoid CCA. Resection was considered, but could not be performed due to intrahepatic metastasis. His family did not desire anticancer drug treatment due to his advanced age. He died 20 days after admission from multiple organ failure with cancer-associated disseminated intravascular coagulation.
A pathological autopsy was performed. Macroscopic findings showed a large gray-white tumor, 8 cm in size, in his right lobe with extensive central necrosis and hemorrhage (Fig. 2). No tumor was found in any other organ.
Microscopic examination revealed proliferation of discohesive polygonal- and spindle-shaped cells with pleomorphic nuclei and prominent nucleoli, accompanied by small adenocarcinomatous foci, and scattered multinucleated cells (Fig. 3a–c). Conventional hepatocellular carcinoma was not detected. Immunohistochemically, the tumor cells were positive for AE1/AE3, cytokeratin-7 (CK7), cytokeratin-19 (CK19) and G-CSF (Fig. 3d–f), but negative for hepatocyte paraffin-1 (Hep par-1; Fig. 3g) and AFP (data not shown). The microscopic examination also confirmed intrahepatic metastasis in the left lobe. The bone marrow displayed hyperplasia, but no abnormal blood cells.
Histopathology. Histopathological examination showed discohesive polygonal- to spindle-shaped cells with multinucleated cells. The tumor cells formed glandular structures in part. a Sarcomatoid area; b adenocarcinomatous area; c transition area (left: sarcomatoid area, right: adenocarcinomatous area; hematoxylin/eosin, × 200 magnification). Immunohistochemical examination showed positive staining for AE1/AE3, Cytokeratin-7 (CK7) and granulocyte-colony stimulating factor (G-CSF). The tumor was not stained with hepatocyte paraffin 1 (Hep par 1). d AE1/AE3; e CK7; f G-CSF (× 400 magnification). g Hep par 1 (d–f, g × 100 magnification)
The patient showed elevated serum G-CSF (111 pg/ml [normal value: < 18.1 pg/ml]) and serum interleukin-6 (IL-6: 952 pg/ml [normal value: < 4.0 pg/ml]; Table 1).
The tumor was thus diagnosed as G-CSF-producing intrahepatic sarcomatoid CCA.
Discussion
Few cases of G-CSF-producing intrahepatic CCA have been reported [10,11,12,13,14,15,16,17,18]. Intrahepatic sarcomatoid CCA that produces G-CSF is especially rare among these cases. Most sarcomatoid carcinomas in the liver are thought to be sarcomatoid hepatocellular carcinoma [19]. One report has demonstrated sarcomatous changes in CCAs [9]. This type of tumor is defined as a “sarcomatous intrahepatic CCA” in the WHO classification of tumors [20]. Expression of AE1/AE3, which is the characteristic marker of sarcomatous transformation, was observed in this case. Intrahepatic sarcomatoid CCA is an aggressive malignancy with a very poor prognosis [9]. Only nine cases of intrahepatic sarcomatoid CCA have been reported. In most cases, the tumor had invaded adjacent organs or metastasized to distant organs [9].
The present case is only the second known case of intrahepatic sarcomatoid CCA that produces G-CSF, along with immunohistochemical proof of G-CSF expression.
All cases of G-CSF-producing intrahepatic CCA reported in the English literature are listed in Table 2 [10,11,12,13,14,15,16,17,18]. As G-CSF-producing intrahepatic CCA with sarcomatoid change is extremely rare, only one case has been documented before now [10,11,12,13,14]. The following findings are indicative of G-CSF-producing tumors: elevated serum G-CSF with an increased leukocyte count, transient decreases in G-CSF and leukocyte count to normal ranges after tumor resection, simultaneous increase in G-CSF and neutrophil count with tumor recurrence, and elevation in G-CSF levels in resected specimens, as shown by immunohistochemical staining or real-time reverse transcriptase polymerase chain reaction. A direct way to prove G-CSF production by tumor cells is immunohistochemical techniques [21]. G-CSF is very difficult to detect by immunostaining because the G-CSF protein is generally retained in the cytoplasm for a short time and its antigenicity is fragile and brief. However, we could clearly demonstrate that this hepatic tumor produced G-CSF by immunohistochemical analysis of autopsy specimens in this case (Fig. 2). We were unable to confirm whether the G-CSF would be reduced, because the tumor was not resected.
The prognosis of G-CSF-producing tumors is generally considered to be poor and depends largely on the primary disease, as in our case [22]. In support of this, G-CSF was shown to stimulate growth of a non-hematopoietic malignant cell line in vivo, and is considered to be an autocrine growth factor that promotes rapid tumor proliferation and metastasis [23]. Secretion of G-CSF and sarcomatoid changes could be related in the present case, but if so, the mechanism is unclear.
Lastly, this patient’s serum IL-6 was elevated. Co-production of G-CSF and IL-6 is reportedly associated with the production of IL-1, an inflammatory cytokine, in G-CSF-producing cancer cell lines [10,11,12,13,14,15,16,17,18, 24]. This patient’s high levels of serum IL-6 and CRP may have caused his chief complaint of fever. IL-6 can act as an endogenous pyrogen that regulates the synthesis of acute-phase proteins, including CRP [25]. The high level of IL-6 in this G-CSF-producing tumor might have affected the cancer-associated disseminated intravascular coagulation. The patient had a cough, but his lungs were normal on CT, and no wheezing was heard on auscultation. The cough disappeared with the use of dexamethasone, and was thought to be due to diaphragmatic irritation from the tumor's inflammatory cytokines.
In conclusion, clinicians should consider G-CSF-producing intrahepatic sarcomatoid CCA when encountering patients with leukocytosis and a hepatic tumor. Radical surgery may provide a more favorable prognosis in such instances. Further cases are needed to clarify the clinical findings for G-CSF-producing intrahepatic sarcomatoid CCA. The patient had intrahepatic metastases and was not operable in this case, but resection is advisable in operable cases. Other effective treatments, such as immunological and cytokine therapies, should be investigated for patients with G-CSF-producing intrahepatic sarcomatoid CCA.
Abbreviations
- AFP:
-
α-Fetoprotein
- CA19-9:
-
Carbohydrate antigen 19-9
- CCA:
-
Cholangiocarcinoma
- CEA:
-
Carcinoembryonic antigen
- CK19:
-
Cytokeratin 19
- CK7:
-
Cytokeratin 7
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- G-CSF:
-
Granulocyte colony-stimulating factor
- Hep par1:
-
Hepatocyte paraffin 1
- IL-6:
-
Interleukin-6
- MRI:
-
Magnetic resonance imaging
- PIVKA:
-
Protein induced by absence of vitamin K
- WBC:
-
White blood cell count
References
Lieschke GJ, Burgess AW. Granulocyte-colony stimulating factor and granulocyte-macrophage colony stimulating factor. N Engl J Med. 1992;327(28–35):99–106.
Asano S, Urabe A, Okabe T, et al. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood. 1977;49:845–52.
Ito N, Matsuda T, Kakehi Y, et al. Bladder cancer producing granulocyte colony-stimulating factor. N Engl J Med. 1990;323:1709–10.
Tachibana M, Miyakawa A, Tazaki H, et al. Autocrine growth of transitional cell carcinoma of the bladder induced by granulocyte-colony stimulating factor. Cancer Res. 1995;55:3438–43.
Thacker JD, Dedhar S, Hogge DE. The effect of GM-CSF and G-CSF on the growth of human osteosarcoma cells in vitro and in vivo. Int J Cancer. 1994;56:236–43.
Iwasa K, Noguchi M, Mori K, et al. Anaplastic thyroid carcinoma producing granulocyte colony-stimulating factor (G-CSF): report of a case. Surg Today. 1995;25:158–60.
Furihata M, Sonobe H, Ohtsuki Y, et al. An immunohistochemical study on a case of granulocyte-colony stimulating factor-producing gall-bladder carcinoma. Pathol Int. 1999;49:1010–3.
Kyo S, Kanaya T, Takakura M, et al. A case of cervical cancer with aggressive tumor growth: possible autocrine growth stimulation by G-CSF and IL-6. Gynecol Oncol. 2000;78:383–7.
Shimada M, Takenaka K, Rikimaru T, et al. Characteristics of sarcomatous cholangio-carcinoma of the liver. Hepatogastroenterology. 2000;47:956–61.
Tamai O, Matsumoto M, Nakamoto T, et al. Cholangiocellular carcinoma with pyrexia and leukocytosis [in Japanese with English abstract]. Jpn J Gastroenterol Surg. 1995;28:1100–4.
Aizawa M, Koshiyama H, Inoue D, et al. Postoperative aggravation of hypercalcemia-leukocytosis syndrome in a case of squamous cell type cholangiocarcinoma. Intern Med. 1997;36:232.
Masuda J, Omagari T, Nomoto T, et al. An autopsy case of cholangiocellular carcinoma producing granulocyte colony-stimulating factor [in Japanese]. Nihon Shokakibyo Gakkai Zasshi. 2000;97:347–52.
Kakinoki K, Takemori Y, Noda Y, et al. An autopsy case of intrahepatic cholangiocarcinoma producing granulocyte-colony stimulating factor. Nihon Shokakibyo Gakkai Zasshi. 2000;97:1165–9.
Sohda T, Shiga H, Nakane H, et al. Cholangiocellular carcinoma that produced both granulocyte-colony-stimulating factor and parathyroid hormone-related protein. Int J Clin Oncol. 2006;11:246–9.
Suzumura K, Iimuro Y, Hirano T, et al. Granulocyte colony-stimulating factor-producing cholangiocellular carcinoma. Int Surg. 2015;100:123–7.
Shinojima Y, Toma Y, Terui T. Sweet syndrome associated with intrahepatic cholangiocarcinoma producing granulocyte colony-stimulating factor. Br J Dermatol. 2006;155:1103–4.
Ozawa N, Doi S, Tsujikawa T, et al. Intrahepatic cholangiocarcinoma producing granulocyte colony-stimulating factor and parathyroid hormone-related protein. Nihon Shokakibyo Gakkai Zasshi. 2017;114:1285–92.
Takenaka M, Akiba J, Kawaguchi T, et al. Intrahepatic cholangiocarcinoma with sarcomatous change producing granulocyte-colony stimulating factor. Pathol Int. 2013;63:233–5.
Wang QB, Cui BK, Weng JM, et al. Clinicopathological characteristics and outcome of primary sarcomatoid carcinoma and carcinosarcoma of the liver. J Gastrointest Surg. 2012;16:1715–26.
Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system. Lyon: WHO, International Agency for Research on Cancer; 2000.
Joshita S, Nakazawa K, Koike S, et al. A case of granulocyte-colony stimulating factor-producing hepatocellular carcinoma confirmed by immunohistochemistry. J Korean Med Sci. 2010;25:476–80.
Berdel WE, Danhauser-Riedl S, Steinhauser G, et al. Various human hematopoietic growth factors (interleukin-3, GM-CSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood. 1989;73:80–3.
Noda I, Fujieda S, Ohtsubo T, et al. Granulocyte- colony-stimulating factor enhances invasive potential of human head-and-neck-carcinoma cell lines. Int J Cancer. 1999;80:78–84.
Suzuki A, Takahashi T, Okuno Y, et al. IL-1 production as a regulator of G-CSF and IL-6 production in CSF-producing cell lines. Br J Cancer. 1992;65:515–8.
Dinarello CA. Cytokines as endogenous pyrogens. J Infect Dis. 1999;179:294–304.
Acknowledgements
We thank Marla Brunker, from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Akifumi Kuwano, Fumiya Narutomi, Shigehiro Nagasawa, Kosuke Tanaka, Yusuke Morita, Masayoshi Yada, Yoshihiro Ohishi, Akihide Masumoto and Kenta Motomura declare that they have no conflict of interest.
Human rights
All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuwano, A., Narutomi, F., Nagasawa, S. et al. A case of granulocyte colony-stimulating factor-producing intrahepatic sarcomatoid cholangiocarcinoma. Clin J Gastroenterol 14, 881–887 (2021). https://doi.org/10.1007/s12328-021-01405-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-021-01405-7