Abstract
We present an extremely rare case of carcinosarcoma with 4 different tumor components in an 88-year-old female. After a diagnosis of acute cholecystitis, we performed percutaneous transhepatic gallbladder drainage in the patient without success, followed by a cholecystectomy and choledocholithotomy. The mass was a 60 × 25 mm polypoid lesion of the gallbladder identified histologically as a carcinosarcoma with adenocarcinoma, neuroendocrine carcinoma, undifferentiated carcinoma and chondrosarcoma components. The biliary-type adenocarcinoma portion exhibited acinar growth patterns with columnar cells having large and markedly hyperchromatic nuclei. These tumor cells were immunohistochemically positive for MUC1 and CDX2. The neuroendocrine carcinoma, small cell type, cells were densely packed and small, with scant cytoplasm, finely granular nuclear chromatin and absence of nucleoli. The mitotic index was high. These tumor cells were immunohistochemically positive for synaptophysin, Ki-67 (index 40%), MUC1, CDX2 and c-Kit. The undifferentiated carcinoma consisted exclusively of spindle cells containing large, markedly hyperchromatic nuclei with a high mitotic index. These tumor cells were immunohistochemically positive for AE1/AE3. The chondrosarcoma was composed of blue-gray chondroid matrix and atypical chondrocytes containing large, hyperchromatic nuclei. These tumor cells were immunohistochemically positive for S100. Its attributes might be suggestive of a greater malignant potential and pathogenesis of carcinosarcoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most patients with carcinoma of the gallbladder are in the 6th or 7th decade of life, and the female to male ratio is 1.77 [1]. Cancer of the gallbladder accounts for 51% of all cancers arising in the biliary tract in women and 28% in men [1]. The most important risk factors for carcinoma of the gallbladder are genetic background, gallstones and an abnormal choledochopancreatic junction [1]. Adenocarcinoma is the most common type of malignant tumor generated in the gallbladder, whereas carcinosarcomas, which are composed of variable proportions of both carcinomatous and sarcomatous elements, are rare having an incidence of less than one percent of all malignant gallbladder neoplasms [2]. Prognosis is poor following curative resection for carcinosarcoma of the gall bladder, because of recurrence as systemic metastasis of the liver and peritoneal dissemination, and a large proportion of these patients have recurrence during the postoperative early period [2]. Although most reported cases of gallbladder carcinosarcoma have adenocarcinoma as the epithelial component, squamous cell carcinoma is also often present, and the mesenchymal component varies from homogeneous sarcoma to more heterotopic elements such as malignant bone, cartilage, and other mesenchymal tissues [2]. We present an extremely rare example of carcinosarcoma consisting of adenocarcinoma, neuroendocrine carcinoma, undifferentiated carcinoma and chondrosarcoma components.
Case report
An 88-year-old female presented with chill, tremor and vomiting. Her medical history indicated tuberculosis in her 20s and pneumonia in her 30s. The abdominal ultrasonography and computed tomography (CT) scanning showed gallbladder enlargement, dilation of the common bile duct, cholecystolithiasis and choledocholith. We made a diagnosis of acute cholecystitis and performed percutaneous transhepatic gallbladder drainage. The procedure was not successful, so a cholecystectomy with biliary and abdominal drainages without lymph node dissection and choledocholithotomy were performed. Retrospectively, we reviewed the CT image taken prior to the original diagnosis of acute cholecystitis and visualized a tumor (Fig. 1) that protruded into the gallbladder lumen with wall thickening; both the tumor and wall appeared mildly contrast enhanced. The intra-luminal mass had a cyst-like portion which was not enhanced after contrast injection. The cystic parts corresponded to intra-tumoral hemorrhage observed in the histopathological specimen.
Contrast-enhanced CT in axial (a) and coronal (b) planes. The tumor protruded into the gallbladder lumen (arrows) with wall thickening (arrow head). The tumor and the thickened wall were mildly contrast enhanced. The intra-luminal mass had a cyst-like part that was not enhanced after contrast injection. The cystic parts corresponded to intra-tumoral hemorrhage observed in the histopathological specimen
Gross examination showed a polypoid lesion, 60 × 25 mm in the corpus of the gallbladder (Fig. 2a). The cut surface of the tumor was white, and broad hemorrhage was seen (Fig. 2b). The gallbladder had diffuse mural thickening (Fig. 2b).
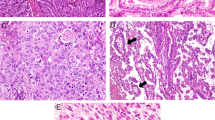
Histological examination identified a carcinosarcoma with adenocarcinoma, neuroendocrine carcinoma, undifferentiated carcinoma and chondrosarcoma components that had invaded the subserosal layer. There was biliary-type adenocarcinoma (occupancy 15%) composed of acinar growth patterns with columnar cells having large and markedly hyperchromatic nuclei (Fig. 3a). These tumor cells were immunohistochemically positive for MUC1 (Fig. 3b), CDX2 (Fig. 3c). According to the WHO classification [1], histological phenotype of a gallbladder adenocarcinoma is divided into three groups: (1) the biliary type is composed of short or long tubular glands lined by cells that vary in height from cuboidal to tall columnar (superficially resembling biliary epithelium). (2) The intestinal type is composed of tubular glands closely resembling those of colonic adenocarcinomas, or consisting of glands lined predominantly with goblet cells and usually with a variable number of neuroendocrine and Paneth cells. About one-third of well-differentiated adenocarcinomas show focal intestinal differentiation with goblet and neuroendocrine cells. (3) The gastric foveolar type is composed of tall columnar cells with basally oriented nuclei and abundant mucin-containing cytoplasm. The neuroendocrine carcinoma, small cell type, (occupancy 25%) had cells that were densely packed and small, with scant cytoplasm, finely granular nuclear chromatin and absence of nucleoli (Fig. 3d). The mitotic index was high (25 per 10 high power fields). These tumor cells were immunohistochemically positive for synaptophysin (Fig. 3e), Ki-67 (index 40%) (Fig. 3f), MUC1 (Fig. 3g), CDX2 (Fig. 3h) and c-Kit (Fig. 3i). The undifferentiated carcinoma (occupancy 50%) was composed exclusively of spindle cells containing large and markedly hyperchromatic nuclei with a high mitotic index (Fig. 4a). Hemorrhage was remarkable in this carcinoma area. These tumor cells were immunohistochemically positive for AE1/AE3 (Fig. 4b). The chondrosarcoma (occupancy 10%) was composed of blue-gray chondroid matrix and atypical chondrocytes containing large, hyperchromatic nuclei (Fig. 4c). These tumor cells were immunohistochemically positive for S100 (Fig. 4d). The postoperative treatment was not performed, because this patient did not hope for it. She was alive for 10 months postoperatively.
Representative images of immunohistochemistry on tissue sections of the adenocarcinoma and neuroendocrine carcinoma components of carcinosarcoma of the gallbladder. Adenocarcinoma (a) that exhibits MUC1 immunoreactivity (b) in the cytoplasm and CDX2 immunoreactivity (c) in the nucleus. Neuroendocrine carcinoma (d) that exhibits synaptophysin immunoreactivity (e) in the cytoplasm, Ki-67 immunoreactivity (f) in the nucleus, MUC1 immunoreactivity (g) in the cytoplasm, CDX2 immunoreactivity (h) in the nucleus and c-Kit immunoreactivity (i) in the cytoplasmic membrane
Representative images of immunohistochemistry on tissue sections of undifferentiated carcinoma and chondrosarcoma components of carcinosarcoma of the gallbladder. Undifferentiated carcinoma (a) that exhibits AE1/AE3 immunoreactivity (b) in the cytoplasm. Chondrosarcoma (c) that exhibits S100 immunoreactivity (d) in the nucleus
Discussion
The carcinosarcoma tumor from our patient contained a biliary-type adenocarcinoma component. Toba et al. reported on the aggressiveness of gallbladder adenocarcinoma classified histologically as a biliary, gastric foveolar, or intestinal type. They found the biliary type and MUC1 expression correlated significantly with a wall invasion pattern they termed a destructive growth type in which cancer cells invade the subserosal layer with destruction of the muscle layer [3]. This was a more aggressive wall invasion pattern than the infiltrative growth type in which cancer cells infiltrate the muscle layer through the intermuscular space without muscle layer destruction [3]. Our patient’s tumor also had a c-Kit immunopositive neuroendocrine carcinoma component. Although the primary organ was lung not gallbladder, a Kaplan–Meier survival analysis developed by Erler et al. for pulmonary neuroendocrine carcinoma revealed a statistically significant difference in survival rates between neuroendocrine carcinoma samples scored as negative or positive for c-Kit overexpression, P = 0.0003 [4]. In their study, the median survival was 19.0 months for c-Kit negative patients and 6.7 months for c-Kit positive patients [4]. These results may reflect a greater malignant potential for carcinosarcomas compared to other malignant tumors. The selective inhibitor of c-Kit may be very effective in the treatment of the metastatic lesion if this neuroendocrine carcinoma component metastasizes to the other organs, because Raymond et al. have showed that continuous daily administration of sunitinib (a multi-target agent which effectively inhibits VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-a, PDGFR-b, and c-Kit) at a dose of 37.5 mg improved progression-free survival, overall survival, and the objective response rate as compared with placebo among patients with advanced pancreatic neuroendocrine tumors [5]. Nevertheless, it is unclear that the expression of c-Kit in gallbladder neuroendocrine carcinomas is a characteristic and general phenomenon, so future studies with a larger number of patients will be required to precisely define the correlation of c-Kit expression and gallbladder neuroendocrine carcinomas for constructing an additional treatment strategy.
Okabayashi et al. performed a literature review of patients that received surgical treatment of their carcinosarcomas of the gallbladder with intent to cure. They found that the current overall 5-year survival rate was 31.0%, which included an in-hospital mortality rate of 8.3% [2]. This was comparable to or worse than the reported rates for adenocarcinoma which is the most common type of malignant tumor of the gallbladder [2]. We analyzed data from 14 patients recorded in the literature in this decade (from 2010 to 2019) who underwent surgical management for carcinosarcoma of the gall bladder (Table 1). These patients consisted of 6 males and 8 females with an age range 50–88 years [6,7,8,9,10,11,12,13,14,15,16,17].
The pathogenesis of carcinosarcoma is controversial. Several theories have been proposed to explain the admixture of epithelial and mesenchymal tissues in these neoplasms: (1) mesenchymal reaction, (2) true sarcoma (including the collision neoplasm hypothesis), (3) malignant proliferation of epithelial origin (including the stromal induction/metaplasia model), (4) an embryonic cell rest origin, and (5) the totipotential stem cell hypothesis [2]. We confirmed the MUC1 and CDX2 immunopositive stainings in both the adenocarcinoma and neuroendocrine carcinoma components in our case, which demonstrated the possibility of an identical origin of at least two components of the carcinosarcoma. In general, MUC1 expression indicates biliary epithelium differentiation and CDX2 expression indicates intestinal epithelium differentiation. Scardoni et al. also reported on 5 of 6 mixed adenoendocrine carcinomas that presented with an overlapping mutational profile (TP53, RB1 and KRAS) in both components, suggesting a monoclonal origin of the adenocarcinoma and neuroendocrine carcinoma components [18].
Accurate preoperative diagnosis of carcinosarcoma of the gallbladder is very difficult, because imaging studies can not differentiate it from carcinoma of the gallbladder, and abdominal angiography often shows neovascularity and staining of carcinosarcomas of the gall bladder, whereas CT shows an enhanced solid mass lesion [2]. Our case having gallstones could not also obtain the preoperative diagnosis of carcinosarcoma of the gallbladder.
In conclusion, there have been no previously reported gallbladder neoplasms having the complex combination of adenocarcinoma, neuroendocrine carcinoma, undifferentiated carcinoma and chondrosarcoma components [19,20,21,22,23,24]. We present an extremely rare example of carcinosarcoma, and its attributes might be suggestive of a greater malignant potential and pathogenesis of carcinosarcoma.
References
Albores-Saavedra J, Adsay NV, Crawford JM, et al. Carcinoma of the gallbladder and extrahepatic bile ducts. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010. p. 266–73.
Okabayashi T, Sun ZL, Montgomey RA, et al. Surgical outcome of carcinosarcoma of the gall bladder: a review. World J Gastroenterol. 2009;15:4877–82.
Toba T, Kijima H, Hakamada K, et al. Histological phenotype is correlated with the wall-invasion pattern of gallbladder adenocarcinoma. Biomed Res. 2014;35:295–302.
Erler BS, Presby MM, Finch M, et al. CD117, Ki-67, and p53 predict survival in neuroendocrine carcinomas, but not within the subgroup of small cell lung carcinoma. Tumour Biol. 2011;32:107–11.
Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–13.
Pu JJ, Wu W. Gallbladder carcinosarcoma. BMJ Case Rep. 2011;1:2011.
Ishida J, Ajiki T, Hara S, et al. Gallbladder calcification leads to discovery of carcinosarcoma of the gallbladder. Surgery. 2012;152:934–5.
Sadamori H, Fujiwara H, Tanaka T, et al. Carcinosarcoma of the gallbladder manifesting as cholangitis due to hemobilia. J Gastrointest Surg. 2012;16:1278–81.
Kim HH, Hur YH, Jeong EH, et al. Carcinosarcoma of the gallbladder: report of two cases. Surg Today. 2012;42:670–5.
Parreira JM, Siqueira DE, Menacho AM, et al. Carcinosarcoma of the gallbladder: case report. Arq Bras Cir Dig. 2012;25:65–6.
Li BF, Li PW, Shi R, et al. Clinicopathologic analysis of gallbladder carcinosarcoma. Am Surg. 2013;79:E37–9.
Kishino T, Mori T, Kawai S, et al. Carcinosarcoma, an atypical subset of gallbladder malignancies. J Med Ultrason. 2001;2014(41):487–90.
Faujdar M, Gupta S, Jain R, et al. Carcinosarcoma of the gallbladder with heterologous differentiation: a case report. J Gastrointest Cancer. 2015;46:175–7.
Tonouchi A, Yokoyama N, Hashidate H, et al. Education and imaging. Gastroenterology: carcinosarcoma of the gallbladder presenting as a cholecysto-colic fistula. J Gastroenterol Hepatol. 2015;30:1112.
Wada Y, Takami Y, Tateishi M, et al. Carcinosarcoma of the gallbladder: report of a case. Clin J Gastroenterol. 2014;7:455–9.
Gao S, Huang L, Dai S, et al. Carcinosarcoma of the gallbladder: a case report and review of the literature. Int J Clin Exp Pathol. 2015;8:7464–9.
Ansari FA, Bhatnagar M, Katiyar DC, et al. Carcinosarcoma of gall bladder. Trop Gastroenterol. 2016;37:63–5.
Scardoni M, Vittoria E, Volante M, et al. Mixed adenoneuroendocrine carcinomas of the gastrointestinal tract: targeted next-generation sequencing suggests a monoclonal origin of the two components. Neuroendocrinology. 2014;100:310–6.
Inoshita S, Iwashita A, Enjoji M. Carcinosarcoma of the gallbladder: report of a case and review of the literature. Acta Pathol Jpn. 1986;36:913–20.
Maitra A, Tascilar M, Hruban RH, et al. Small cell carcinoma of the gallbladder: a clinicopathologic, immunohistochemical, and molecular pathology study of 12 cases. Am J Surg Pathol. 2001;25:595–601.
Takahashi Y, Fukushima J, Fukusato T, et al. Sarcomatoid carcinoma with components of small cell carcinoma and undifferentiated carcinoma of the gallbladder. Pathol Int. 2004;54:866–71.
Shintaku M, Kataoka K, Kawabata K. Mixed adenoneuroendocrine carcinoma of the gallbladder with squamous cell carcinomatous and osteosarcomatous differentiation: report of a case. Pathol Int. 2013;63:113–9.
Park HJ, Jang KT, Choi DW, et al. Clinicopathologic analysis of undifferentiated carcinoma of the gallbladder. J Hepatobiliary Pancreat Sci. 2014;21:58–63.
Lee SW, Baek SY, Sung SH. Combined undifferentiated and neuroendocrine carcinomas of the gallbladder appearing as two separate lesions: a case report with radiological-pathological correlation. J Radiol Case Rep. 2015;9:14–21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human/animal rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from this patient to be included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mochizuki, K., Hata, H., Naitou, K. et al. Carcinosarcoma (adenocarcinoma, neuroendocrine carcinoma, undifferentiated carcinoma and chondrosarcoma) of the gallbladder. Clin J Gastroenterol 13, 110–115 (2020). https://doi.org/10.1007/s12328-019-01012-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-019-01012-7