Abstract
A 66-year-old man who was on oral medication for type 2 diabetes experienced a rapid decline in glycemic control (increase in glycosylated hemoglobin level from 7.7 to 10.2% over 3 months). Abdominal ultrasonography revealed a 20-mm hypoechoic mass in the pancreatic tail. Serum tumor marker carbohydrate antigen 19–9 and DUPAN2 levels were within the respective normal ranges; serum IgG4 level was also normal at 21.8 mg/dL. Abdominal contrast computed tomography revealed a 26-mm tumor in the pancreatic tail. Magnetic resonance cholangiopancreatography revealed disruption of the main pancreatic duct and dilation of the caudal pancreatic duct. Endoscopic ultrasonography revealed a near-round-shaped hypoechoic mass with interspersed hyperechoic areas. Endoscopic ultrasonography-guided fine needle aspiration was performed using a 22-G needle, but no malignant findings were observed. There were no signs of sialadenitis, retroperitoneal fibrosis, nephropathy, or other conditions associated with IgG4-related diseases. Distal pancreatectomy was performed; a 23-mm white mass was resected from the pancreatic tail. A histopathological examination showed advanced inflammatory cell infiltration mainly involving lymphocytes/plasma cells along with storiform fibrosis and obliterative phlebitis. No more than five IgG4-positive cells were observed per high-power field. These were level 1 pathological findings, and a definitive diagnosis of type 1 autoimmune pancreatitis (AIP) was made according to the International Consensus Diagnostic Criteria. Type 1 AIP associated with normal serum IgG4 levels and absence of IgG4-positive cells on histological examination is a rare clinical entity, which is very difficult to distinguish from pancreatic cancer. Here we report such a case and present a review of the relevant literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autoimmune pancreatitis (AIP), a term used to describe pancreatitis of autoimmune etiology, was first proposed by Yoshida et al. [1]. Several sets of diagnostic criteria for AIP have been proposed worldwide. In 2011, the disease was classified into type 1 and type 2 by the International Consensus Diagnostic Criteria (ICDC) [2]. Type 1 AIP usually occurs in middle-aged and older men; it typically exhibits imaging findings of pancreatic enlargement and narrowing of the pancreatic duct and histological findings of lymphoplasmacytic sclerosing pancreatitis (LPSP). It is recognized as a pancreatic lesion associated with IgG4-related diseases and is characterized by elevated serum IgG4 level and invasion of pancreatic tissue by IgG4-positive cells.
In recent years, cases of type 1 AIP with no serum IgG4 level elevation or histologically detectable IgG4-positive cells have been reported, albeit extremely rarely. The involvement of IgG4 may not be essential in the pathogenesis of type 1 AIP.
Here we report the case of a patient who exhibited typical LPSP findings but had normal serum IgG4 level and histologically undetectable IgG4-positive cells.
Case presentation
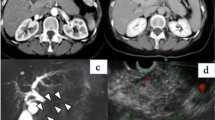
The patient was a 66-year-old man. He had a past medical history of appendectomy. His family history was unremarkable. He had a history of alcohol consumption (2 cups daily for 30 years) and cigarette smoking (10 cigarettes per day for 25 years). He was on oral medication for the treatment of type 2 diabetes at the Department of Diabetic Medicine for 4 years. Abdominal ultrasonography (AUS) was performed due to rapid worsening of glycemic control (HbA1c 7.7–10.2% over 3 months), which revealed a 20-mm hypoechoic mass in the pancreatic tail (Fig. 1). He was referred to our department for further evaluation. On physical examination, there was no abdominal tenderness or a palpable abdominal mass.
All blood test results were within the respective normal range, including serum amylase, 78 U/L; carbohydrate antigen 19–9, 21 mg/dL; and DUPAN2, 21 mg/dL. Serum IgG and IgG4 level were also normal at 1028 mg/dl and 21.8 mg/dL. Abdominal contrast enhanced computed tomography (CT) revealed a 26-mm tumor in the pancreatic tail; the observed contrast effect was ischemic in the arterial phase and prolonged in the portal vein and parallel phases (Fig. 2). No signs of sialadenitis, retroperitoneal fibrosis, nephropathy, or other findings associated with IgG4-related diseases were observed. Magnetic resonance cholangiopancreatography (MRCP) revealed a disrupted main pancreatic duct and dilated caudal pancreatic duct (diameter: 4.8 mm) (Fig. 3a). Diffusion-weighted magnetic resonance images (MRI) showed a diffusion decrease corresponding to the mass (Fig. 3b). Endoscopic retrograde cholangiopancreatography (ERCP) was not performed as the patient refused. Endoscopic ultrasonography (EUS) revealed a near-round-shaped hypoechoic mass with interspersed hyperechoic areas (Fig. 4). Endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) was performed with a 22-G needle (Expect™ SlimLine, Boston Scientific Japan, Tokyo, Japan). The histological examinations showed pancreatic duct epithelium with scarce atypia and fibrotic tissue. The typical findings of AIP (lymphoplasmacytic infiltration with fibrosis, storiform fibrosis, obliterative phlebitis, or abundant (> 10 cells/HPF) IgG4-positive cells) were not revealed (Fig. 5).
The specimen obtained by EUS-FNA. The histological examinations showed pancreatic duct epithelium with scarce atypia and fibrotic tissue. The typical findings of AIP (lymphoplasmacytic infiltration with fibrosis, storiform fibrosis, obliterative phlebitis, or abundant (> 10 cells/HPF) IgG4-positive cells) were not revealed (hematoxylin–eosin stain, × 200)
As pancreatic cancer could not be ruled out, distal pancreatectomy was performed after informed consent; a 23-mm white mass was resected from the pancreatic tail (Fig. 6). On histopathological examination, advanced inflammatory cell infiltration mainly involving lymphocytes and plasma cells was observed along with storiform fibrosis and obliterative phlebitis (Fig. 7). On IgG4 staining, no more than five IgG4-positive cells were observed per high-power field (Fig. 8a). On IgG staining, IgG-positive cells (weakly positive) were scattered (Fig. 8b). Pathologically, there was no findings of AIP on the resected margin. There were level 1 pathological findings, and a definitive diagnosis of type 1 AIP was made according to ICDC.
Three years after the operation, there have been no signs of relapse of AIP in the remnant pancreas. IgG4-related diseases are not observed in other organs, and serum IgG4 level is 32 mg/dL, which is also within the normal range.
Discussion
The concept of AIP was proposed by Yoshida et al. [1]. It is believed to have an autoimmune etiology due to the typical findings of hyper-IgG-emia, autoantibody positivity, and rapid response to steroid therapy. In 2001, Hamano et al. reported serum IgG4 level as an AIP-specific marker, and they confirmed the presence of IgG4-positive cells in the pancreatic tissue [3, 4]. Furthermore, similar pathological conditions accompanied by serum IgG4 elevation and histological IgG4-positive cell infiltration have been reported in organs such as the salivary glands, retroperitoneum, kidneys, and bile duct. This led to a comprehensive disease concept of IgG4-related diseases [5, 6]. ICDC was then created in 2011 based on the accumulation of cases, and AIP was classified into type 1 and type 2 [2].
Type 1 AIP usually occurs in middle-aged and older men and shows pancreatic enlargement and pancreatic duct narrowing on imaging and histological findings of LPSP. Type 1 AIP is characterized by elevated serum IgG4 level and invasion of pancreatic tissue by IgG4-positive cells.
Type 2 AIP typically occurs in younger men and women, is often complicated by acute pancreatitis, and shows some association with inflammatory bowel disease. Pathological findings include granulocytic epithelial lesion (GEL) characterized by the destruction of pancreatic duct epithelium. Serum IgG4 level is rarely elevated and IgG4-positive cells are not usually detected on histological examination.
As described above, type 1 and type 2 AIP exhibit distinct clinical features. Type 1 is a pancreatic lesion of IgG4-related disease, whereas type 2 is not considered to be an IgG4-related disease.
However, IgG4 is not an absolute criterion for the diagnosis of type 1 AIP. In a recent international study conducted across eight countries, serum IgG4 levels were normal in 37% of 204 patients with histologically proven type 1 AIP [7]. On the other hand, histological findings of IgG4-positive cells occurred in 84–100% of patients with AIP and are more specific [8,9,10].
Recently, a small number of patients with typical findings of LPSP, but who had normal serum IgG4 levels and histologically undetectable IgG4-positive cells have been reported (Table 1) [11, 12]. All patients were middle-aged or older; two patients were male and one patient was a female. All patients had extra-pancreatic lesions. Serum IgG4 ranged from 40.1 to 78.2 mg/dL, and all patients showed ˂ 10 IgG4-positive cells per high-power field upon histological examination. The involvement of IgG4 may not be essential in the pathology of type 1 AIP.
In the first place, the role of IgG4 in AIP is not clear. It may play a direct role in the pathogenesis as a hindrance factor, an anti-inflammatory role as a protective factor, or some other role; however, there is no consensus [13]. Shiokawa et al. reported that both IgG1 and IgG4 from patients with IgG4-related disease have pathogenic activities through binding affected tissues in neonatal mice [14]. In the recent study, the same group identified laminin 511 is a target antigen in AIP [15].
Paik et al. compared the serum IgG4-positive group and -negative group of patients with type 1 AIP [16]. They found fewer IgG4-positive cells on histological examination and lesser involvement of other organs in the serum IgG4-negative group.
Nakano et al. reported a patient with type 1 AIP who had normal serum IgG4 levels and IgG4-positive cells that were histologically undetectable [11]. T-cells and B-cells in the pancreatic tissue were evaluated using anti-CD3 and anti-CD20 antibodies. In IgG4-negative AIP, B-cell invasion was less notable than T-cell invasion. This finding indicates the possibility that suppression of the T-cell-independent pathway may be involved in reducing serum IgG4 level and histological IgG4-positive cells.
The most serious concern for IgG4-negative AIP is that differentiation between pancreatic cancer and AIP is more difficult. In the present case, serum IgG4 level was normal, and no lesions were found in other organs. Pancreatic cancer was suspected owing to the rapid progression of diabetes and enlargement of the caudal pancreatic duct; the findings were not readily suggestive of AIP. In patients with suspected AIP, steroid treatment can be an option; however, it was difficult to consider steroid therapy in this case.
Although extremely rare, type 1 AIP with no concomitant serum IgG4 level elevation or histologically detectable IgG4-positive cells does exist. IgG4 may not play an essential role in the pathogenesis of type 1 AIP. With accumulating cases in the future, the significance of IgG4-negative autoimmune pancreatitis is expected to be clarified.
References
YoshidaK, TokiF, TakeuchiT, et al. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–8.
ShimosegawaT, ChariST, FrulloniL, et al. International Association of Pancreatology. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–8.
HamanoH, KawaS, HoriuchiA, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–8.
HamanoH, KawaS, OchiY, et al. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002;359:1403–4.
HamanoH, ArakuraN, MurakiT, et al. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol. 2006;41:1197–205.
StoneJH, KhosroshahiA, DeshpandeV, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:3061–7.
KamisawaT, ChariST, GidaySA, et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas. 2011;40:809–14.
KamisawaT, FunataN, HayashiY, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–4.
ZhangL, NotoharaK, LevyMJ, et al. IgG4-positive plasma cell infiltration in the diagnosis of autoimmune pancreatitis. Mod Pathol. 2007;20:23–8.
DhallD, SuriawinataAA, TangLH, et al. Use of immunohistochemistry for IgG4 in the distinction of autoimmune pancreatitis from peritumoral pancreatitis. Hum Pathol. 2010;4:643–52.
NakanoE, KannoA, MasamuneA, et al. IgG4-unrelated type 1 autoimmune pancreatitis. World J Gastroenterol. 2015;21:9808–9816.
HartPA, SmyrkTC, ChariST. Lymphoplasmacytic sclerosing pancreatitis without IgG4 tissue infiltration or serum IgG4 elevation: IgG4-related disease without IgG4. Mod Pathol. 2015;28:238–47.
NirulaA, GlaserSM, KalledSL, et al. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr Opin Rheumatol. 2011;23:119–24.
ShiokawaM, KodamaY, KuriyamaK, et al. Pathogenicity of IgG in patients with IgG4-related disease. Gut. 2016;65:1322–32.
Shiokawa M, Kodama Y, Sekiguchi K, et al. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci Transl Med. 2018;8:10.
PaikWH, RyuJK, ParkJM, et al. Clinical andpathological differences between serum immunoglobulin G4-positive and -negative type 1 autoimmune pancreatitis. World J Gastroenterol. 2013;19:4031–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yuichi Takano, Takahiro Kobayashi, Fumitaka Niiya, Eiichi Yamamura, Naotaka Maruoka, Kazuaki Yokomizo, Hiroki Mizukami, Jun-ichi Tanaka2 Tomoko Norose, Nobuyuki Ohike, and Masatsugu Nagahama declare that they have no conflict of interest.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
Takano, Y., Kobayashi, T., Niiya, F. et al. Serum and histological IgG4-negative type 1 autoimmune pancreatitis. Clin J Gastroenterol 12, 232–238 (2019). https://doi.org/10.1007/s12328-018-0919-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-018-0919-4