Abstract
Hepatocellular carcinoma (HCC) can be difficult to diagnose and treat in patients with Osler–Rendu–Weber disease due to vascular malformation and regenerative nodular hyperplasia. In addition, percutaneous liver puncture should be avoided for the diagnosis and treatment as the procedure carries a high risk of bleeding. We herein report the successful treatment of HCC in a patient with Osler–Rendu–Weber disease using radiofrequency ablation (RFA) under laparoscopy. A 71-year-old man with Osler–Rendu–Weber disease was admitted to our hospital for the treatment of HCC. He also had chronic hepatitis C virus infection. The arterioportal shunts in the liver were detected by computed tomography (CT) and angiography. A tumor 20 mm in size was detected as a defected-lesion in the hepatic segment IV during the portal phase by CT. RFA under laparoscopy was performed for the curative treatment for HCC, with sufficient ablation obtained. Although the blood gushed out from the needle tract at the end of the procedure, complete hemostasis was achieved promptly using coagulation forceps. The post-operative course was favorable. Thus, laparoscopic RFA is a useful treatment modality for HCC in patients with Osler–Rendu–Weber disease, as a hemostasis device can be used with direct visualization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osler–Rendu–Weber disease, also known as hereditary hemorrhagic telangiectasia, is characterized by cutaneous telangiectases and visceral vascular malformations, affecting several organs, including the lung, brain and liver. Approximately 30–73% of patients with Osler–Rendu–Weber disease have involvement of the liver, with 8% of these patients presenting with hepatic symptoms [1]. Hepatic manifestations are categorized into three distinct clinical types: arteriovenous shunts that cause high-output cardiac failure, arterioportal shunts that cause portal hypertension and biliary type, characterized by deformities in the bile ducts. The most common type is high-output cardiac failure, followed by portal hypertension and biliary type. Female patients show a more severe clinical course and a higher rate of complications than male [2].
Although the development of malignant liver tumors, including hepatocellular carcinoma (HCC), is rare in patients with Osler–Rendu–Weber disease, it is difficult to diagnose and treat HCC due to regenerative nodular hyperplasia and vascular malformations. While a few cases of HCC have been reported in association with Osler–Rendu–Weber disease, the treatment was not always successful.
We herein report a case of HCC successfully treated using radiofrequency ablation (RFA) under laparoscopy without any serious complications.
Case report
A 71-year-old Japanese man with Osler–Rendu–Weber disease was admitted to our hospital for the treatment of HCC. He had been suffering from repeated episodes of epistaxis, and his mother had Osler–Rendu–Weber disease. The patient also had chronic hepatitis C virus (HCV) infection, and a sustained viral response had not been achieved by interferon monotherapy about 15 years earlier. On a physical examination, several angiomas were found on his lip. There were no symptoms related to liver cirrhosis or heart failure, including flapping tremor, edema, ascites, splenomegaly, and dyspnea. An esophagogastroduodenoscopic examination showed no gastroesophageal varices. Table 1 shows the laboratory data on admission. Tumor markers for HCC, including Des-γ-carboxy prothrombin, were elevated.
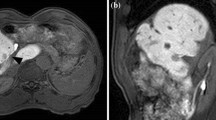
On contrast bolus dynamic computed tomography, the most peripheral portal vein as well as the portal trunk was enhanced during the arterial phase (Fig. 1a, b), indicating that arterioportal shunts were present. A tumor was detected as a defected-lesion with a diameter of 20 mm during the portal phase in hepatic segment IV, although enhancement was not observed during the arterial phase (Fig. 1a–d).
Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI) showed that the tumor was not enhanced in the arterial and portal phase (Fig. 2a, b). Then the tumor was detected as a hypointense nodule in the hepatobiliary phase (Fig. 2c). Angiographic imaging showed that right and the left hepatic arteries diverged from the superior mesenteric artery and the left gastric artery, respectively. Angiography of the right hepatic artery showed that the contrast medium promptly appeared in the portal vein (Fig. 3a), confirming the presence of the arterioportal shunts. In addition, dilated and meandering collateral veins appeared immediately after the bolus injection of the contrast to the right hepatic artery (Fig. 3b). Thus, the present case was diagnosed as portal hypertension type. On an angiography of the middle hepatic artery, a thin tumor stain was noted (Fig. 3c). We highly suspected HCC based on the findings obtained from CT, MRI and angiography. We selected RFA under laparoscopy after the discussion on the treatment plan with liver surgeons. Under general anesthesia, laparoscopy was performed for RFA with sufficient informed consent from the patient. On laparoscopic findings, nodal changes were observed on the liver surface (Fig. 4a). In addition, spider angioma-like capillary dilation was present on the liver surface (Fig. 4b), which is specific for Osler–Rendu–Weber disease. To determine the safest insertion route, vascular malformations were screened using ultrasonography equipped with a color Doppler examination (TUS A-300, TOSHIBA, Japan) under laparoscopy (Fig. 4c). A 21-gauge guiding needle was inserted near the target under ultrasonographic guidance in combination with laparoscopic visualization (Fig. 4d). A metallic sheath was inserted along the guiding needle, and then the radiofrequency applicator was accurately replaced in the planned position. We performed ablation twice for HCC using a monopolar applicator with an adjustable active tip (VIVA RF SYSTEM; STARmed Co., Goyang, Korea) for 17 min 53 s in total. Although the blood gushed out from the needle tract even after the ablation for the hepatic surface, complete hemostasis was achieved using coagulation forceps (Fig. 4e, f). Postoperative CT scan showed that the tumor had been sufficiently ablated without post-treatment hemorrhage on the liver surface (Fig. 1e, f). A histological examination revealed that the tumor was moderately differentiated HCC (Fig. 5a). The background liver was compatible with liver cirrhosis cause by chronic HCV infection, with stages of inflammation and liver fibrosis of grades 2 and 3–4 (Fig. 5b, c), respectively. The patient was discharged without any complications 5 days after laparoscopic RFA. No recurrence of HCC has been observed at 1 year after the procedure. At present, alpha-fetoprotein and Des-γ-carboxy prothrombin dropped to 9 ng/ml and 30 mAU/ml, respectively. In addition, a sustained virological response was achieved with 12 weeks of elbasvir/grazoprevir treatment for the chronic HCV infection.
Abdominal contrast-enhanced CT scans (a, c, e axial view; b, d, f coronal view). a, b Arterial phase. Contrast bolus dynamic CT scans show enhancement of the most peripheral portal veins as well as the portal trunk (arrow), indicating the presence of arterioportal shunts. c, d Portal phase. A 20-mm tumor was detected as a defected-lesion in hepatic segment IV (arrow). e, f Post-RFA. The tumor is sufficiently ablated
Angiography of the right hepatic artery (a, b) and the middle hepatic artery (c). a The portal vain (arrow head) is enhanced after a few seconds by bolus injection of the contrast medium to the right hepatic artery (arrow). b Dilated and meandering collateral veins (arrow head) are noted, indicating portal hypertension. Extrahepatic blood flow was refluxed from the portal vein to the left gastric vein. c A thin tumor stain is noted after the bolus injection of contrast to the middle hepatic artery (arrow)
RFA under laparoscopy. a There are multiple nodal changes on the liver surface. b Spider angioma-like capillary dilation on the liver surface (arrow). c Imaging obtained by color Doppler ultrasonography shows the dilated and meandering portal vein (arrow) and intrahepatic vessels. d A RFA applicator is inserted into the target under ultrasonographic guidance (arrow). e Hemorrhage from the needle tract at the removal of the RFA applicator. f Complete hemostasis is achieved using coagulation forceps
Discussion
We successfully treated HCC that developed in a patient with Osler–Rendu–Weber disease using laparoscopic RFA without any serious complications. To the best of our knowledge, this is the first such report.
HCC can be difficult to diagnose and treat in patients with Osler–Rendu–Weber disease due to vascular malformation and regenerative nodular hyperplasia. The high activity of hepatocellular regeneration in Osler–Rendu–Weber disease results in the development of nodular lesions, including focal nodular hyperplasia (FNH) [3]. Indeed, the frequency of FNH in patients with Osler–Rendu–Weber disease is 2.9%, which is 100-fold greater than that of the general population [4]. Hepatologists may have difficulty in differentiating the diagnosis between HCC and FNH due to vascular malformation. In addition, a percutaneous liver biopsy for the diagnosis should be avoided because the procedure carries a high risk of bleeding. HCC was highly suspected based on the findings of CT, MRI and angiography, which showed a lack of typical FNH, including hypodense scarring at the center and fine radiating septations. In addition, the background was HCV-infected liver, which is prone to HCC development. A histological examination also confirmed that the tumor was moderately differentiated HCC.
Although RFA is recommended for the treatment of a single HCC lesions < 3 cm in diameter according to the BCLC therapeutic algorithm [5], there is a high risk of hemorrhage due to intrahepatic shunts in Osler–Rendu–Weber disease. To the best of our knowledge, no reports have described the treatment of HCC using RFA. Although transcatheter arterial chemoembolization (TACE) therapy may be an alternative modality for treating HCC, Lee et al. [6] reported that three sessions of TACE were ineffective for treating HCC that developed in a patients with Osler–Rendu–Weber disease and severe deterioration of the liver function was observed after the TACE procedures. The presence of shunts may induce biliary ischemia after TACE [7]. While orthotopic liver transplantation is another choice of treatment for HCC in Osler–Rendu–Weber disease patients [8], the shortage of donor livers remains a serious issue. Although Gaujoux et al., reported that liver tumors were successfully resected in patients with Osler–Rendu–Weber disease. However, liver resection in patients with Osler–Rendu–Weber disease is limited for selected cases [9].
In the present case, we performed RFA under laparoscopy. One of the merits of laparoscopy is the easy control of hemorrhagic complications [10]. Indeed, while hemorrhage did occur at the needle tract in this case, we were able to achieve complete hemostasis using coagulation forceps under direct visualization. This method can be applied to manage bleeding from the abdominal wall. Casaccia et al. [11] also support the benefit of direct visualization in the laparoscopy when active bleeding developed at the puncture or trocar access sites. Santambrogis et al. [12] reported 64 cases of complete hemostasis in 88 patients with cirrhosis who received laparoscopic RFA for HCCs. In fact, laparoscopic RFA are safe and effective therapeutic option when patients are ineligible for percutaneous RFA due to abnormal coagulation tests and thrombocytopenia [13, 14]. Thus, locoregional modalities, such as RFA under laparoscopy are useful for treating HCC when available.
In conclusion, we propose that RFA under laparoscopy is a useful modality for treating malignant tumors, including HCC, in patients with Osler–Rendu–Weber disease.
References
Garcia-Tsao G. Liver involvement in hereditary hemorrhagic telangiectasia (HHT). J Hepatol. 2007;46:499–507.
Buscarini E, Danesino C, Olivieri C, et al. Liver involvement in hereditary haemorrhagic telangiectasia or Rendu-Osler-Weber disease. Dig Liver Dis. 2005;37:635–45.
Wanless IR, Gryfe A. Nodular transformation of the liver in hereditary hemorrhagic telangiectasia. Arch Pathol Lab Med. 1986;110:331–5.
Buscarini E, Danesino C, Plauchu H, et al. High prevalence of hepatic focal nodular hyperplasia in subjects with hereditary hemorrhagic telangiectasia. Ultrasound Med Biol. 2004;30:1089–97.
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55.
Lee JH, Lee YS, Kim PN, et al. Osler-Weber-Rendu disease presenting with hepatocellular carcinoma: radiologic and genetic findings. Korean J Hepatol. 2011;17:313–8.
Blewitt RW, Brown CM, Wyatt JI. The pathology of acute hepatic disintegration in hereditary haemorrhagic telangiectasia. Histopathology. 2003;42:265–9.
Scelzo C, Greco S, Bonanni L, et al. The role of liver transplantation in the treatment of hereditary hemorrhagic telangiectasia: a short literature review. Transplant Proc. 2007;39:2045–7.
Gaujoux S, Bucau M, Ronot M, et al. Liver resection in patients with hepatic hereditary hemorrhagic telangiectasia. Dig Surg. 2013;30:410–4.
Morimoto N, Isoda N, Takaoka Y, et al. Short-term results of laparoscopic radiofrequency ablation using a multipolar system for localized hepatocellular carcinoma. Liver Cancer. 2017;6:137–45.
Casaccia M, Santori G, Bottino G, et al. Laparoscopic resection vs laparoscopic radiofrequency ablation for the treatment of small hepatocellular carcinomas: a single-center analysis. World J Gastroenterol. 2017;23:653–60.
Santambrogio R, Podda M, Zuin M, et al. Safety and efficacy of laparoscopic radiofrequency ablation of hepatocellular carcinoma in patients with liver cirrhosis. Surg Endosc. 2003;17:1826–32.
Cillo U, Vitale A, Dupuis D, et al. Laparoscopic ablation of hepatocellular carcinoma in cirrhotic patients unsuitable for liver resection or percutaneous treatment: a cohort study. PLoS One. 2013;8:e57249.
Herbold T, Wahba R, Bangard C, et al. The laparoscopic approach for radiofrequency ablation of hepatocellular carcinoma–indication, technique and results. Langenbecks Arch Surg. 2013;398:47–53.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Research involving human participants
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from the patient for being included in the study.
Rights and permissions
About this article
Cite this article
Takaoka, Y., Morimoto, N., Miura, K. et al. A successful treatment for hepatocellular carcinoma with Osler–Rendu–Weber disease using radiofrequency ablation under laparoscopy. Clin J Gastroenterol 11, 501–506 (2018). https://doi.org/10.1007/s12328-018-0877-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-018-0877-x