Abstract
A 70-year-old woman underwent upper gastrointestinal endoscopy and was found to have a 25-mm protruded lesion in the gastric body. A biopsy revealed malignant cells. Endoscopic submucosal dissection was performed. Histopathologically, the tumor was mainly composed of poorly differentiated adenocarcinoma (PDA), while moderately differentiated adenocarcinoma was observed on its superficial layer. The tumor was located within the mucosal layer. PDAs rarely form a protruded lesion. Here, the presence of a moderately differentiated adenocarcinoma element in the superficial layer of the tumor might have protected the tumor cells from erosion, and solid proliferation of the PDA also contributed to its outward growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is rare for a poorly differentiated gastric adenocarcinoma to present as a protruded lesion [1,2,3,4]. In addition, poorly differentiated adenocarcinomas (PDAs) tend to invade into the submucosal layer, and most cases are detected in more advanced stages [4]. Thus, protruded intramucosal PDAs are extremely rare, and therefore little is known about their pathological and endoscopic characteristics [5,6,7,8,9,10,11]. In this case report, we present a case of a protruded intramucosal PDA of the stomach. We also compare the previous findings on poorly differentiated types of early gastric cancer presenting as types 0-I and discuss the pathobiological nature of the tumor based on its morphological and immunohistological findings.
Case report
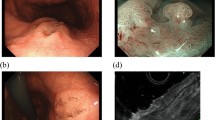
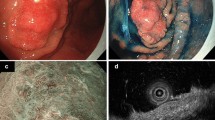
A 70-year-old woman, who underwent upper gastrointestinal endoscopy to determine the cause of her anemia, was found to have a protruded gastric lesion on the greater curvature of the lower gastric body. The patient was referred to our hospital for treatment. The blood test results were all within normal limits. Contrast-enhanced computed tomography revealed no remarkable lymph node metastasis. Conventional endoscopy revealed a 25-mm protruded lesion formed by aggregated nodules of varying sizes in the greater curvature of the lower gastric body (Fig. 1a, b). As the height of the protrusion of the nodules was >3 mm, we considered the morphology type of the tumor to be type 0-I (the Paris classification [12]). The surface of the distal side of the lesion was slightly depressed and covered with whitish exudate. Magnifying endoscopy with narrow-band imaging (M-NBI) revealed that the surface microstructure of the individual nodular components contained barely visible irregular structures, and the microvessels exhibited dilation, twisting, and caliber changes, forming closed-loop vessels with repeated irregular anastomoses (Fig. 1c, d). The microstructure and microvessels of the nodules with the whitish exudate could not be examined because the exudate was adhering to its superficial layer. The M-NBI findings suggested that the tumor consisted mainly of moderately differentiated adenocarcinoma. A biopsy specimen revealed that the tumor cells had proliferated in a fused glandular to alveolar structure.
Endoscopic findings of the lesion. a Upper gastrointestinal endoscopy shows a 25-mm protruded lesion formed by aggregated nodules of varying sizes in the greater curvature of the lower gastric body. The surface of part of the lesion was covered with whitish exudate, and faded in color. b Chromoendoscopy findings with indigo carmine staining. The surface of some nodules was covered with whitish exudate. c, d Magnifying endoscopy with M-NBI reveals that the surface microstructure of the individual nodular components contained barely visible irregular structures, and the microvessels exhibited dilation, twist, and caliber change, forming closed-loop vessels with repeated irregular anastomoses
On the basis of these findings, we decided to perform endoscopic submucosal dissection (ESD) for histological evaluation. The lesion was safely and completely removed en bloc by ESD without severe complications, such as bleeding and perforation. The resected specimen showed a protruded lesion measuring 26 × 19 mm and formed by aggregated nodules of varying sizes (Fig. 2). Histopathological examination revealed that the tumor showed an outward growth pattern that had resulted in the protruded lesion (Fig. 3a). In the surface areas of the tumor, most of the tumor cells showed a fused glandular structure, suggesting a moderately differentiated adenocarcinoma. On the contrary, inner areas of the protruded tumor lesion showed tumor cells forming solid nodular or acinar-like structures (Figs. 3b, 4a, b). There was also a transitional appearance between the moderately differentiated and poorly differentiated components. In some areas corresponding to the endoscopically nodular appearance with the whitish exudate, a superficial, moderately differentiated adenocarcinoma component was eluded and the PDA exposed to the surface. The background mucosa exhibited moderate atrophic gastritis and showed intestinal epithelial metaplasia. A microscopic examination of the biopsy specimen revealed the presence of Helicobacter pylori. Immunohistochemical examination revealed that some of the PDA cells were positive for chromogranin A and pepsinogen-I, indicating differentiation into endocrine cells and fundic gland cells, respectively (Fig. 5). The tumor showed a partly intestinal mucin phenotype, being focally positive for MUC6 and MUC2 and negative for MUC5AC. The tumor was negative for alpha fetoprotein. The pathological diagnosis was a PDA associated with moderately differentiated adenocarcinoma and macroscopic type 0-I + IIa. The tumor was limited to the lamina propria. Lymphatic but not venous invasion was observed. The horizontal and vertical resection margins were free of cancer.
a, b In the surface areas of the tumor, most of tumor cells show a fused glandular structure, suggesting moderately differentiated adenocarcinoma (×40), and a high Ki-67 positive cell ratio in superficial moderately differentiated adenocarcinoma than in PDA (×40). c, d Inner areas of the protruded tumor lesion show tumor cells forming a solid nodular structure, and a lower Ki-67 positive cell ratio than in superficial moderately differentiated adenocarcinoma (×40)
Discussion
It is rare for gastric PDAs to exhibit a protruded lesion. Oyamada reported that only 6.5% of poorly differentiated early adenocarcinomas of the stomach exhibit an elevated form [2]. The incidence of PDAs exhibiting type 0-I is rare among poorly differentiated early adenocarcinomas. Among the early gastric cancers infiltrating the submucosal layer resected by surgery at our hospital between July 2012 and June 2017, the incidence of PDAs was 24.2% (136 in 561 cases), and that of PDAs exhibiting type 0-I was 0.8% (1 in 136 cases). Adachi et al. reported that the incidence of PDAs among type 0-I early gastric cancer was 4.3% (the tumor depth of all cases was SM invasion) [5]. Takeshita et al. reported that among 77 cases of elevated-type early gastric cancer exhibiting type 0-I or type 0-IIa, 6 (7.8%) were PDAs, and only 1 case (1.3%) was a poorly differentiated early adenocarcinoma showing type 0-I [6]. Differentiated carcinomas have a strong tendency for glandular formation, and they tend to form protrusions by growing while replacing existing normal glands. On the other hand, PDAs are amorphous, have only weak intercellular bonds, and grow rapidly into deeper tissues. Most protruded-type PDAs are detected in advanced stages after invading the submucosal layer. Thus, it is extremely rare to observe the protruded form of PDA limited to the mucosal layer, and very few previous cases have been reported (Table 1).
PDAs generally show weak intercellular bonds and do not tend to grow upward [4, 13,14,15]. Sakane et al. [9] reported that the following four mechanisms have been postulated to explain how PDAs might develop into an elevated form—(1) marked proliferation of tumor cells and strong intercellular bonds within the mucosal and submucosal layer, (2) localized fibrosis in deep submucosal tissue, (3) marked lymphocytic infiltration of the stroma, and (4) neoplastic transformation of hyperplastic polyps. In the present case, no evidence of severe fibrosis, pronounced lymphocyte infiltration, or hyperplastic components in the mucosa and submucosa were detected.
PDAs are generally categorized as solid type and non-solid type. Because of weak intercellular bonds, non-solid-type PDAs may not be resistant to physicochemical stress (caused by gastric acid), and often have erosions or ulcerations on the surface of the tumor. The PDA in the present case was a solid tumor. Ikeda et al. [15] reported four cases of elevated early PDAs of the stomach, and all cases were solid-type PDAs proliferating mainly in the submucosal layer that formed the protrusion. This study also suggested that solid-type PDAs, rather than non-solid-type PDAs, tend to form a protruded lesion, and the present case was also consistent with that report.
In the present case, the superficial layer of the PDA was covered with a moderately differentiated adenocarcinoma, which may also have contributed to the formation of the protruded lesion. Maguchi et al. [16] also reported a case of type 0-IIa early gastric carcinoma with a superficial layer of a differentiated carcinoma in which signet ring cell carcinoma proliferated in the mucosal layer. The differentiated carcinoma component covering the superficial layer of the tumor protected against digestion by gastric acid, helping to prevent the formation of erosion and ulceration. Interestingly, the present case showed a higher Ki-67-positive cell ratio in the superficial moderately differentiated adenocarcinoma element than in the PDA (Fig. 4c). Based on this finding, the differentiated adenocarcinoma component had a higher proliferative capacity than the PDA; this was also thought to be a factor contributing to the preservation of the differentiated carcinoma component covering the superficial layer.
In the present case, the tumor remained in the mucosal layer. Generally, it is said that PDAs forming a protruded-type lesion, especially type 0-I, frequently infiltrate the submucosal layer. It is also extremely rare for gastric PDAs exhibiting type 0-I to remain in the mucosal layer. Solid-type PDAs frequently include differentiated adenocarcinoma components in the mucosal layer and are derived from differentiated adenocarcinomas [17]. In terms of the growth pattern, the moderately differentiated adenocarcinoma that formed the superficial layer in the present case exhibited a transitional appearance to the PDA in the lamina propria. This observation suggested that the PDA may have directly been derived from the moderately differentiated adenocarcinoma that had proliferated in the superficial layer and dedifferentiated to the PDA in the deep mucosal layer, so that the PDA might remain in the mucosal layer because there was space for the tumor to proliferate in the mucosal lamina propria. However, considering the malignancy of poorly differentiated adenocarcinoma, the tumor might infiltrate the submucosal layer within a few years.
Tumor cells in other areas also stained positive for pepsinogen-1. Ueyama et al. [18, 19] collected cases of gastric carcinoma exhibiting differentiation into fundic glands, and they proposed a new category of fundic gland-type gastric adenocarcinoma. The endoscopic findings of the present case differed from those reported for fundic gland-type gastric carcinoma, and the clonal proliferation of tumor cells exhibiting differentiation into fundic glands could not be confirmed. There has been no previous report of PDAs exhibiting differentiation into fundic glands, and its significance is unclear.
There have only been a small number of reports on the endoscopic findings of elevated PDAs. Kawaura et al. [20] reported the following endoscopic characteristics of elevated PDAs—(1) the tumor is formed by an aggregation of nodules of varying sizes, with an irregular surface frequently covered with whitish exudate; (2) faded in color; and (3) its morphology is similar to that of a submucosal tumor. In the present case, the elevation was formed by the aggregation of nodules of varying sizes, and some of these nodules were faded in color. The surface of some nodules was also covered with whitish exudate, consistent with the endoscopic characteristics reported by Kawaura et al. Pathologically, the area in which the superficial layer was covered with whitish exudate was exposed PDA.
In our case report, we have presented a case of elevated PDA of the stomach that was limited to the mucosal layer. The elevation may have been maintained by the structure of the solid-type PDA and the moderately differentiated adenocarcinoma element on the superficial layer. Additional reports of cases similar to this will be required in future investigations of their appearance under magnifying endoscopy, growth patterns, and histological schemes.
References
Mishima T, Okuda S, Tatsuta M, et al. Elevated type early gastric cancers presenting as signet ring cell carcinoma. Jpn J Gastroenterol. 1978;75:1504.
Oyamada Y. Clinicopathological study for diffuse type gastric carcinomas presenting as elevated formation. Prog Dig Endosc. 1987;30:153–8.
Yanagisawa A, Kato Y, Sugano H. Histogenetic backgrounds and growth pattern of undifferentiated type microcarcinoma of the stomach-in comparison with differentiated type microcarcinoma. Stomach Intest. 1989;24:1335–43.
Anzai H, Takagi K, Ohta H, et al. Clinicopathological studies of elevated early gastric cancer showing histologically undifferentiated adenocarcinoma. Jpn J Gastroenterol Surg. 1988;21:2099–105.
Adachi Y, Mori M, Enjoji M. Type I early gastric carcinoma consisting of poorly differentiated adenocarcinoma − a clinicopathologic study. Stomach Intest. 1988;23:577–81.
Takeshita K, Nakajima A, Mori S, et al. Endoscopic characteristics of early gastric cancers related to the types of histology. Gastroenterol Endosc. 1982;24:440–7.
Tomimatusu H, Ide K, Koga T, et al. Early gastric signet ring cell carcinoma (Type IIc + I): a case report. Gastroenterol Endosc. 1991;33:562–6.
Ichiyoshi Y, Saku M, Maekawa S, et al. Poorly differentiated adenocarcinoma developed in a hyperplastic polyp of the stomach, report of a case. Stomach Intest. 1992;27:696–9.
Sakane M, Kashiwagi R, Mitsutsufi M, et al. A case of poorly differentiated early adenocarcinoma of the stomach forming a protruded type lesion. Fig Endosc. 1997;9:300–4.
Tadatomo H, Ashizawa T, Andoh M, et al. A case of undifferentiated type of early gastric cancer presenting as type I. Jpn J Gastroenterol Surg. 2002;35:156–60.
Yasuda M, Torisu R, Aoki R, et al. Poorly differentiated early gastric adenocarcinoma presenting as a pedunculated lesion. Gastroenterol Endosc. 2002;44:1162–7.
Inoue H, Kashida H, Kudo S, et al. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–43.
Nakamura K. Structure of the gastric cancer. Tokyo: Igakushoin; 1990. p. 33–5.
Nakahara K, Watanabe Y, Tamiya Y, et al. Macroscopic type of early gastric cancer, type 0-I and type 0-IIa cancer. Stomach Intest. 2009;44:507–21.
Ikeda A, Yasuhiro O, Kuwata T, et al. Elevated type gastric cancer formed by non-solid poorly differentiated adenocarcinoma, report of a case. Stomach Intest. 2012;47:1435–45.
Maguchi H, Orii Y, Saitoh Y, et al. Ila-aggregated polypoid type of poorly differentiated adenocarcinoma in the gastric mucosa, report of a case. Stomach Intest. 1991;26:209–15.
Tanaka H, Horie S, Kashiwagi R, et al. A case of poorly differentiated gastric adenocarcinoma appearing polyposis lesions. Gastroenterol Endosc. 2004;46:1051–6.
Ueyama H, Yao T, Nakashima Y, et al. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609–19.
Ueyama H, Matsumoto K, Nagahara A, et al. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014;46:153–7.
Kawaura Y, Kaneko Y, Iwa T. A comparative study between endoscopic findings and the histological findings of elevated gastric cancer. Gastroenterol Endosc. 1981;23:686–90.
Acknowledgements
We thank Dr. Hiroya Ueyama of the Department of Gastroenterology, and Dr. Takashi Yao of the Department of Pathology at the Juntendo University School of Medicine for evaluating the pathological findings.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
Nakajo, K., Oono, Y., Kuwata, T. et al. A case of a protruded lesion formed by a poorly differentiated intramucosal adenocarcinoma of the stomach: an immunohistochemical analysis. Clin J Gastroenterol 11, 127–132 (2018). https://doi.org/10.1007/s12328-017-0804-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-017-0804-6