Abstract
Introduction
To compare the effects of a preservative-free (PF) ophthalmic solution containing hyaluronic acid (HA) 0.4% and taurine (TAU) 0.5% with those of a PF ophthalmic solution containing HA 0.2% on ocular surface signs, symptoms, and morphological parameters in glaucoma patients under multiple long-term topical hypotensive therapy.
Methods
Eligible patients underwent evaluation of ocular surface parameters by ocular surface disease index (OSDI) and glaucoma symptom scale (GSS) questionnaires, breakup time test (BUT), Schirmer I test, corneal and conjunctival staining (Oxford scale), and conjunctival in vivo confocal microscopy (Heidelberg Retina Tomograph 3, Heidelberg Engineering GmbH, Heidelberg, Germany). After the baseline visit, patients were randomized to use a PF ophthalmic solution containing HA 0.4% and TAU 0.5%, QID, in both eyes (group 1) or to use a PF ophthalmic solution containing HA 0.2%, QID (group 2) in addition to the ongoing preserved hypotensive treatment. Follow-up visits were scheduled at 30 and 90 days.
Results
Thirty-nine eyes of 39 glaucoma patients were included in the study. At baseline, results of study tests of both groups were similar. After 90 days in group 1 the BUT (p = 0.01), the Oxford score (p = 0.03), the conjunctival goblet cells (CGC) density (p = 0.0005) ,and the two questionnaires score significantly improved (OSDI, p = 0.003; GSS, p = 0.003) compared to baseline values, while in group 2 all these parameters did not differ from baseline (BUT, p = 0.39; Oxford score, p = 0.54; CGC density, p = 0.33, OSDI p = 0.65, GSS, p = 0.25). The BUT and the CGC density were statistically different between groups both at 30 and 90 days (p = 0.04 and p = 0.04, respectively). The Schirmer I test did not statistically change after 90 days in both groups.
Conclusions
The PF ophthalmic solution with HA 0.4% and TAU 0.5% seems to improve CGC density and reduce signs and symptoms of dry eye in glaucoma patients under long-term multiple preserved hypotensive therapy.
Trial registration
ClinicalTrials.gov identifier, NCT03480295.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaucoma is a chronic, progressive, potentially blinding, optic neuropathy and lowering the intraocular pressure (IOP) is the only evidence-based method to reduce the risk of visual field progression [1,2,3]. According to the European Glaucoma Society Guidelines, topical monotherapy is the first step in the therapeutic algorithm for glaucoma and if target pressure is not reached, it is recommended to switch drugs or to add another drug in combination [4].
The use of combination therapy is necessary in a high proportion of patients at any stage of the disease; for example, the Ocular Hypertension Treatment Study and the Collaborative Initial Glaucoma Treatment Study reported that up to 50% and 75% of patients required two or more drugs to reach their target intraocular pressure [1, 3].
Nevertheless, the chronic use of multiple hypotensive eye drops with repeated daily instillations exposes the ocular surface to the simultaneous actions of the active compounds and the preservatives with consequences on the health status of the ocular surface, subjective symptoms, and reduced patient quality of life [5,6,7].
Benzalkonium chloride (BAK) is the most widely used preservative in ophthalmic preparations and its epithelial toxic effects have been well established. The chronic use of BAK has been associated with dry eye characterized by inflammation of the ocular surface, conjunctival squamous metaplasia, apoptosis and disruption of the corneal epithelium barrier, decrease of conjunctival goblet cells (CGC), and tear film instability [8, 9].
One of the most common treatments to relieve signs and symptoms related to dry eye is the use of lubricants containing hypotonic or isotonic buffered solutions with electrolytes, surfactants, and various types of viscosity agents such as hyaluronic acid (HA). HA is a naturally occurring polysaccharide that has an excellent water-retaining and lubricant properties, as well as viscoelastic effects that aid vision during blinks and maintain hydration and lubrication of the ocular surface between blinks [10]. For dry eye therapy, higher HA concentrations, between 0.1% and 0.4% are usually used in clinical practice [11,12,13].
Several studies have demonstrated that taurine (TAU), an essential amino acid highly expressed in the anterior segment of the eye, and the most abundant amino acid in the tear fluids, exerts a protective activity against damage caused by oxidative agents to the ocular surface [14,15,16,17]. Furthermore, it has been demonstrated that TAU plays a key role in regulating epithelial barrier function [18].
The purpose of the present study was to compare the effects of a preservative-free ophthalmic solution containing HA 0.4% and TAU 0.5% (Oftaial Plus®, Alfa Intes, S.r.l.) on ocular surface signs, symptoms, and morphological parameters with those of a preservative-free ophthalmic solution containing lower concentration of HA (0.2%) (Zerodue®, Alfa Intes, S.r.l.) without TAU in glaucoma patients undergoing multiple long-term topical preserved hypotensive therapy.
Methods
This was a 3-month, prospective, randomized, single masked, parallel study.
The study protocol was approved by the ethics committee of the IRCCS Fondazione G.B. Bietti (Trial Registry N.51/FB/16) where it was conducted in accordance with the Declaration of Helsinki. The trial was retrospectively registered at ClinicalTrials.gov, ID NCT03480295.
Only patients older than 18 years old of both genders and able to understand and sign the written informed consent were enrolled.
Inclusion criteria were a diagnosis of glaucoma and ongoing topical therapy with two or more preserved ocular hypotensive eye drops per day for at least 2 years.
Primary open angle glaucoma was defined as the presence of a repeatable visual field (VF) defect, corresponding to optic nerve damage. A glaucomatous VF change was defined as the consistent presence of a cluster of three or more non-edge points on the pattern deviation plot, with a probability of occurring in less than 5% of the normal population, and with one of these points having the probability of occurring in less than 1% of the normal population, a pattern standard deviation with p < 5%, a glaucoma hemifield test result outside normal limits. VF defects had to be reliable (false positive < 15%; fixation losses and false negative responses < 25%) and confirmed in at least two tests no more recent than 1 month.
Exclusion criteria were best corrected visual acuity ≤ 20/40, history of active or past ophthalmological diseases different than glaucoma, contraindications to use of topical solution components used in this study, current use of contact lenses, current use or use in the past 6 months of ocular medications other than hypotensive eye drops, systemic treatments known to affect tear secretion, any history or slit lamp evidence of eye surface diseases different from dry eye, history of ocular trauma, surgery or laser treatments, rheumatologic and autoimmune diseases, diabetes, peripheral neuropathies, use of systemic steroids or immunosuppressants.
Clinical Examination
At the screening visit all patients underwent comprehensive ophthalmological examination including best corrected visual acuity assessment, slit lamp evaluation, IOP measurement using Goldmann applanation tonometry, and indirect dilated ophthalmoscopy with a 90-diopters lens. Visual field testing using Humphrey 24-2 SITA-Standard program (Carl Zeiss Meditec, Inc., Dublin, CA 24-2 program) was performed only for those patients with the last exam older than 3 months.
Baseline visit was split into two visits scheduled 1 week apart ±2 days to evaluate ocular surface alterations by means of several tests.
To minimize the influence of each test on the subsequent test, we performed first the least invasive and then the most invasive test. During the first baseline visit patients were asked to complete two questionnaires, the ocular surface disease index (OSDI) and the glaucoma symptom scale (GSS), and then underwent the breakup time test (BUT), corneal and conjunctival staining evaluation using Oxford Staining Scheme, and Schirmer I test after 15 min of rest. During the second baseline visit, patients underwent conjunctival confocal laser microscopy with the Rostock Module of Cornea of the Heidelberg Retina Tomograph (HRT3, Heidelberg Engineering GmbH, Heidelberg, Germany).
At the end of the second baseline visit patients were randomized into two groups with a 1:1 ratio according to a computer-generated randomization list. Group 1 was asked to self-administer one drop four times/day of a preservative-free ophthalmic solution containing 750 kDa HA 0.4% and TAU 0.5% in both eyes, while group 2 was asked to self-administer one drop four times/day of a preservative-free ophthalmic solution containing only 750 kDa HA 0.2%, in addition to the ongoing hypotensive therapy.
Follow-up visits were scheduled at 30 and 90 days and all the exams were repeated in the same order as they were performed at the baseline visits.
Both eyes were tested but only one randomly selected eye per patient was included in the analysis.
The primary endpoint was the comparison of the changes over time of the CGC density between the two groups. Secondary endpoints were the between-group comparisons of the changes over time of BUT, Schirmer test, conjunctival staining, OSDI score, and GSS score.
All clinical measurements as well the statistical analysis were performed by investigators masked to the patients’ treatment group. A brief description of the study tests is reported below.
OSDI
OSDI is a questionnaire with good test–retest reliability and validity used to evaluate the impact of dry eye on vision-related quality of life [19]. OSDI is a 12-item questionnaire divided into three different subgroups: ocular discomfort (five items), ocular symptoms during daily activities (four items), and ocular symptoms caused by environmental triggers (three items) within the past 4 weeks. The 12 items are graded on a scale of 0–4 where 0 indicates none of the time; 1, some of the time; 2, half of the time; 3, most of the time; 4, all the time. The total OSDI score is then calculated according to the following formula: OSDI = [(sum of scores for all questions answered) × 100]/(total number of questions answered) × 4]. Thus, the OSDI is scored on a scale of 1–100, with higher scores representing greater disability.
GSS
The GSS questionnaire is a modified version of a symptom checklist that was developed by the investigators of the Ocular Hypertension Treatment Study [20]. In this study, the Italian version of the GSS, which has been previously validated, has been used [21]. The items include 10 ocular concerns, some of nonvisual nature (burning/smarting/stinging, tearing, dryness, itching, soreness/tiredness, feeling of something in the eye) and some of visual nature (blurry/dim vision, hard to see in daylight, hard to see in dark places, halos around lights). The 10 items investigate each eye separately. Each item consists of a yes/no response choice for the presence of a specific symptom within the past 4 weeks, plus a 4-level bothersome scale for those who reported having a given symptom. Therefore, for each eye, a 5-level score is generated, ranging from 0 (complaint present and very bothersome) to 4 (complaint absent). This score is then transformed to a 0–100 scale, with 0 representing presence of a very bothersome problem and 100 representing absence of a problem. The final GSS score is averaged between the two eyes.
BUT
The BUT was performed to quantify the tear film stability, by instilling a drop of fluorescein in the inferior fornix. After several blinks, the tear film was examined using a broad beam of the slit lamp with cobalt blue illumination. The time lapse between the last blink and the appearance of the first randomly distributed dark discontinuity in the fluorescein-stained tear film was measured three times, and the mean value of the measurements was calculated.
Corneal and Conjunctival Staining
Corneal and conjunctival staining was evaluated 2 min after the sodium fluorescein application, using a slit lamp with broad slit width and ×16 magnification with cobalt blue illumination. The light source of the slit lamp was also set to high intensity.
Corneal and conjunctival staining was graded according to the Oxford staining scheme, a chart consisting of a series of panels, labeled A–E in order of increasing severity (grade 0 = absent; grade 1 = minimal; grade 2 = mild; grade 3 = moderate; grade 4 = marked; grade 5 = severe). In each chart, staining is represented by punctate dots. The number of dots increases by 1 log unit between panel A and B and by 0.5 log units between B and E. To grade staining, comparisons are made between the panels and the appearance of staining on the exposed interpalpebral conjunctiva and cornea of the patient [22].
Schirmer I
The Schirmer I test was used to analyze the production of aqueous tears. It was performed by measuring the amount of wetting of a special filter paper (5 mm wide and 35 mm long) placed in the inferior fornix. To minimize the artifacts from this test, the patients gently closed their eyelids until 5 min had elapsed. Then patients opened their eyes, and the strips were removed.
In Vivo Confocal Microscopy
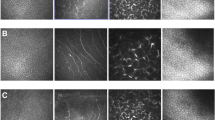
The HRT3/Rostock Cornea Module is composed of a ×60 water immersion objective lens and of a diode laser with a wavelength of 670 nm. The size of image acquired consists of 384 × 384 pixels including an area of 400 × 400 µm with transversal optical resolution of approximately 1 µm/pixel. For a successful exam, a large drop of high viscosity contact gel (Recugel; Bausch & Lomb Inc.) was applied on the surface of the microscope lens. Then, for each subject, an appropriate and sterilized plastic cup was placed on the microscope (TomoCap; Heidelberg Engineering GmbH). Before examination, the eye was topically anesthetized using 0.4% oxybuprocaine hydrochloride. Then patients were accommodated with appropriate positioning of the head and a drop of high viscosity contact gel was instilled in the lower conjunctival fornix. Patients were invited to fix the red light straight ahead and, once the focal plane was adjusted, they were invited to observe the most extreme temporal field of view. The sequences of the nasal bulbar conjunctival images were taken 5 mm away from the limbus along the z-axis in the manual mode. At the end of each examination, one drop of the antibiotic was instilled. The images were taken between 5 and 25 µm of the conjunctival tissue and the best sequence with the highest quality was considered for the analysis. As described by previous studies, the goblet cells appeared large, hypereflective, and oval-shaped with hypereflective nuclei, larger than the surrounding epithelial non-goblet cells, crowded in groups or dispersed within the epithelium [23, 24]. The images were examined by two different investigators using the Cell Count Software (Heidelberg Engineering GmbH) in the manual mode and the results were expressed as cells per square millimeter.
Statistical Analysis
The normality of the distribution of the data was checked by Shapiro–Wilk test and parametric continuous variables were expressed as mean ± standard deviation and non-parametric variables as median and interquartile range.
To compare changes over time of the study parameters between the two groups, multivariate analysis of variance (MANOVA) for repeated measures was used.
Within-group paired parametric and non-parametric continuous variables were compared by means of the paired samples t test and Wilcoxon signed rank test. Categorical variables were compared between groups by means of Chi-square test or Fisher’s exact test as appropriate. A p value less than 0.05 was considered statistically significant. The statistical analysis was performed using JMP software ver. 9.0.1 (SAS Institute Inc.).
Results
Forty eyes of 40 glaucoma patients under multiple long-term preserved topical hypotensive therapy were included in the study. Group 1 included 19 eyes of 19 patients, while group 2 included 20 eyes of 20 patients. One patient of group 1 was lost to follow-up after the baseline visit without performing any follow-up visit and was not included in the analysis.
Clinical and demographic data of both groups were statistically similar and are shown in Table 1. Moreover, no statistically significant differences were detected between groups in baseline ocular surface signs, symptoms, and morphological parameters (Table 2).
Changes over time of BUT and the CGC density were statistically different between groups throughout the follow-up (p = 0.045 and p = 0.048, respectively) while changes of Schirmer I, OSDI, and GSS scores of over time were statistically similar between groups (full details are reported in Table 2).
In group 1 after 90 days of treatment, but not after 30 days, both the GSS score and the OSDI score were statistically significantly improved compared to baseline (GSS score, 56.95 ± 14.64 at baseline vs 63.71 ± 13.69 at 90 days, p = 0.0032; OSDI score, 43.76 ± 19.17 at baseline vs 34.47 ± 15.48 at 90 days, p = 0.007). In group 2 neither GSS nor OSDI showed statistically significant changes at 30 and 90 days compared to baseline (full details in Table 2).
In group 1 after 90 days, BUT, Oxford score, and CGC density were significantly improved compared to baseline values (BUT, 7.63 ± 2.98 vs 8.84 ± 2.52 s, p = 0.01; Oxford score, 1.31 ± 0.74 vs 0.89 ± 0.56, p = 0.03; CGC density, 50.89 ± 20.75 vs 67.47 ± 23.68 cells/mm2, p = 0.0005). In group 1, Oxford score was the only parameter showing statistically significant changes at 30 days compared to baseline (full details in Table 2).
In group 2, BUT, Oxford score, and CGC density after 90 days were not statistically different from baseline (BUT, 7.25 ± 2.14 at baseline vs 7.65 ± 2.0 s at 90 days, p = 0.39; Oxford score, 1.15 ± 0.98 at baseline vs 1.00 ± 0.85 at 90 days, p = 0.54; CGC density, 49.65 ± 19.88 at baseline vs 53.60 ± 19.86 cells/mm2 at 90 days, p = 0.33).
No significant changes compared to baseline of Schirmer I test both after 30 days and after 90 days were observed in either group (group 1, 12.05 ± 6.40 vs 12.78 ± 7.10 mm/s, p = 0.59; group 2, 11.25 ± 2.3 vs 11.40 ± 3.97 mm/s, p = 0.76).
Discussion
Medical treatment is used as first-line therapy in glaucoma to modulate the progression of the disease and, when effective, is administered for decades or a lifetime in the form of single or multiple topical eye drops. Nevertheless, despite being essential for controlling the progression of the disease, chronic topical treatment exposes the ocular surface to the simultaneous deleterious actions of the active compounds and their preservatives with significant impact on the health of the ocular surface with consequent symptoms and decreased quality of life [5,6,7]. Dry eye, inflammation of the ocular surface, conjunctival squamous metaplasia, apoptosis, disruption of the corneal epithelium barrier, and decrease of CGC are all described potential consequences of chronic topical glaucoma treatment [8, 9].
In the present study, we compared the effects of a preservative-free topical solution containing HA 0.4% and TAU 0.5% with those of a preservative-free topical solution containing lower concentration of HA (0.2%) without TAU on ocular surface signs, symptoms, and morphological parameters in glaucoma patients under multiple long-term topical preserved hypotensive therapy.
In our sample population at baseline, a reduced BUT was detected in both groups (group 1, 7.63 ± 2.98; group 2, 7.25 ± 2.14) indicating a tear film instability associated with minimal to mild alterations of corneal and conjunctival epithelium as expressed by the Oxford score (group 1, 1.31 ± 0.74; group 2, 1.15 ± 0.98). This finding is consistent with previous findings of a reduced BUT in patients under chronic topical medications for glaucoma [25].
In our study BUT showed a statistically significant trend of improvement throughout the follow-up in patients treated with HA 0.4% and TAU 0.5% topical solution, but not in patients treated with a lower concentration of HA 0.2% without TAU. Specifically, we found that after 90 days of treatment with HA 0.4% and TAU 0.5% topical solution, BUT was significantly increased by 15% compared to baseline from 7.63 ± 2.98 to 8.84 ± 2.52 s; while in patients treated with HA 0.2%, BUT showed a non-significant change of +0.5% compared to baseline values.
Additionally, a reduced CGC density at baseline was detected in both groups and this agrees with previous studies evaluating CGC density in glaucoma patients under topical preserved hypotensive therapy [23, 24]. CGC are reported to be decreased in the presence of inflammatory and toxic stimuli on the ocular surface like during chronic topical preserved eye drops exposure. CGC represent the main source of ocular surface mucoproteins and participate in tear film stability and their reduction may lead to tear film instability and ocular surface alterations [25]. Furthermore, it has been reported that CGC integrity plays a critical role in bleb functionality after filtration surgery and that their reduction might compromise the long-term surgical outcome [26].
In the present study CGC density showed a significant trend of improvement throughout the follow-up in patients treated with HA 0.4% and TAU 0.5% topical solution, but not in patients treated with a lower concentration of HA 0.2% without TAU. Within-group analysis of changes revealed that CGC density was significantly increased in patients treated with HA 0.4% and TAU 0.5% topical solution after 90 days, but not after 30 days from baseline.
At baseline, in both groups the OSDI scores suggested that patients were affected by a severe dry eye disease. The OSDI score provides a rapid assessment of the symptoms of ocular irritation consistent with dry eye disease and their impact on vision-related functioning. The within-group analysis showed that OSDI score was significantly improved after 90 days of treatment with HA 0.4% and TAU 0.5% topical solution despite changes from baseline not being statistically different between groups. We did not evaluate the correlation between OSDI score and clinical signs of dry eye which was beyond the scope of the present study. However, previous studies failed to find strong correlations between objective clinical signs of dry eye and patient symptoms probably because these measures lack sufficient sensitivity to capture the full range of ocular surface and tear abnormalities that produce typical dry eye symptoms [19].
The GSS score at baseline was 56.95 ± 14.64 in group 1 and 54.82 ± 17.54 in group 2. The GSS quantitatively assesses ophthalmic symptoms common to patients with glaucoma: a score of 0 represents presence of a very bothersome problem and a score of 100 represents absence of a problem. Although it cannot be determined whether the scale measures symptoms of glaucoma or symptoms of glaucoma treatment, the psychometric properties of the measure show that it provides a valid and reliable estimate of symptoms associated with glaucoma and its treatments and may help clarify the quality of life experienced by glaucomatous patients [20]. The within-group analysis showed that GSS score was significantly improved after 90 days of treatment with HA 0.4% and TAU 0.5% topical solution despite changes from baseline not being statistically different between groups.
The Schirmer I test, which expresses the aqueous component of the tear film production, was within normal limits at baseline and was unaffected by the study interventions in either group throughout the follow-up.
In clinical practice, HA in different concentrations (0.003–0.4%) is frequently prescribed to patients suffering from signs and symptoms of dry eye because of its hydrating and mucomimetic properties. HA increases the stability of the precorneal tear film and improves ocular surface wettability and smoothness thanks to its water-retentive and viscoelastic properties. Furthermore, it increases the healing time of corneal epithelium, promoting epithelial cell proliferation and migration because it is a ligand for CD44, a transmembrane cell surface adhesion molecule [27].
In a study comparing the effect of an artificial tear containing preservative-free HA 0.2% with a 0.9% saline solution in 16 mild dry eye patients which used both products, each for 1 month, the authors found that patients reported a significant improvement in OSDI score, bulbar hyperemia, corneal staining, conjunctival staining, and subjective satisfaction when taking the lubricants containing preservative-free HA 0.2% [13]. As mentioned before, patients included in our study were affected by severe dry eye disease and this might be the reason why ocular surface parameters measured in group 2 under HA 0.2% did not statistically improve throughout the study.
Supplementation of ocular lubricants with amino acids is a recent strategy to treat dry eye disease. Amino acids are naturally present in human tears and among these TAU is the most frequent (46.28% in basal tears and 32.78% in reflex tears) [15].
The beneficial effects of TAU are, in part, a result of its antioxidant properties, as well as its ability to improve mitochondrial function by stabilizing the electron transport chain and inhibiting the generation of reactive oxygen species [28].
It has been demonstrated that TAU favors corneal wound healing, protects ocular surface tissues from chemical damage, reduces ocular inflammation, and induces a regenerative effect on the tear film [18].
Results of our study confirm the beneficial effects of both elements in combination, HA 0.4% and TAU 0.5%, in improving signs and symptoms of dry eye in glaucomatous patients under long-term hypotensive preservative therapy.
Furthermore, this preservative-free ophthalmic solution is a hypotonic solution, so it can reduce tear hyperosmolarity that stimulates a cascade of inflammatory events in the epithelial surface cells, involving mitogen-activated protein kinases and nuclear factor kB signaling pathways, along with generating inflammatory cytokines (interleukin-1α, interleukin-1β, and tumor necrosis factor-α) and matrix metalloproteinases (MMP9) [28]. These inflammatory events usually lead to the apoptotic death of surface epithelial cells, including goblet cells.
On the other hand, the preservative-free ophthalmic solution with HA 0.2% administered to patients in group 2 is an isotonic solution, and this might have contributed to the lower improvements of signs and symptoms of dry eye in those patients, in addition to the low HA concentration and the absence of TAU.
A limit of the present study is that the results leave open the question whether the outcome parameters differ as a result of the higher concentration of HA, or the presence of TAU, or the combination of both. However, it should be observed that the HA molecular weight of the two ophthalmic solutions was the same (750 kDa), so the viscosity could be considered similar.
Additional studies are necessary to further explore whether the between-group differences observed in the present study are related to the higher concentration of HA, to the presence of TAU, or to the combination of both in one single preservative-free hypotonic solution.
Conclusions
A preservative-free hypotonic ophthalmic solution with HA 0.4% and TAU 0.5% seems to reduce signs and symptoms of dry eye in glaucoma patients under multiple long-term preserved hypotensive therapy. It is important to recognize the presence of ocular surface alterations in patients with glaucoma because relieving the subsequent ocular discomfort may help them to improve their adherence to the hypotensive treatment, while preserving their quality of life.
References
De Moraes CG, Demirel S, Gardiner SK, et al. Effect of treatment on the rate of visual field change in the ocular hypertension treatment study observation group. Invest Ophthalmol Vis Sci. 2012;53:1704–9.
Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment. Arch Ophthalmol. 2003;121:48–56.
Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–53.
European Glaucoma Society. Terminology and guidelines for glaucoma. 4th ed. Savona, Italy: Publicomm; 2014.
Rossi GC, Pasinetti GM, Scudeller L, Bianchi PE. Ocular surface disease and glaucoma: how to evaluate impact on quality of life. J Ocul Pharmacol Ther. 2013;29(4):390–4.
Ghosh S, O’Hare F, Lamoureux E, Vajpayee RB, Crowston JG. Prevalence of signs and symptoms of ocular surface disease in individuals treated and not treated with glaucoma medication. Clin Exp Ophthalmol. 2012;40:675–81.
Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153:1–9.
Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86:418–23.
Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15:511–38.
Aragona P, Papa V, Micali A, Santocono M, Milazzo G. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br J Ophthalmol. 2002;86:181–4.
Johnson ME, Murphy PJ, Boulton M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefes Arch Clin Exp Ophthalnol. 2006;244:109–12.
Troiano P, Monaco G. Effect of hypotonic 0.4% hyaluronic acid drops in dry eye patients: a cross-over study. Cornea. 2008;27:1126–30.
Pinto-Fraga J, López-de la Rosa A, Blázquez Arauzo F, Urbano Rodríguez R, González-García MJ. Efficacy and safety of 0.2% hyaluronic acid in the management of dry eye disease. Eye Contact Lens. 2017;43:57–63.
Nakamori K, Koyama I, Nakamura T, Nemoto M, Yoshida T, Umeda M, Inoue K. Quantitative evaluation of the effectiveness of taurine in protecting the ocular surface against oxidant. Chem Pharm Bull. 1993;41:335–8.
ChenZhuo L, Murube J, Latorre A, del Rio RM. Different concentrations of amino acids in tears of normal and human dry eyes. Adv Exp Med Biol. 2002;506:617–21.
Pasantes-Morales H, Wright CE, Gaull GE. Taurine protection of lymphoblastoid cells from iron-ascorbate induced damage. Biochem Pharmacol. 1985;34:2205–7.
Nakatsukasa M, Sotozono C, Shimbo K, et al. Amino acid profiles in human tear fluids analyzed by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. Am J Ophthalmol. 2011;151:799–808.
Rusciano D, Roszkowska AM, Gagliano C, Pezzino S. Free amino acids: an innovative treatment for ocular surface disase. Eur J Pharmacol. 2016;787:9–19.
Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118:615–21.
Lee BL, Gutierrez P, Gordon M, et al. The glaucoma symptom scale. A brief index of glaucoma-specific symptoms. Arch Ophthalmol. 1998;116:861–6.
Rossi GCM, Pasinetti GM, Scudeller L, et al. The Italian version of the glaucoma symptom scale questionnaire: translation, validation, and reliability. J Glaucoma. 2013;22:44–51.
Bron A, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–50.
Mastropasqua L, Agnifili L, Fasanella V, et al. Conjunctival goblet cells density and preservative-free tafluprost therapy for glaucoma: an in vivo confocal microscopy and impression cytology study. Acta Ophthalmol. 2013;91:397–405.
Zhu W, Zhu W, Kong X, Xu J, Sun X. Effects of long-term antiglaucoma eye drops on conjunctival structures: an in vivo confocal microscopy study. J Ophthalmol. 2015;165475.
Doughty MJ, Bergmanson JP. New insights into the surface cells and glands of the conjunctiva and their relevance to the tearfilm. Optometry. 2003;74:485–500.
Agnifili L, Fasanella V, Mastropasqua R, et al. In vivo goblet cell density as a potential indicator of glaucoma filtration surgery outcome. Invest Ophthalmol Vis Sci. 2016;57:2898–905.
Nishida T, Nakamura M, Mishima H, Otori T. Hyaluronan stimulates corneal epithelial migration. Exp Eye Res. 1991;53:753–8.
Jong CJ, Azuma J, Schaffer S. Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids. 2012;42:2223–32.
Acknowledgements
We thank the participants of the study.
Funding
The research for this paper was supported by the Italian Ministry of Health and by Fondazione Roma. No funding or sponsorship was received for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Disclosures
Gloria Roberti, Luca Agnifili, Francesca Berardo, Ivano Riva, Michele Figus, Gianluca Manni, Luciano Quaranta, and Francesco Oddone have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee (IRCCS Fondazione G.B. Bietti, Trial Registry N.51/FB/16) and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data availability
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6120980.
Rights and permissions
About this article
Cite this article
Roberti, G., Agnifili, L., Berardo, F. et al. Prospective, Randomized, Single Masked, Parallel Study Exploring the Effects of a Preservative-Free Ophthalmic Solution Containing Hyaluronic Acid 0.4% and Taurine 0.5% on the Ocular Surface of Glaucoma Patients Under Multiple Long-Term Topical Hypotensive Therapy. Adv Ther 35, 686–696 (2018). https://doi.org/10.1007/s12325-018-0699-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0699-8