Abstract
Endophthalmitis is an intraocular inflammatory condition which may or may not be caused by infective agents. Noninfectious (sterile) endophthalmitis may be attributable to various causes including postoperative retained soft lens matter or toxicity following introduction of other agents into the eye. Infectious endophthalmitis is further subdivided into endogenous and exogenous. In endogenous endophthalmitis there is hematogenous spread of organisms from a distant source of infection whereas in exogenous endophthalmitis direct microbial inoculation may occur usually following ocular surgery or penetrating eye injury with or without intraocular foreign bodies. Acute infective endophthalmitis is usually exogenous induced by inoculation of pathogens following ocular surgery, open-globe injury and intravitreal injections. More infrequently the infective source is internal and septicemia spreads to the eye resulting in endogenous endophthalmitis. Several risk factors have been implicated including immunosuppression, ocular surface abnormalities, poor surgical wound construction, complicated cataract surgery with vitreous loss and certain types of intraocular lens. Comprehensive guidelines and recommendations on prophylaxis and monitoring of surgical cases have been proposed to minimize the risk of acute endophthalmitis. Early diagnosis and prompt management of infective endophthalmitis employing appropriately selected intravitreal antibiotics are essential to optimize visual outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute infective endophthalmitis is an inflammatory ocular condition usually caused by bacteria and more infrequently by fungi, or other parasites. This potentially devastating intraocular infection may arise either exogenously or endogenously. In exogenous endophthalmitis, the infective agents enter the eye through a breach in the globe, most commonly following intraocular surgery or injection, while penetrating eye injury and intraocular extension of infection originating from the cornea or a filtering bleb represent less common causes. In case the infective source is internal and septicemia spreads to the eye, endophthalmitis is endogenous. In this perspective, a comprehensive overview of infective endophthalmitis with emphasis on treatment trends and prophylaxis guidelines will be presented.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Exogenous Endophthalmitis
Post-Cataract Endophthalmitis
Endophthalmitis is a rare but potentially blinding complication of cataract surgery. The incidence of acute-onset postoperative endophthalmitis has steadily declined over the years from 10% in the first half of twentieth century to 0.014–0.08% nowadays [1–10]. The availability of advanced technology, the adoption of refined surgical techniques and the use of intracameral cefuroxime at the end of the operation have meaningfully contributed to this notable reduction in endophthalmitis rate [1–10].
Most endophthalmitis cases present within the first or second week after cataract surgery complaining of reduced vision and ocular pain [8]. Other ocular signs include eyelid swelling, conjunctival injection and intraocular inflammation ranging from mild inflammation to severe panuveitis with hypopyon. The severity and the prognosis of intraocular endophthalmitis is associated with the virulence and inoculum size of the offending organism, the time to presentation and initiation of appropriate therapy and the patient’s immune status [2, 11–13].
The microbial spectrum of post-cataract endophthalmitis includes Gram-positive bacteria, predominantly coagulase-negative staphylococci, Staphylococcus aureus, β-hemolytic streptococci, S. pneumonia and Enterococcus faecalis. More rarely infection can be caused by Gram-negative rods including haemophilus influenza and pseudomonas aeriginosa [5–9]. It is important to point out that visual prognosis is extremely guarded when virulent strains of streptococci or Gram-negative microbes are involved [2, 14, 15]. Bacteria causing endophthalmitis are summarized in Table 1.
Risk Factors
Several risk factors for the development of acute post-cataract endophthalmitis have been suggested [1, 8, 10, 11, 16, 17]. They can be divided into preoperative, including diseased ocular surface, poor hygiene and systemic immunosuppression and intraoperative including poor surgical wound construction, complicated cataract surgery with vitreous loss, type of intraocular lens inserted, topical anesthesia, and prolonged surgical time [11, 16, 17]. The European Society of Cataract and Refractive Surgeons (ESCRS) multicentered study [1] evaluated the above parameters in a large cohort of over 16,000 patients undergoing phacoemulsification cataract surgery and identified 3 factors that significantly increase the risk of postoperative infectious endophthalmitis: a clear corneal incision (CCI), the use of silicone intraocular lens implants (IOLs) and the occurrence of surgical complications. In contrast, the use of intracameral Cefuroxime was associated with significantly reduced risk of postoperative endophthalmitis (Table 2).
Historically, the popularization of sutureless CCIs coincided with a rise of endophthalmitis rate from 0.087% to 0.265% at the end of the previous century [4]. Taban et al. [8] in a large meta-analysis reported an increased risk of endophthalmitis when CCI as opposed to scleral tunnel technique was adopted and the ECSRS study prospectively confirmed those observations reporting that patients who underwent CCI procedure were 5.88 times more likely to present postoperatively with endophthalmitis [1]. Construction of the corneal tunnels, which are more prone to gapping allows exogenous microorganisms to gain easier access to the anterior chamber [1, 11]. However, refinement of corneal incision technique and the use of intracameral cefuroxime have recently been shown to significantly reduce this risk [18].
Implantation of silicone IOLs has also been associated with a threefold increase in the endophthalmitis rate compared to standard acrylic, or Poly(methyl methacrylate) intraocular lenses [19]. It has been postulated that this finding may be attributable to both the hydrophobic nature of silicone and the surface biofilms forming on the IOLs [1]. Finally, intraoperative complications, specifically capsular rupture and consequent vitreous loss has been identified as a consistent risk factor in several studies, increasing the incidence of endophthalmitis by 3–17 times [1, 20].
Prophylaxis
It has been demonstrated that ocular surface abnormalities including blepharitis, or other chronic eyelid or conjunctival inflammation is associated with increased microbial load [16]. While a positive culture does not directly translate into a direct risk of infectious endophthalmitis, these data suggest that it may be beneficial to look for and treat moderate to severe blepharitis prior to cataract surgery [4, 21–24]. The most effective method to ensure preoperative antisepsis, is application of providone–iodine 5–10% to the cornea, conjunctivalsac and periocular surface for a minimum of 3 min prior to ocular surgery. This results in a significant reduction (up to 90%) of the normal ocular surface flora and has been consistently shown to meaningfully reduce postoperative endophthalmitis rates [1, 4, 11, 16, 21, 24, 25]. Povidone–iodine application should be carried out after the removal of the anesthetic gel employed in cases of topical anesthesia, as the latter has been shown to avert povidone–iodine’s access to conjunctival flora and potentially may increase the risk of endophthalmitis [26].
Several, mainly European-controlled studies, have reported that intracameral administration of antibiotics at the end of the surgery has a meaningful protective effect against the development of endophthalmitis [11, 19, 21, 27–29]. In the ESCRS study, patients underwent phacoemulsification cataract surgery and were divided into four treatment groups (povidone–iodine alone, or povidone–iodine plus an additional therapy—intracameral cefuroxime, topical levofloxacin, or both). Results showed that when intracameral cefuroxime (1 mg in 0.1 mL normal saline) was not used there was a 4.92-fold increase (95% confidence interval, 1.87–12.9) in the risk of postoperative endophthalmitis (Table 2). The same study demonstrated the preventive effect of perioperative topical administration of levofloxacin, which can achieve high concentration in the anterior chamber after topical application [1, 19]. However, no study has shown the superiority of preoperative topical antibiotic administration as opposed to the use of povidone–iodine alone. Moreover, the growing rates of antibiotic resistance represent an area of concern [11, 30].
Management
The mainstay of treatment for acute infectious endophthalmitis following cataract surgery remains the prompt injection of suitable intravitreal antibiotics combined with pars planavitrectomy (PPV) for the most severe cases. Collection of a vitreous specimen with vitrector or needle should always precede administration of antibiotics and the samples should be delivered to a forewarned microbiologist for Gram-stain culture and appropriate microbial sensitivity testing [2].
A combination of two intravitreal antibiotics is recommended to ensure sufficient coverage of both Gram-positive and Gram-negative organisms (Table 3). Vancomycin 1 mg and ceftazidime 2.25 mg are currently considered as the optimal first line therapy [19]. Alternatively, vancomycin can be used in conjunction with amicacin specifically in β-lactame sensitive patients. This regimen provides better synergetic effect but has lost popularity due to the increased probability of retinal toxicity [1, 2, 11, 31, 32]. For fungal endophthalmitis, amphotericin B 5 μg, or voriconazole 100 μg is recommended [34]. Repeat intravitreal antibiotic administration is performed after at least 48 h and the selection of regimen is guided by the response to the initial injection and the culture sensitivity results. Systemic antibiotic therapy with the same drugs used for intravitreal injections or oral fluorquinolones may be used as adjunctive treatment to reach and maintain high intraocular concentrations [1, 11, 33, 34].
Although the Endophthalmitis Vitrectomy Study (EVS) recommended performing a pars plana vitrectomy only in patients presenting with visual acuity of Light Perception (LP), recent reports have demonstrated favorable functional and anatomical outcome even when surgical intervention was carried out in cases with better than LP visual acuity (Fig. 1). Prompt management and technical advances in vitrectomy are likely to account for these results [1, 35].
Cornea-Related Endophthalmitis
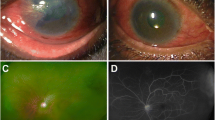
The incidence of endophthalmitis following penetrating keratoplasty ranges from 0.2% to 0.4% [36]. Wound dehiscence, suture abscess and the chronic use of topical steroids have been implicated as causative factors [37] (Fig. 2). The microorganism involved can be either bacterial (Gram-positive or Gram-negative species) or fungal (Candida species) [38–40]. Prolonged duration of cornea storage times have been associated with the development of fungal endophthalmitis [41]. Despite treatment (topical and intraocular antimicrobials, keratoplasty, pars planavitrectomy), the outcomes can be devastating with severe visual loss in more than 50% of cases [42].
Bacterial and fungal endophthalmitis have also been reported following endothelial keratoplasty [43]. Similarly, endophthalmitis can develop following keratoprosthesis (k-Pro) surgery with a reported incidence varying from 0% to 12.5% in the published literature [44–47]. Time from keratoprosthesis procedure to the onset of infection, ranges from 6 weeks to 46 months [48]. Diagnosis is usually challenging due to the development of retroprosthetic membrane and the limited aperture available for posterior segment examination [49]. The disease must be differentiated from sterile vitritis which typically presents with sudden onset of painless visual reduction accompanied by florid anterior chamber and vitreous reaction [44, 46]. In contrast with infectious endophthalmitis there is lack of tenderness or injection and the condition is highly responsive to intensive topical steroids, with frequently complete visual recovery. The microorganisms involved in infections are either Gram-positive cocci (the most common cause), Gram-negative organisms or fungi [48]. Topical antibiotics and soft contact lenses have been used for prophylaxis against endophthalmitis. Vitreous tap and injection of intravitreal antibiotics (vancomycin, ceftazidime) have been used for the treatment of post-k-pro endophthalmitis, however, good postoperative outcomes have also been reported with the use of 25-gauge pars planavitrectomy [11].
Progression of infectious keratitis to endophthalmitis is relatively uncommon (0.5%) [50–52]. Risk factors include age, dry eye, corneal perforation, fungal infection, systemic immune dysfunction, steroid use (topical and oral), dementia and nursing home care [50–52]. Pseudomonas aeruginosa, staphylococcus and streptococcus species are the most common bacterial causes [50] while aspergillus and fusarium species are less frequent [53]. Despite treatment visual outcomes are poor and often evisceration or enucleation is required [51, 52].
Endophthalmitis Associated with Vitreous Procedures
Endophthalmitis following pars plana vitrectomy is usually rare with the incidence ranging from 0.018% to 0.076% [54–59]. Following the introduction of small-gauge vitrectomy, concerns developed regarding an unexpected increase in the incidence of postoperative endophthalmitis [58, 60, 61]. However, review studies could not conclude that small-gauge vitrectomy is associated with higher risk of endophthalmitis [62–64], while two studies comparing endophthalmitis rates following 20-, 23- and 25-gauge vitrectomy failed to demonstrate any significant difference between the three groups [57, 65]. A higher incidence of endophthalmitis has been reported in fluid-filled eyes compared with air or gas filled eyes [66]. Posterior vitreous wick syndrome has also been proposed as a risk factor for postvitrectomy endophthalmitis [67]. Maintaining the infusion bottle at normal levels, removing herniated vitreous and avoiding high intraocular pressure at the end of the operation may prevent the occurrence of this syndrome, thus minimizing the risk of postvitrectomy endophthalmitis [58, 68]. Other risk factors associated with increased susceptibility to infection include the use of intravitreal, or topical corticosteroids [69, 70], the presence of immunosuppression, diabetes and older age [61, 70].
The Microsurgical Safety Task Force has provided suggestions to minimize the risk of post-PPV endophthalmitis [61] (Table 4).
The use of intravitreal antibiotics alone, or in combination with therapeutic vitrectomy has been used for the treatment of post-pars planavitrectomy endophthalmitis. Unfortunately, many cases have a poor visual outcome (vision worse than 20/200), nevertheless, occasionally better visual acuity (>20/40) may be possible [57, 71].
Postintravitreal Injection Endophthalmitis
The use of intravitreal injections represents one of the fastest growing fields in ophthalmology over the past decade. Considering that in 2008, approximately 1 million injections covered by Medicare were given in the United States, the estimated global number currently given in a year worldwide, may exceed 2 million injections [72]. Consequently, the reported prevalence of intravitreal injection-related endophthalmitis has also grown and it is now thought to be the second most common cause of endophthalmitis after cataract surgery [73]. This is especially important given that a patient may undergo many more intravitreal injections in a lifetime than cataract surgeries.
Epidemiology
The rate of endophthalmitis per intravitreal injection is low, with reported rates varying from 0.025% to 0.2% [74–76]. Given the rarity of infection and the effect that a single case can have on a data set, the range unsurprisingly has been wide. However, recent studies with large sample sizes have narrowed down the estimated rate to be between 0.02% and 0.03%. A study using a Medicare database of over 40,000 intravitreal injections found an endophthalmitis rate of 0.09% per injection [77]. A Boston study reviewed 10,208 injections that resulted in three infections, all of which were culture proven. This yields a rate of 0.029% per injection [78]. A review of the British Ophthalmological Surveillance unit found 47 cases in an estimated 186,972 injections, giving a per-injection rate of 0.025% [74]. The number of total injections in this study was extrapolated. These two studies note that the first infection occurred after the 7th and 5th injections, respectively, suggesting that the rate of infection is not affected by whether it is early or later on during the treatment course. A study from Miami documented 11 endophthalmitis cases in 60,322 injections, a rate of 0.02% [79]. Out of these data, a rate of approximately 0.7% chance of endophthalmitis has been derived for a patient undergoing a 24-injection course of treatment.
Microbiology
There are three possible sources of infection implicated in the injection-related endophthalmitis, including the normal ocular flora, the injection environment, and the clinical staff providing the injection. A study conducted in England reported 47 post-injection endophthalmitis cases, with 60% positive yield of the evaluated cultures [74], and more than 95% of them caused by Gram-positive bacteria [80]. Coagulase-negative staphylococci and Viridians streptococci were implicated in 60% and 25% of the cases, respectively [3, 80]. The above incidence of streptococcal endophthalmitis is considerably higher as opposed to post-cataract endophthalmitis (Fig. 3). V. streptococci was also the infective isolated agent in an outbreak of 12 post-injection endophthalmitis cases caused by contaminated Avastin syringes made up by a single compounding pharmacy [81]. Similar to those were the results of a recent meta-analysis, which examined the bacterial isolates from 26 cases, reporting that coagulase-negative staphylococcus was the cause of infection in 65.4% of cases and streptococcus species were implicated in 30.8% [82]. When compared, the microbiologic profile of injection-related infection cases and post-cataract infection cases was different in that a significantly higher rate of streptococcus infection was identified in the former group (24.53% vs 6.24%; P = 0.022) [3]. Considering this finding it has been postulated that contamination from oral flora may be the cause of the higher rates of streptococcus in intravitreal injections as compared to cataract surgery [82].
Guidelines for Prophylaxis
Reported risk factors for postintravitreal injection endophthalmitis include old age, diabetes mellitus, blepharitis, subconjunctival anesthesia, patient’s poor cooperation during injection and the use of compounded medication [83]. In 2014, an expert panel of retinal specialists updated the previously published guidelines on prophylaxis and monitoring of intravitreal injections. Part of the recommendations which were based on the existing literature referred to perioperative strategy to minimize the risk of acute postintravitreal injection endophthalmitis.
Importantly, eyelid, adnexal and ocular surface abnormalities should be considered as a potential risk factor for endophthalmitis since most endophthalmitis cases following intravitreal injections in patients with such abnormalities were attributed to S. epidermidis [84]. Therefore, eyes with active external infection should have the injection postponed until the infection is adequately managed. A prospective-controlled study confirmed this approach reporting that blepharitis was present in 6.5% of 47 postintravitreal injection endophthalmitis cases (P = 0.006) [74]. In general, patients undergoing intravitreal injections should meet the same hygiene considerations as those patients undergoing intraocular surgery a fact that is consistent with standards of good medical practice.
Povidone–iodine (5–10%) should be the last agent applied to the intended injection site and should be left in place for at least 30 s prior to the injection [85]. Povidone–iodine may also be applied gently to the eyelids, including the eyelid margins and eyelashes. However, it has been shown that eyelid scrubbing or eyelid pressure may dislodge bacteria from the accessory glands and therefore should be avoided. In case an anesthetic gel is used, povidone–iodine should be instilled both before and after the application of the anesthetic gel. Ocular surface preparation with povidone–iodine appears to be advantageous as compared with topical antibiotics, since it does not seem to promote bacterial resistance [86]. It has also been proposed that povidone–iodine flush is more effective in reducing bacterial population compared to a single drop application [87].
There is little scientific evidence to support that routine use of sterile gloves is associated with a reduced risk of endophthalmitis following intravitreal injections. Although there are no controlled data, the use of gloves is considered as a part of general infection avoidance clinical practice. In two prospective randomized controlled trials performed by the Diabetic Retinopathy Clinical Research Network, the use of gloves, or a drape was not essential and the endophthalmitis rate was relatively low (0.078%, 3 out of 3838 injections) [88]. An endophthalmitis rate of 0.057% was reported in a retrospective case series of 15,895 injections involving ranibizumab, bevacizumab, triamcinolone acetonide, and pegaptanib in which gloves were not used [89]. Despite the fact that the use of gloves is not strictly required by either evidence-based studies or regulations, this step is routinely adopted by the majority of physicians who find it consistent with good clinical practice. In a survey of 765 retina specialists, 58% reported use of gloves, and of those, 58% used sterile gloves [90]. Regardless of the use or nonuse of gloves, careful handwashing before and after patient contact and adhesion to aseptic injection technique is recommended for intravitreal injections.
Although the use of sterile drape is considered part of the routine preparation for major or minor operations, there is no body of literature to justify its use when performing routine intravitreal injections since studies failed to reveal an increased endophthalmitis rate when drape was not used [91]. Moreover, in nonophthalmic surgical procedures, a Cochrane meta-analysis showed that the use of plastic adhesive drape may even be harmful as it is related to a significant increase in local infections [92]. The rationale behind characterizing the use of drape unnecessary and problematic is further reinforced by its potential to upregulate patient’s stress and discomfort [93].
The impact of wearing facial masks or minimizing talking during the procedure of injections has not been evaluated by randomised controlled trials. Nevertheless, current literature suggests that the notably high rate of streptococcus isolates implies that causative organisms are likely to be transmitted from oropharyngeal droplets [94, 95]. Previous studies confirmed that a procedure protocol involving use of a face mask and avoidance of talking is associated with a significant decrease in culture yield of plates positioned next to the mouth [96, 97]. Adopting such a policy should reduce the risk of endophthalmitis minimizing the spread of aerosolized droplets containing oral contaminants.
The eyelashes and the lid margins may represent primary source of infection because of needle contact during entry into the vitreous. Therefore, it is logical to prevent contact of the lids and lashes with the injection needle and ocular surface. Although the beneficial role of speculum has not been adequately confirmed by the current literature and despite patient discomfort that might generate, it should be considered as part of the optimized injection protocol, providing a more sterile environment and potentially minimizing the risk of endophthalmitis [98, 99].
The use of pre- or postintravitreal injection treatment with topical antibiotics has been the standard clinical practice for many years. However, several studies that evaluated their role, failed to demonstrate any significant benefit regarding endophthalmitis prevention. Moss et al. conducted a randomised controlled study showing that a 3-day course of gatifloxacin prior to injection did reduce the positive yield of conjunctival cultures, but there was no additional benefit in combination with povidone–iodine versus eyes that only received povidone–iodine. Similarly, other large retrospective studies did not demonstrate a clinical benefit for the use of topical antibiotics after an injection [100]. This is consistent with reports showing that following an injection, topical antibiotics fail to attain sufficient therapeutic levels within the vitreous cavity. The lack of benefit along with the potential for development of antibiotic resistance and concomitant cost, render perioperative use of antibiotics probably unnecessary.
Management
Similar to post-cataract endophthalmitis, the most common etiologic agent in injection-related endophthalmitis is coagulase-negative staphylococcus. Consequently, some clinicians have used the EVS recommendations to guide their treatment strategy. Although the ideal timing of vitrectomy remains as yet unclear, it is widely accepted that initial vitreous tap and antibiotic injection is a reasonable therapeutic approach. Vancomycin 1.0 mg/0.1 ml with either ceftazidime 2.25 mg/0.1 ml, or amikacin 0.4 mg/0.1 ml, remains the mainstay of current treatment. Subsequent injections should be tailored to culture results avoiding amikacin due to retinal toxicity [101].
Endophthalmitis associated with intravitreal injections can have variable outcomes depending on the virulence of the infective organisms. A large retrospective study of 27,736 injections showed that 78% of the 23 presumed infectious endophthalmitis cases regained baseline visual acuity. The authors reported no difference in the visual recovery rate based on positive or negative yield of cultures. Endophthalmitis patients following intravitreal injections exhibit worse prognosis compared to those post-cataract surgery. It has been postulated that is attributable to the increased incidence of Streptococcus spp. in the former group, or alternatively it may reflect differences in the ability of inoculated organisms to multiply in the vitreous as opposed to the anterior chamber [11].
Endophthalmitis after glaucoma surgery
One of the most dramatic complications after glaucoma filtering surgery is bleb-related endophthalmitis. The reported rate varies from 1.3%/patient/year for superior blebs to 7.8%/patient/year for inferior blebs [102–104]. It is critical to clearly differentiate between blebitis, which is an isolated bleb infection with varying degrees of anterior segment inflammation and bleb-associated endophthalmitis with coexisting vitreous involvement. Risk factors may include the use of antifibrotic agents (5-fluorouracil and mitomycin-C), chronic bleb leak, a thin avascular bleb, bleb manipulation, bacterial conjunctivitis and blepharitis, accidental minor trauma, epithelial drying, young age and male sex [105–109]. In late-onset endophthalmitis the microorganisms are often more virulent, with streptococcus species implicated in nearly 50% of these cases, compared to coagulase-negative staphylococcus, which is the most common pathogen in the early postoperative endophthalmitis [11, 110]. Treatment of bleb-associated endophthalmitis includes vancomycin, or amikacin (as topical eye-drops and intravitreal injections), oral fluoroquinolone, and topical corticosteroids [111–115]. PPV is likely to be required given the aggressive organisms involved and the extremely poor visual outcomes anticipated [110]. The Tube versus Trabeculectomy Study observed that the occurrence of endophthalmitis, or blebitis were more frequent in the trabeculectomy group compared to the tube group. This result raises concern, but the power of that study was limited with regard to identifying rare complications [116].
Late endophthalmitis may also develop after valve implantation as mentioned before. Insufficient conjunctival coverage and exposure of the device increase the risk. Immediate treatment with topical antibiotics and scheduling of a new surgery to cover the implant is essential [117].
Endophthalmitis Secondary to Trauma
The frequency of post-traumatic endophthalmitis following open-globe injuries (Figs. 3, 4) varies among different studies between 1% and 18.9% [118–122]. The development of infection can be acute, or delayed while risk factors identified include delayed presentation for eye examination, worse visual acuity at presentation, the presence of intraocular foreign body and the species of microorganisms involved [119, 123–125]. Especially for trauma cases associated with the presence of intraocular foreign bodies, the risk of endophthalmitis depends on the size and composition of the foreign body, the presence of contaminating materials such as soil, the speed of the foreign body, the path within the eye, the length of time between injury and removal of foreign body, the state of the immune system of the patient and the treatment regimen undertaken [125].
Staphylococcus and streptococcus species have been most frequently identified in post-traumatic endophthalmitis cases [34, 126]. P. aeruginosa and bacillus cereus are also frequently identified but their prevalence varies with geographic location [120, 127]. Bacillus species are associated with worse prognosis as they can cause visual loss and destruction of ocular tissue even within hours after the injury [128, 129]. Fungi species have been also isolated in a small percentage of post-traumatic endophthalmitis [124, 130].
The use of systemic and intraocular antibiotics for prophylaxis against post-traumatic endophthalmitis remains controversial. Nevertheless, when systemic antibiotics are not employed after open-globe injuries there is a greater risk for endophthalmitis development [131, 132]. In a prospective, randomized study, cases of intraocular foreign bodies that have been managed with intracameral and intravitreal antibiotics have been associated with a reduced risk of endophthalmitis compared with the control group treated with intravitreal balanced salt solution [133].
Treatment in post-traumatic endophthalmitis should be aggressive, with vitrectomy combined with intravitreal antibiotics (e.g. vancomycin plus ceftazidime) and systemic antibiotic therapy. Topical and subconjunctival antibiotics can also be used. However, the optimal primary closure of an open-globe injury remains an essential step to improve the prognosis of these challenging cases [134, 135].
Endogenous Endophthalmitis
Endogenous endophthalmitis develops when microorganisms that originate from an obvious (e.g. pneumonia, meningitis, endocarditis, hepatic abscess) or, more rarely, a non-obvious (e.g. osteomyelitis, sinusitis) extra-ocular septic focus gain access to the eye via the blood stream [136, 137]. This is usually associated with the presence of systemic risk factors, although rarely it can develop in the absence of concomitant risk factors [138]. Well-known risk factors include diabetes mellitus, malignancies, intravenous drug abuse, systemic lupus erythematosus, endoscopy, chemotherapy, acquired immune deficiency syndrome, sickle-cell anemia, dental procedures, and other immunocompromised states [73, 139].
Endogenous bacterial endophthalmitis is responsible for less than 10% of endophthalmitis cases [140]. Gram-positive (S. pneumoniaes, aureus, Bacillus cereus) and Gram-negative microorganisms (Neisseria meningitidis, Haemophilus influenzae, Escherichia coli, Klebsiella pneumoniae) have been isolated in these rare cases [138–143].
Apart from the ocular findings suggestive of endophthalmitis, systemic symptoms and signs including fever and malaise. Further, positive blood cultures may be elicited in these cases. Retinal detachment, cataract, perivascular hemorrhages, low intraocular pressure and phthisis, sepsis and death can develop during the course of the disease. Prognosis depends on the general state of health of the patient [139, 140].
Endogenous fungal infections are less common than endogenous bacterial endophthalmitis and are usually caused by Candida albicans, C. grabrata [144, 145]. It is well documented that when immunosuppression co-exists, many organs, including the eye, can be affected. The infection presents either with visual loss (macular involvement), or with ocular pain (anterior uveitis, scleritis). Retinal foci less than 1 mm in diameter can become coalescent and develop into a mushroom-shaped lesion, which projects to the vitreous body. Furthermore, typical findings include the appearance of fluffy white vitreous opacities, described as a “string of pearls” appearance [146]. A second etiological agent is aspergillus (fumigatus, flavus) which is found in the loam (soil) and produces large choroidal infiltrates, subretinal hypopyon, retinal necrosis, vitreous exudates and vasculitis [147]. Intraocular infection caused by fungi can possibly present as masquerade syndrome and be treated erroneously with corticosteroids, which will aggravate the disease and negatively impact prognosis. Macular involvement is associated with poor prognosis with final visual acuity less than 20/200 in most cases of fungal endophthalmitis.
Ophthalmologist review for patients at risk of developing eye involvement during the course of a systemic infection is indicated. The diagnosis of endogenous endophthalmitis is typically confirmed following microbiologic evidence of infection in an intraocular specimen (aqueous or vitreous), and from blood samples (positive cultures) [148, 149]. Fungal growth can be confirmed by Giemsa stains. Polymerase chain reaction (PCR) primers and diagnostic vitrectomy can help in case of negative cultures [150]. For endogenous bacterial endophthalmitis, systemic antibiotics are supplemented with intravitreal antibiotics [137]. On the other hand, endogenous fungal endophthalmitis is treated with intravenous fluconazole, or voriconazole combined with systemic and intravitreal caspofungin. The need for vitrectomy (to obtain a sample of tissue for Giemsa, PCR for Candida, cultures) can be imperative and has to be accompanied with concomitant intravitreal injection of amphotericin B or voriconazole to avoid the development of adhesions [151].
Conclusion
Acute infective endophthalmitis remains a devastating complication following intraocular surgery and trauma. Although not entirely preventable, advances in surgical techniques and equipment along with new prophylactic measures have significantly reduced its incidence. Management of ocular surface disease prior to surgery, preoperative antisepsis with povidone–iodine, meticulous equipment sterilization and intracameral antibiotic use are essential to minimize the risk of infection.
Prompt diagnosis and accurate identification of the causative organism is important especially in cases that fail to respond to initial broad spectrum antibiotic treatment. Intravitreal antibiotic injections remain the mainstay of treatment and although Endophthalmitis Vitrectomy Study guidelines refer to post-cataract endophthalmitis they generally apply to all endophthalmitis categories. However, advances in vitreoretinal surgery may expand the role of vitrectomy in the management of acute infections attaining improved treatment outcomes.
References
Barry P, Cordovés L, Gardner S. ESCRS guidelines for prevention and treatment of endophthalmitis following cataract surgery: data, dilemmas and conclusions. Dublin: European Society of Cataract and Refractive Surgeons; 2013.
Pathengay A, Flynn HW Jr, Isom RF, Miller D. Endophthalmitis outbeaks following cataract surgery: causative organisms, etiologies and visual acuity outcomes. J Cataract Refract Surg. 2012;38(7):1278–82.
Simunovic MP, Rush RB, Hunyor AP, Chang AA. Endophthalmitis following intravitreal injection versus endophthalmitis following cataract surgery: clinical features, causative organisms and post-treatment outcomes. Br J Ophthalmol. 2012;96(6):862–6.
Sadaka A, Durand ML, Gilmore MS. Bacterial endophthalmitis in the age of outpatient intravitreal therapies and cataract surgeries: host–microbe interactions in intraocular infection. Prog Retin Eye Res. 2012;31(4):316–31.
Keay L, Gower EW, Cassard SD, Tielsch JM, Schein OD. Postcataract surgery endophthalmitis in the United States: analysis of the complete 2003 to 2004 Medicare database of cataract surgeries. Ophthalmology. 2012;119(5):914–22.
Freeman EE, Roy-Gagnon M-H, Fortin E, Gauthier D, Popescu M, Boisjoly H. Rate of endophthalmitis after cataract surgery in Quebec, Canada, 1996–2005. Arch Ophthalmol. 2010;128(2):230–4.
Kodjikian L, Salvanet-Bouccara A, Grillon S, Forestier F, Seegmuller J-L, Berdeaux G, et al. Postcataract acute endophthalmitis in France: national prospective survey. J Cataract Refract Surg. 2009;35(1):89–97.
Taban M, Behrens A, Newcomb RL, Nobe MY, Saedi G, Sweet PM, et al. Acute endophthalmitis following cataract surgery: a systematic review of the literature. Arch Ophthalmol. 2005;123(5):613–20.
Leopold I. Incidence of endophthalmitis after cataract surgery. Trans Ophthalmol Soc UK. 1971;191:575–609.
Aaberg TM, Flynn HW, Schiffman J, Newton J. Nosocomial acute-onset postoperative endophthalmitis survey: a 10-year review of incidence and outcomes. Ophthalmology. 1998;105(6):1004–10.
Levison AL, Mendes TS, Bhisitkul R. Post procedural endophthalmitis: a review. Expert Rev Ophthalmol. 2013;8(1):45–62.
Chen X, Adelman RA. Microbial spectrum and resistance patterns in endophthalmitis: a 21-year (1988–2008) review in Northeast United States. J Ocul Pharmacol Ther. 2012;28(4):329–34.
Lemley CA, Han DP. Endophthalmitis: a review of current evaluation and management. Retina. 2007;27(6):662–80.
Han DP, Wisniewski SR, Wilson LA, Barza M, Vine AK, Doft BH, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122(1):1–17.
Johnson MW, Doft BH, Kelsey SF, Barza M, Wilson LA, Barr CC, et al. The endophthalmitis vitrectomy study: relationship between clinical presentation and microbioloaic spectrum. Ophthalmology. 1997;104(2):261–72.
Charles QY, Ta CN. Prevention of postcataract endophthalmitis: evidence-based medicine. Curr Opin Ophthalmol. 2012;23(1):19–24.
Al-Mezaine HS, Kangave D, Al-Assiri A, Al-Rajhi AA. Acute-onset nosocomial endophthalmitis after cataract surgery: incidence, clinical features, causative organisms, and visual outcomes. J Cataract Refract Surg. 2009;35(4):643–9.
Lundström M, Wejde G, Stenevi U, Thorburn W, Montan P. Endophthalmitis after cataract surgery: a nationwide prospective study evaluating incidence in relation to incision type and location. Ophthalmology. 2007;114(5):866–70.
Group EES. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978–88.
Brazitikos P, Balidis M, Tranos P, Androudi S, Papadopoulos N, Tsinopoulos I, Stangos N. Sulcus implantation of a 3-piece, 6.0 mm optic, hydrophobic foldable acrylic intraocular lens in phacoemulsification complicated by posterior capsule rupture. J Cataract Refract Surg. 2002;28(9):1618–22.
Galor A, Goldhardt R, Wellik SR, Gregori NZ, Flynn HW. Management strategies to reduce risk of postoperative infections. Curr Ophthalmol Rep. 2013;1(4):161–8.
Miño DKH, Ta CN, Froehlich SJ, Schaller UC, Engelbert M, Klauss V, et al. Prospective study of risk factors for conjunctival bacterial contamination in patients undergoing intraocular surgery. Eur J Ophthalmol. 2008;19(5):717–22.
de Kaspar HM, Shriver EM, Nguyen EV, Egbert PR, Singh K, Blumenkranz MS, et al. Risk factors for antibiotic-resistant conjunctival bacterial flora in patients undergoing intraocular surgery. Graefe’s Arch Clin Exp Ophthalmol. 2003;241(9):730–3.
Tan CS, Wong HK, Yang FP. Epidemiology of postoperative endophthalmitis in an Asian population: 11-year incidence and effect of intracameral antibiotic agents. J Cataract Refract Surg. 2012;38(3):425–30.
Keynan Y, Finkelman Y, Lagacé-Wiens P. The microbiology of endophthalmitis: global trends and a local perspective. Eur J Clin Microbiol Infect Dis. 2012;31(11):2879–86.
Miller JJ, Scott IU, Flynn HW, Smiddy WE, Newton J, Miller D. Acute-onset endophthalmitis after cataract surgery (2000–2004): incidence, clinical settings, and visual acuity outcomes after treatment. Am J Ophthalmol. 2005;139(6):983–7.
Parmar P, Salman A, Kaliamurthy J, Prasanth DA, Thomas PA, Jesudasan CAN. Anterior chamber contamination during phacoemulsification and manual small-incision cataract surgery. Am J Ophthalmol. 2006;141(6):1160–1.
Romero-Aroca P, Méndez-Marin I, Salvat-Serra M, Fernández-Ballart J, Almena-Garcia M, Reyes-Torres J. Results at seven years after the use of intracamerular cefazolin as an endophthalmitis prophylaxis in cataract surgery. BMC Ophthalmol. 2012;12(1):2.
Barreau G, Mounier M, Marin B, Adenis J-P, Robert P-Y. Intracameral cefuroxime injection at the end of cataract surgery to reduce the incidence of endophthalmitis: French study. J Cataract Refract Surg. 2012;38(8):1370–5.
Ta CN, Chang RT, Singh K, Egbert PR, Shriver EM, Blumenkranz MS, et al. Antibiotic resistance patterns of ocular bacterial flora: a prospective study of patients undergoing anterior segment surgery. Ophthalmology. 2003;110(10):1946–51.
Group EVS. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479–96.
Sheng Y, Sun W, Gu Y, Lou J, Liu W. Endophthalmitis after cataract surgery in China, 1995–2009. J Cataract Refract Surg. 2011;37(9):1715–22.
Gribomont A. General review-post-cataract surgery endophthalmitis: an update. Bull Soc Belge Ophtalmol. 2009;311:43.
Wykoff CC, Flynn HW, Miller D, Scott IU, Alfonso EC. Exogenous fungal endophthalmitis: microbiology and clinical outcomes. Ophthalmology. 2008;115(9):1501–7. e2.
Kuhn F, Gini G. Ten years after … are findings of the Endophthalmitis Vitrectomy Study still relevant today? Graefe’s Arch Clin Exp Ophthalmol. 2005;243(12):1197–9.
Taban M, Behrens A, Newcomb RL, Nobe MY, McDonnell PJ. Incidence of acute endophthalmitis following penetrating keratoplasty: a systematic review. Arch Ophthalmol. 2005;123(5):605–9.
Hood CT, Lee BJ, Jeng BH. Incidence, occurrence rate, and characteristics of suture-related corneal infections after penetrating keratoplasty. Cornea. 2011;30(6):624–8.
Chen JY, Jones MN, Srinivasan S, Neal TJ, Armitage WJ, Kaye SB, et al. Endophthalmitis after penetrating keratoplasty. Ophthalmology. 2015;122(1):25–30.
Kloess PM, Stulting RD, Waring GO, Wilson LA. Bacterial and fungal endophthalmitis after penetrating keratoplasty. Am J Ophthalmol. 1993;115(3):309–16.
Kunimoto DY, Tasman W, Rapuano C, Recchia F, Busbee B, Pearlman R, et al. Endophthalmitis after penetrating keratoplasty: microbiologic spectrum and susceptibility of isolates. Am J Ophthalmol. 2004;137(2):343–5.
Hassan SS, Wilhelmus KR, Dahl P, Davis GC, Roberts RT, Ross KW, et al. Infectious disease risk factors of corneal graft donors. Arch Ophthalmol. 2008;126(2):235–9.
Bor E, Kremer I. Endophthalmitis and wound dehiscence following late removal of penetrating keratoplasty sutures. Ophthalmic Surg Lasers Imaging. 2010;42(3):234–40.
Shih CY, Ritterband DC, Rubino S, Palmiero P-M, Jangi A, Liebmann J, et al. Visually significant and nonsignificant complications arising from Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2009;148(6):837–43.
Ramchandran RS, DiLoreto DA, Chung MM, Kleinman DM, Plotnik RP, Graman P, et al. Infectious endophthalmitis in adult eyes receiving Boston type I keratoprosthesis. Ophthalmology. 2012;119(4):674–81.
Greiner MA, Li JY, Mannis MJ. Longer-term vision outcomes and complications with the Boston type 1 keratoprosthesis at the University of California. Davis. Ophthalmology. 2011;118(8):1543–50.
Klufas MA, Yannuzzi NA, D’Amico DJ, Kiss S Vitreoretinal aspects of permanent keratoprosthesis. Surv Ophthalmol. 2015;60(3):216–28.
Robert M-C, Moussally K, Harissi-Dagher M. Review of endophthalmitis following Boston keratoprosthesis type 1. Br J Ophthalmol. 2012;96(6):776–80.
Durand ML, Dohlman CH. Successful prevention of bacterial endophthalmitis in eyes with the Boston keratoprosthesis. Cornea. 2009;28(8):896–901.
Chew HF, Ayres BD, Hammersmith KM, Rapuano CJ, Laibson PR, Myers JS, et al. Boston keratoprosthesis outcomes and complications. Cornea. 2009;28(9):989–96.
Scott IU, Flynn HW, Feuer W, Pflugfelder SC, Alfonso EC, Forster RK, et al. Endophthalmitis associated with microbial keratitis. Ophthalmology. 1996;103(11):1864–70.
O’Neill EC, Yeoh J, Fabinyi DC, Cassidy D, Vajpayee RB, Allen P, et al. Risk factors, microbial profiles and prognosis of microbial keratitis-associated endophthalmitis in high-risk eyes. Graefe’s Arch Clin Exp Ophthalmol. 2014;252(9):1457–62.
Henry CR, Flynn HW, Miller D, Forster RK, Alfonso EC. Infectious keratitis progressing to endophthalmitis: a 15-year study of microbiology, associated factors, and clinical outcomes. Ophthalmology. 2012;119(12):2443–9.
Bharathi MJ, Ramakrishnan R, Shivakumar C, Meenakshi R, Lionalraj D. Etiology and antibacterial susceptibility pattern of community-acquired bacterial ocular infections in a tertiary eye care hospital in south India. Indian J Ophthalmol. 2010;58(6):497.
Taylor S, Aylward G. Endophthalmitis following 25-gauge vitrectomy. Eye. 2005;19(11):1228–9.
Eifrig CW, Scott IU, Flynn HW, Smiddy WE, Newton J. Endophthalmitis after pars plana vitrectomy: incidence, causative organisms, and visual acuity outcomes. Am J Ophthalmol. 2004;138(5):799–802.
Recchia FM, Scott IU, Brown GC, Brown MM, Ho AC, Ip MS. Small-gauge pars plana vitrectomy: a report by the American Academy of Ophthalmology. Ophthalmology. 2010;117(9):1851–7.
Scott IU, Flynn HW Jr, Acar N, Dev S, Shaikh S, Mittra RA, et al. Incidence of endophthalmitis after 20-gauge vs 23-gauge vs 25-gauge pars plana vitrectomy. Graefe’s Arch Clin Exp Ophthalmol. 2011;249(3):377–80.
Scott IU, Harry W, Flynn J, Dev S, Shaikh S, Mittra RA, Arevalo JF, et al. Endophthalmitis after 25-gauge and 20-gauge pars plana vitrectomy: incidence and outcomes. Retina. 2008;28(1):138–42.
Hu AY, Bourges J-L, Shah SP, Gupta A, Gonzales CR, Oliver SC, et al. Endophthalmitis after pars plana vitrectomy: a 20-and 25-gauge comparison. Ophthalmology. 2009;116(7):1360–5.
Kunimoto DY, Kaiser RS, Service WER. Incidence of endophthalmitis after 20-and 25-gauge vitrectomy. Ophthalmology. 2007;114(12):2133–7.
Shah RE, Gupta O. The microsurgical safety task force: guidelines for minimizing endophthalmitis with vitrectomy surgery. Curr Opin Ophthalmol. 2012;23(3):189–94.
Oshima Y, Kadonosono K, Yamaji H, Inoue M, Yoshida M, Kimura H, et al. Multicenter survey with a systematic overview of acute-onset endophthalmitis after transconjunctival microincision vitrectomy surgery. Am J Ophthalmol. 2010;150(5):716–25. e1.
Bahrani HM, Fazelat AA, Thomas M, Hirose T, Kroll AJ, Lou PL, Ryan EA. Endophthalmitis in the era of small gauge transconjunctival sutureless vitrectomy-meta analysis and review of literature. Semin Ophthalmol. 2010;25(5–6):275–82.
Govetto A, Virgili G, Menchini F, Lanzetta P, Menchini U. A systematic review of endophthalmitis after microincisional versus 20-gauge vitrectomy. Ophthalmology. 2013;120(11):2286–91.
Wu L, Berrocal MH, Arévalo JF, Carpentier C, Rodriguez FJ, Alezzandrini A, et al. Endophthalmitis after pars plana vitrectomy: results of the Pan American Collaborative Retina Study Group. Retina. 2011;31(4):673–8.
Chiang A, Kaiser RS, Avery RL, Dugel PU, Eliott D, Shah SP, et al. Endophthalmitis in microincision vitrectomy: outcomes of gas-filled eyes. Retina. 2011;31(8):1513–7.
Venkatesh P, Verma L, Tewari H. Posterior vitreous wick syndrome: a potential cause of endophthalmitis following vitreo-retinal surgery. Med Hypotheses. 2002;58(6):513–5.
Singh A, Chen JA, Stewart JM. Ocular surface fluid contamination of sutureless 25-gauge vitrectomy incisions. Retina. 2008;28(4):553–7.
Cochereau I. Endophthalmitis following new procedures. J Fr Ophtalmol. 2007;30(10):1067–9.
Park JC, Ramasamy B, Shaw S, Prasad S, Ling RH, Foot B, et al. A prospective and nationwide study investigating endophthalmitis following pars plana vitrectomy: incidence and risk factors. Br J Ophthalmol. 2014;98(4):529–33.
Bacon AS, Davison CR, Patel BC, Frazer DG, Ficker LA, Dart J. Infective endophthalmitis following vitreoretinal surgery. Eye. 1993;7(pt 4):529–34.
Brechner RJ, Rosenfeld PJ, Babish JD, Caplan S. Pharmacotherapy for neovascular age-related macular degeneration: an analysis of the 100% 2008 medicare fee-for-service part B claims file. Am J Ophthalmol. 2011;151(5):887–95. e1.
Durand ML. Endophthalmitis. Clin Microbiol Infect. 2013;19(3):227–34.
Lyall D, Tey A, Foot B, Roxburgh S, Virdi M, Robertson C, et al. Post-intravitreal anti-VEGF endophthalmitis in the United Kingdom: incidence, features, risk factors, and outcomes. Eye. 2012;26(12):1517–26.
Bhatt SS, Stepien KE, Joshi K. Prophylactic antibiotic use after intravitreal injection: effect on endophthalmitis rate. Retina. 2011;31(10):2032–6.
Inoue M, Kobayakawa S, Sotozono C, Komori H, Tanaka K, Suda Y, et al. Evaluation of the incidence of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor. Ophthalmologica. 2011;226(3):145–50.
Day S, Acquah K, Mruthyunjaya P, Grossman DS, Lee PP, Sloan FA. Ocular complications after anti-vascular endothelial growth factor therapy in medicare patients with age-related macular degeneration. Am J Ophthalmol. 2011;152(2):266–72.
Englander M, Chen TC, Paschalis EI, Miller JW, Kim IK. Intravitreal injections at the Massachusetts Eye and Ear Infirmary: analysis of treatment indications and postinjection endophthalmitis rates. Br J Ophthalmol. 2013;97(4):460–5.
Moshfeghi AA, Rosenfeld PJ, Flynn HW Jr, Schwartz SG, Davis JL, Murray TG, et al. Endophthalmitis after intravitreal anti-vascular endothelial growth factor antagonists: a six-year experience at a University Referral Center. Retina. 2011;31(4):662–8.
Hoevenaars N, Gans D, Missotten T, van Rooij J, Lesaffre E, Van Meurs J. Suspected bacterial endophthalmitis following intravitreal anti-VEGF injection: case series and literature review. Ophthalmologica. 2012;228(3):143–7.
Goldberg RA, Flynn HW, Isom RF, Miller D, Gonzalez S. An outbreak of streptococcus endophthalmitis after intravitreal injection of bevacizumab. Am J Ophthalmol. 2012;153(2):204–8. e1.
Mccannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti–vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31(4):654–61.
Vaziri K, Schwartz SG, Kishor K, Flynn Jr HW. Endophthalmitis: state of the art. Clin Ophthalmol (Auckl, NZ). 2015;9:95.
Scott IU, Flynn HW, Feuer W. Endophthalmids after secondary intraocular lens implantation: a case–control study. Ophthalmology. 1995;102(12):1925–31.
Friedman DA, Mason JO III, Emond T, Mcgwin G Jr. Povidone–iodine contact time and lid speculum use during intravitreal injection. Retina. 2013;33(5):975–81.
Hsu J, Gerstenblith AT, Garg SJ, Vander JF. Conjunctival flora antibiotic resistance patterns after serial intravitreal injections without postinjection topical antibiotics. Am J Ophthalmol. 2014;157(3):514–8. e1.
Safar A, Dellimore MC. The effect of povidone iodine flush versus drops on conjunctival colonization before intravitreal injections. Int Ophthalmol. 2007;27(5):307–12.
Bhavsar A, Googe J Jr, Stockdale C, Bressler N, Brucker A, Elman M, et al. Diabetic Retinopathy Clinical Research Network. Risk of endophthalmitis after intravitreal drug injection when topical antibiotics are not required: the diabetic retinopathy clinical research network laser-ranibizumab-triamcinolone clinical trials. Arch Ophthalmol. 2009;127(12):1581–3.
Cheung CS, Wong AW, Lui A, Kertes PJ, Devenyi RG, Lam W-C. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology. 2012;119(8):1609–14.
Green-Simms AE, Ekdawi NS, Bakri SJ. Survey of intravitreal injection techniques among retinal specialists in the United States. Am J Ophthalmol. 2011;151(2):329–32.
Pilli S, Kotsolis A, Spaide RF, Slakter J, Freund KB, Sorenson J, et al. Endophthalmitis associated with intravitreal anti-vascular endothelial growth factor therapy injections in an office setting. Am J Ophthalmol. 2008;145(5):879–82.
Webster J, Alghamdi A. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;4:CD006353.
Tailor R, Beasley R, Yang Y, Narendran N. Evaluation of patients’ experiences at different stages of the intravitreal injection procedure–what can be improved? Clin Ophthalmol (Auckl, NZ). 2011;5:1499.
Shimada H, Hattori T, Mori R, Nakashizuka H, Fujita K, Yuzawa M. Minimizing the endophthalmitis rate following intravitreal injections using 0.25% povidone–iodine irrigation and surgical mask. Graefe’s Arch Clin Exp Ophthalmol. 2013;251(8):1885–90.
Brynskov T, Kemp H, Sørensen TL. No cases of endophthalmitis after 20,293 intravitreal injections in an operating room setting. Retina. 2014;34(5):951–7.
Doshi RR, Leng T, Fung AE. Reducing oral flora contamination of intravitreal injections with face mask or silence. Retina. 2012;32(3):473–6.
Wen JC, McCannel CA, Mochon AB, Garner OB. Bacterial dispersal associated with speech in the setting of intravitreous injections. Arch Ophthalmol. 2011;129(12):1551–4.
Shah C, Garg S, Vander J, Brown G, Kaiser R, Haller J. Post-Injection Endophthalmitis (PIE) Study Team. Outcomes and risk factors associated with endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmology. 2011;118(10):2028–34.
Fineman MS, Hsu J, Spirn MJ, Kaiser RS. Bimanual assisted eyelid retraction technique for intravitreal injections. Retina. 2013;33(9):1968–70.
Avery RL, Bakri SJ, Blumenkranz MS, Brucker AJ, Cunningham ET Jr, D’Amico DJ, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina. 2014;34:S1–18.
Charles QY, Ta CN. Prevention and treatment of injection-related endophthalmitis. Graefe’s Arch Clin Exp Ophthalmol. 2014;252(7):1027–31.
Wolner B, Liebmann JM, Sassani JW, Ritch R, Speaker M, Marmor M. Late bleb-related endophthalmitis after trabeculectomy with adjunctive 5-fluorouracil. Ophthalmology. 1991;98(7):1053–60.
DeBry PW, Perkins TW, Heatley G, Kaufman P, Brumback LC. Incidence of late-onset bleb-related complications following trabeculectomy with mitomycin. Arch Ophthalmol. 2002;120(3):297–300.
Vaziri K, Kishor K, Schwartz SG, Maharaj AS, Moshfeghi DM, Moshfeghi AA, et al. Incidence of bleb-associated endophthalmitis in the United States. Clin Ophthalmol (Auckl, NZ). 2015;9:317.
Jampel HD, Quigley HA, Kerrigan-Baumrind LA, Melia BM, Friedman D, Barron Y. Risk factors for late-onset infection following glaucoma filtration surgery. Arch Ophthalmol. 2001;119(7):1001–8.
Soltau JB, Rothman RF, Budenz DL, Greenfield DS, Feuer W, Liebmann JM, et al. Risk factors for glaucoma filtering bleb infections. Arch Ophthalmol. 2000;118(3):338–42.
Mochizuki K, Jikihara S, Ando Y, Hori N, Yamamoto T, Kitazawa Y. Incidence of delayed onset infection after trabeculectomy with adjunctive mitomycin C or 5-fluorouracil treatment. Br J Ophthalmol. 1997;81(10):877–83.
Leng T, Miller D, Flynn HW Jr, Jacobs DJ, Gedde SJ. Delayed-onset bleb-associated endophthalmitis (1996–2008): causative organisms and visual acuity outcomes. Retina. 2011;31(2):344–52.
Wallin Ö, Al-ahramy AM, Lundström M, Montan P. Endophthalmitis and severe blebitis following trabeculectomy. Epidemiology and risk factors; a single-centre retrospective study. Acta Ophthalmol. 2014;92(5):426–31.
Al-Turki TA, Al-Shahwan S, Al-Mezaine HS, Kangave D, Abu El-Asrar AM. Microbiology and visual outcome of bleb-associated endophthalmitis. Ocul Immunol Inflamm. 2010;18(2):121–6.
Brown RH, Yang LH, Walker SD, Lynch MG, Martinez LA, Wilson LA. Treatment of bleb infection after glaucoma surgery. Arch Ophthalmol. 1994;112(1):57–61.
Chen PP, Gedde SJ, Budenz DL, Parrish RK. Outpatient treatment of bleb infection. Arch Ophthalmol. 1997;115(9):1124–8.
Reynolds AC, Skuta GL, Monlux R, Johnson J. Management of blebitis by members of the American Glaucoma Society: a survey. J Glaucoma. 2001;10(4):340–7.
Song A, Scott IU, Flynn MHW, Budenz DL. Delayed-onset bleb-associated endophthalmitis: clinical features and visual acuity outcomes. Ophthalmology. 2002;109(5):985–91.
Busbee BG, Recchia FM, Kaiser R, Nagra P, Rosenblatt B, Pearlman RB. Bleb-associated endophthalmitis: clinical characteristics and visual outcomes. Ophthalmology. 2004;111(8):1495–503.
Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–14.e1.
Al-Torbak A, Al-Shahwan S, Al-Jadaan I, Al-Hommadi A, Edward D. Endophthalmitis associated with the Ahmed glaucoma valve implant. Br J Ophthalmol. 2005;89(4):454–8.
Fan JC, Niederer RL, Von Lany H, Polkinghorne PJ. Infectious endophthalmitis: clinical features, management and visual outcomes. Clin Exp Ophthalmol. 2008;36(7):631–6.
Andreoli CM, Andreoli MT, Kloek CE, Ahuero AE, Vavvas D, Durand ML. Low rate of endophthalmitis in a large series of open globe injuries. Am J Ophthalmol. 2009;147(4):601–8. e2.
Chhabra S, Kunimoto DY, Kazi L, Regillo CD, Ho AC, Belmont J, et al. Endophthalmitis after open globe injury: microbiologic spectrum and susceptibilities of isolates. Am J Ophthalmol. 2006;142(5):852–4.
Faghihi H, Hajizadeh F, Esfahani MR, Rasoulinejad SA, Lashay A, Mirshahi A, et al. Posttraumatic endophthalmitis: report No. 2. Retina. 2012;32(1):146–51.
Zhang Y, Zhang M, Jiang C, Yao Y, Zhang K. Endophthalmitis following open globe injury. Br J Ophthalmol. 2010;94(1):111–4.
Essex RW, Yi Q, Charles PG, Allen PJ. Post-traumatic endophthalmitis. Ophthalmology. 2004;111(11):2015–22.
Al-Omran AM, Abboud EB, El-Asrar AMA. Microbiologic spectrum and visual outcome of posttraumatic endophthalmitis. Retina. 2007;27(2):236–42.
Bhagat N, Nagori S, Zarbin M. Post-traumatic infectious endophthalmitis. Surv Ophthalmol. 2011;56(3):214–51.
Al-Rashaed SA, Abu El-Asrar AM. Exogenous endophthalmitis in pediatric age group. Ocul Immunol Inflamm. 2006;14(5):285–92.
Vedantham V, Nirmalan PK, Ramasamy K, Prakash K, Namperumalsamy P. Clinico-microbiological profile and visual outcomes of post-traumatic endophthalmitis at a tertiary eye care center in South India. Indian J Ophthalmol. 2006;54(1):5.
Das T, Choudhury K, Sharma S, Jalali S, Nuthethi R, Endophthalmitis Research Group. Clinical profile and outcome in Bacillus endophthalmitis. Ophthalmology. 2001;108(10):1819–25.
Callegan MC, Kane ST, Cochran DC, Novosad B, Gilmore MS, Gominet M, et al. Bacillus endophthalmitis: roles of bacterial toxins and motility during infection. Invest Ophthalmol Vis Sci. 2005;46(9):3233–8.
Gupta A, Srinivasan R, Kaliaperumal S, Saha I. Post-traumatic fungal endophthalmitis—a prospective study. Eye. 2008;22(1):13–7.
Schmidseder E, de Kaspar HM, Kampik A, Klauß V. Post-traumatic endophthalmitis after penetrating eye injury. Risk factors, microbiological diagnosis and functional outcome. Ophthalmolge. 1998;95(3):153–7.
Woodcock MG, Scott RA, Huntbach J, Kirkby GR. Mass and shape as factors in intraocular foreign body injuries. Ophthalmology. 2006;113(12):2262–9.
Soheilian M, Rafati N, Mohebbi M-R, Yazdani S, Habibabadi HF, Feghhi M, et al. Prophylaxis of acute posttraumatic bacterial endophthalmitis: a multicenter, randomized clinical trial of intraocular antibiotic injection, report 2. Arch Ophthalmol. 2007;125(4):460–5.
Ahmed Y, Schimel A, Pathengay A, Colyer M, Flynn H. Endophthalmitis following open-globe injuries. Eye. 2012;26(2):212–7.
Sternberg P, Martin DF. Management of endophthalmitis in the post-endophthalmitis vitrectomy study era. Arch Ophthalmol. 2001;119(5):754–5.
Vaziri K, Pershing S, Albini TA, Moshfeghi DM, Moshfeghi AA. Risk factors predictive of endogenous endophthalmitis among hospitalized patients with hematogenous infections in the United States. Am J Ophthalmol. 2015;159(3):498–504.
Jackson TL, Paraskevopoulos T, Georgalas I. Systematic review of 342 cases of endogenous bacterial endophthalmitis. Surv Ophthalmol. 2014;59(6):627–35.
Shankar K, Gyanendra L, Hari S, Dev Narayan S. Culture proven endogenous bacterial endophthalmitis in apparently healthy individuals. Ocul Immunol Inflamm. 2009;17(6):396–9.
Wong J-S, Chan T-K, Lee H-M, Chee S-P. Endogenous bacterial endophthalmitis: an east Asian experience and a reappraisal of a severe ocular affliction. Ophthalmology. 2000;107(8):1483–91.
Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48(4):403–23.
Chung KS, Kim YK, Song YG, Kim CO, Han SH, Chin BS, et al. Clinical review of endogenous endophthalmitis in Korea: a 14-year review of culture positive cases of two large hospitals. Yonsei Med J. 2011;52(4):630–4.
Okada AA, Johnson RP, Liles WC, D’Amico DJ, Baker AS. Endogenous bacterial endophthalmitis: report of a ten-year retrospective study. Ophthalmology. 1994;101(5):832–8.
Chen K-J, Hwang Y-S, Chen Y-P, Lai C-C, Chen T-L, Wang N-K. Endogenous Klebsiella endophthalmitis associated with Klebsiella pneumoniae pneumonia. Ocul Immunol Inflamm. 2009;17(3):153–9.
Takebayashi H, Mizota A, Tanaka M. Relation between stage of endogenous fungal endophthalmitis and prognosis. Graefe’s Arch Clin Exp Ophthalmol. 2006;244(7):816–20.
Connell P, O’Neill E, Fabinyi D, Islam F, Buttery R, McCombe M, et al. Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye. 2011;25(1):66–72.
Shah C, McKey J, Spirn M, Maguire J. Ocular candidiasis: a review. Br J Ophthalmol. 2008;92(4):466–8.
Riddell Iv J, Mcneil SA, Johnson TM, Bradley SF, Kazanjian PH, Kauffman CA. Endogenous Aspergillus endophthalmitis: report of 3 cases and review of the literature. Medicine. 2002;81(4):311–20.
Almeida DR, Miller D, Alfonso EC. Anterior chamber and vitreous concordance in endophthalmitis: implications for prophylaxis. Arch Ophthalmol. 2010;128(9):1136–9.
Bispo PJM, de Melo GB, Hofling-Lima AL, Pignatari ACC. Detection and gram discrimination of bacterial pathogens from aqueous and vitreous humor using real-time PCR assays. Invest Ophthalmol Vis Sci. 2011;52(2):873–81.
Sugita S, Kamoi K, Ogawa M, Watanabe K, Shimizu N, Mochizuki M. Detection of Candida and Aspergillus species DNA using broad-range real-time PCR for fungal endophthalmitis. Graefe’s Arch Clin Exp Ophthalmol. 2012;250(3):391–8.
Sridhar J, Flynn HW Jr, Kuriyan AE, Miller D, Albini T. Endogenous fungal endophthalmitis: risk factors, clinical features, and treatment outcomes in mold and yeast infections. J Ophthalmic Inflamm Infect. 2013;3(1):60.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Paris Tranos, Nikolaos Dervenis, Athanasios N. Vakalis, Solon Asteriadis, Panagiotis Stavrakas and Anastasios G. P. Konstas have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content To view enhanced content for this article go to http://www.medengine.com/Redeem/F744F0607E2CF57F.
Rights and permissions
About this article
Cite this article
Tranos, P., Dervenis, N., Vakalis, A.N. et al. Current Perspectives of Prophylaxis and Management of Acute Infective Endophthalmitis. Adv Ther 33, 727–746 (2016). https://doi.org/10.1007/s12325-016-0307-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-016-0307-8